Abstract
The global spread of carbapenem-resistant Enterobacteriaceae (CRE) is increasingly becoming a major challenge in clinical and public health settings. To date, the treatment for serious CRE infections remains difficult. The intelligent use of antimicrobials and effective infection control strategies is crucial to prevent further CRE spread. Early consultation with experts in the treatment of infections with multidrug-resistant organisms is valuable in patient management. This brief review will focus on the current, yet limited, treatment options for CRE infections.
Similar content being viewed by others
Review
Introduction
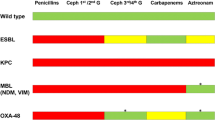
The global spread of carbapenem-resistant Enterobacteriaceae (CRE) has become a major challenge in clinical and public health settings. Infections with CRE organisms that are multidrug-resistant (that is, non-susceptible to at least one antimicrobial in at least three antimicrobial classes), extensively drug-resistant (that is, non-susceptible to at least one antimicrobial from all but one or two antimicrobial classes), or pan-drug-resistant (that is, non-susceptible to all antimicrobial agents) are difficult to treat [1]. As a result, severe infections with CRE have significant morbidity, mortality, and health-care costs [2–4]. Most CRE have beta-lactamases belonging to the Ambler class A, B, and D [5]. Table 1 summarizes main examples from each class of carbapenemase-producing organisms.
Carbapenems are no longer fully effective in the CRE epidemic. The paucity of novel antimicrobials in development escalates the antimicrobial resistance problem, severely reducing the available therapeutic choices. In this review, we will summarize the main treatment options used in clinical practice as well as the few antimicrobials currently in development. For issues related to the epidemiology, detection, and prevention of infections with CRE, the reader is referred to several excellent reviews published on this topic [6, 10].
Current treatment options for infections with carbapenem-resistant Enterobacteriaceae
The optimal treatment of infections with CRE is uncertain, as most data come from retrospective case series and anecdotal case reports; few prospective studies or randomized controlled trials are published on this topic. Since carbapenem-producing organisms are often resistant to other antimicrobial classes in addition to most beta-lactams, additional susceptibility testing to antimicrobials such as colistin, fosfomycin, tigecycline, aztreonam, and rifampin is needed [11, 12]. Consultations from experts in the treatment of infections with multidrug-resistant organisms may also prove valuable in patient management. The following antimicrobial therapies have been used with various levels of success in the treatment of CRE infections.
Colistin (polymyxin E)
Colistin (polymyxin E) is an old bactericidal antibiotic with cationic detergent properties. It disrupts the outer cell membrane of the Gram-negative bacilli by binding to the lipid A component of the lipopolysaccharide, causing leakage of cytoplasmic contents and bacterial cell death [13]. The antibacterial spectrum includes most of the Enterobacteriaceae (Escherichia coli, Klebsiella, Salmonella, Shigella, and Enterobacter), Pseudomonas, Acinetobacter, and Stenotrophomonas species. However, colistin is not active against Burkholderia cepacia, Serratia marcescens, Moraxella catarrhalis, pathogenic Neisseria spp, Proteus spp, Providencia spp, or Morganella morganii[14]. Colistin is also not active in vitro against anaerobes and aerobic Gram-positive cocci.
The ideal dose for colistin in the treatment of severe CRE infections is uncertain. In addition, significant confusion may arise because of differences in formulations between the intravenous (IV) product available in the US (colistin-based) and the one available in Europe and other regions (colistimethate sodium) (Table 2). Recent studies suggest that higher treatment doses [15] or an initial loading dose followed by higher maintenance dosing regimens may be needed for improved clinical outcomes, especially for infections with organisms with high minimum inhibitory concentrations (MICs) [16]. Specifically, for organisms with a colistin MIC of not more than 2 mg/L, some authors recommend a loading dose of 2.5 mg/kg given over a 2-hour infusion, followed by a maintenance dose of 3 mg/kg per day, based on population pharmacokinetic studies in critically ill patients [16]. Colistin monotherapy is not recommended for organisms with MICs to colistin of at least 4 mg/L [16]. Dalfino and colleagues [17], in their prospective cohort study of 25 critically ill patients with bacteremia or ventilator-associated pneumonia caused by CRE (Klebsiella) and other carbapenem-resistant bacteria (Acinetobacter and Pseudomonas), used a regimen of 9 million IU of colistimethate sodium loading dose (270 mg colistin base), followed by a maintenance dose of 4.5 million IU of colistimethate sodium (135 mg colistin base) every 12 hours in patients with normal renal function. For patients with underlying renal injury, the dosing interval was adjusted appropriately based on their renal clearance. The clinical cure achieved in this high-dose study was 82.1%, with a 17.8% rate of colistin-related acute kidney injury which was reversible within 10 days of discontinuing the drug. Of note, colistin monotherapy was administered to less than half of the patients in this study. Most patients received combination therapy with a carbapenem or aminoglycoside in addition to colistin, although only eight Klebsiella pneumoniae isolates were susceptible to gentamicin and none of the isolates was susceptible to carbapenems [17]. The colistin dosing strategy used by Dalfino and colleagues in this study of critically ill patients does seem to validate the recommendations from recent population pharmacokinetic analyses [18–20], suggesting that for severe infections in ICU patients the most effective bacterial killing is obtained with a loading dose, followed by higher overall maintenance doses given at extended intervals.
The importance of combination therapy is also suggested by several other retrospective studies. In a cohort of patients with bloodstream infections caused by K. pneumoniae carbapenemase (KPC)-producing organisms, none of the 14 patients treated with colistin in combination with one or more antimicrobials (tigecycline ± carbapenem ± gentamicin) died, whereas four of the seven patients treated with colistin alone died from their infection [21]. Similarly, colistin-polymyxin B combined with carbapenem had a mortality of 12.5% (1 out of 6) versus 66.7% (8 out of 12) in a study of patients with bacteremia caused by KPC-producing K. pneumoniae bacteremia [22]. Finally, in a large retrospective study of 125 patients with KPC-producing K. pneumoniae sepsis from three hospitals in Italy, the combination of colistin with tigecycline and extended-infusion meropenem (2 g IV infused over 3 hours every 8 hours) had the lowest mortality (13%) versus 50% mortality for those patients receiving colistin monotherapy [23].
The most common adverse event with colistin is nephrotoxicity, which can develop in up to half of the patients treated with high parenteral doses but which seems to be reversible in most cases [20, 24]. Reports of resistance to colistin among KPC-producing K. pneumoniae strains [25, 26], though rare, are concerning, especially for combination treatment regimens where colistin is intended as the major active component.
Polymyxin B
Polymyxin B differs from colistin by one amino acid [27]. In contrast to colistin, however, it is administered as its active form and thus achieves higher plasma concentrations faster, making the need for a loading dose less stringent [16]. Polymyxin B is not cleared by the kidney and therefore does not require renal dose adjustment [28]. The clinical experience with polymyxin B in treatment of CRE infections is limited to small case series. Bergamasco and colleagues [29], in their description of a KPC-producing K. pneumoniae nosocomial outbreak among solid organ transplant patients, reported a survival rate of 67% (6 out of 9) for the patients treated with polymyxin B alone or in combination with tigecycline or carbapenem. These patients with pneumonia, bloodstream, urinary tract, or skin and soft tissue infections were given a polymyxin B dose of 25,000 or 15,000 IU/kg for a creatinine clearance of at least 50 mL/minute or less than 50 mL/minute, respectively. As is true for colistin, polymyxin B used in combination therapy for severe infections may be more effective, especially when one considers the possibility of resistance development during monotherapy. In this regard, Lee and colleagues [30] described the emergence of resistance to polymyxin B for three out of 12 patients treated with polymyxin B for their KPC-producing K. pneumoniae bloodstream infections; in contrast, none of the four patients treated with polymyxin B in combination with tigecycline developed resistance during therapy [30].
Carbapenems
Carbapenems have been used, though counter-intuitively, in the treatment of infections with CRE, usually as the adjuvant component of a combination drug regimen. This strategy is potentially useful only when the MICs of the infecting carbapenem-resistant organisms are still relatively low (that is, not more than 4 to 8 mg/L) [31]. Thus, the MICs should always be determined and taken into account if carbapenems are contemplated as a potential treatment option. Bacterial killing for isolates with MICs of 4 mg/L is more likely with high-dose, prolonged-infusion regimens (that is, meropenem 2 g IV infused over 3 hours every 8 hours) [32]. The outcomes of carbapenem treatment in patients infected with multidrug-resistant Gram-negative organisms, including CRE, as reported anecdotally, in small case series or small retrospective clinical studies are summarized in the excellent review by Daikos and Markogiannakis [31]. A systematic review of 34 studies compiling a total of 298 patients treated for infections with KPC or metallo-beta-lactamase-producing K. pneumoniae found a combination regimen of at least two active drugs, one of which was a carbapenem, to be associated with the lowest failure rate (8%) compared with other regimens studied [10] (Table 3). As previously mentioned, Tumbarello and colleagues [23] found the triple-combination regimen of colistin, tigecycline, and meropenem to be associated with the highest odds of survival in their multicenter retrospective cohort study of 125 patients with KPC-producing K. pneumoniae bloodstream infections. Meropenem was administered as an extended infusion over at least 3 hours, at 2 g IV every 8 hours, with renal adjustment as needed. However, although more than 50% of the isolates in this study were fully resistant to meropenem (MIC of at least 16 mg/L), the vast majority of the isolates were susceptible to colistin (88% with MICs of not more than 2 mg/L) and tigecycline (91.2% with MICs of not more than 2 mg/L).
Recently, a double-carbapenem combination (ertapenem-doripenem) has been proposed as a potential treatment strategy for KPC-producing bacteria [38, 39]. Data come from in vitro experiments on a murine animal model [38] as well as in vivo. Regarding the latter, three patients with bacteremia or urinary tract infection (UTI) caused by pan-resistant KPC-producing K. pneumoniae[39] and one ICU patient with bacteremia and sepsis caused by colistin-resistant KPC-producing K. pneumoniae were reported to have been successfully treated with a double-carbapenem combination [40]. Most recently, Karaiskos and colleagues [41] reported treating 14 patients with bacteremias and UTIs, including two patients with septic shock caused by KPC-producing K. pneumoniae with double-carbapenems therapy, as follows: 1 g ertapenem IV daily, followed 1 hour later by meropenem at 2 g every 8 hours infused over 3 hours. All treated patients experienced clinical and microbiological cure at 1-month follow-up, although four patients experienced a recurrence of their UTI [41]. Nevertheless, since the clinical experience with this salvage therapy is still limited, concerns for promoting further carbapenem resistance remain [33], and the MICs of many carbapenem-producing organisms are sufficiently high to render carbapenems ineffective, this treatment strategy is not routinely recommended for clinical practice at the present time.
Tigecycline
Tigecycline has been shown to have in vitro activity against multidrug-resistant Enterobacteriaceae isolates [42]. Tigecycline has been used in the treatment of infections with CRE primarily as an adjuvant drug in combination therapy (Table 3) [43–45]. However, the clinical experience with tigecycline has been somewhat disappointing, especially for severe infections such as bloodstream infections or nosocomial pneumonias, for which the drug does not have US Food and Drug Administration (FDA) approval. For example, Kontopidou and colleagues [46], in their study of 127 ICU patients with bacteremias or ventilator-associated pneumonias caused by carbapenem-resistant K. pneumoniae, found that patients treated with tigecycline, especially as monotherapy (at doses of 100 to 200 mg/day), had the highest failure rates compared with other drug combinations. Most patients treated with tigecycline in this cohort had an MIC of 2 μg/mL (which is the cutoff for susceptibility) and severe infections with high Acute Physiology and Chronic Health Evaluation II scores, which may explain why tigecycline was ineffective [46]. Post-approval meta-analyses have shown that tigecycline had lower cure rates and higher mortality compared with other treatment regimens in pooled randomized controlled trials of various infectious syndromes [47–49]. When evaluated in a randomized controlled trial of hospital-acquired pneumonia, tigecycline plus ceftazidime was inferior to vancomycin and imipenem-cilastatin for the treatment of ventilator-associated pneumonia [50]. The problem may be related to the low plasma serum concentrations achieved by the dose recommended by the manufacturer (100 mg loading dose followed by a maintenance dose of 50 mg every 12 hours), which is likely ineffective against pathogens with an MIC of between 0.4 and 1 mg/L. Higher doses have been used in clinical practice [51]. In fact, a recent phase 2 randomized controlled trial of patients with hospital-acquired pneumonia studied higher doses of tigecycline (150 mg loading followed by 75 mg every 12 hours, and 200 mg loading followed by 100 mg maintenance dose every 12 hours) versus imipenem/cilastatin. Clinical cure rates were the highest in the arm with the highest dose regimen of tigecycline, whereas the safety profile was similar to that of the lower dose regimens [52]. Nevertheless, in 2010, the FDA added a warning regarding the risk of increased mortality with tigecycline treatment, especially for non-approved indications such as hospital- or ventilator-associated pneumonias (found at [53]). This safety concern was upgraded to a stronger Boxed Warning in 2013, after analysis of 10 clinical trials of tigecycline use for FDA-approved indications, including trials conducted after drug approval, still showed a higher (0.6%) risk of death for patients treated with tigecycline versus other antimicrobials (found at [54]). As a result, many clinicians have chosen tigecycline-based regimens only when other therapies were not available. The low concentration of tigecycline in the urine further limits the use of this antimicrobial for the treatment of UTIs. Unless more compelling evidence of improved clinical outcomes in well-designed studies of high-dose tigecycline becomes available, tigecycline monotherapy is not routinely recommended for severe infections such as bacteremia or hospital-acquired pneumonia.
Fosfomycin
Fosfomycin is another old broad-spectrum antibiotic that inhibits bacterial cell wall synthesis and has in vitro activity against CRE [12, 55]. The oral formulation achieves high concentrations in the urine and is usually effective in the treatment of non-complicated UTIs [56]. The IV formulation (fosfomycin disodium) is not available in the US and other countries, although it has been used successfully in Greece, mostly as an adjuvant drug in combination therapies [57]. For example, a study of 11 critically ill patients with nosocomial infections caused by KPC K. pneumoniae were treated with IV fosfomycin (2 to 4 g every 6 hours) in combination with colistin (n = 6), gentamicin (n = 3), and piperacillin/tazobactam (n = 1). All patients were reported to have good treatment-related microbiological and clinical outcomes, while the all-cause hospital mortality was 18.2% (two patients) [34]. The emergence of resistance to fosfomycin during therapy for bacteremia with KPC K. pneumoniae has been reported and is especially concerning since fosfomycin was used as an adjunct in combination therapy in these cases [58]. Recently, parenteral fosfomycin administered in combination with colistin or tigecycline was studied in a prospective observational multicenter trial in 11 ICUs in Europe. In total, 41 patients with bacteremia or ventilator-associated pneumonia caused by carbapenemase-producing K. pneumoniae were treated with a median dose of 24 g of fosfomycin per day for a total of 14 days. Microbiological cure was reported in 56.5% of cases, with an all-cause 28-day mortality of 43.5% and emergence of resistance in three patients [35].
Antimicrobials in development
Several parenteral antimicrobial therapies are currently under investigation for the treatment of multidrug-resistant Gram-negative infections, including CRE. Ceftazidime-avibactam (a new beta-lactamase inhibitor) is active against extended-spectrum beta-lactamase-producing organisms, some resistant Pseudomonas aeruginosa strains, and CRE of the KPC type, but not against metallo-beta-lactamases such as New Delhi metallo-beta-lactamase and verona integron-encoded metallo-beta-lactamase. It is currently undergoing phase 3 studies for complicated UTI and intra-abdominal infections [59]. Ceftaroline-avibactam, entering phase 3 trials, is similarly active against KPC-producing strains, but not against P. aeruginosa or other metallo-beta-lactamase-producing organisms. Neither one of these drugs in development has activity against Acinetobacter species [6, 59]. Imipenem in combination with another novel beta-lactamase inhibitor, MK-7655, appears active in vitro against serine carbapenemase-producing organisms and against P. aeruginosa, but not against metallo-carbapenemase-producing organisms or Acinetobacter baumannii[59]. Plazomycin (ACHN-490), a new aminoglycoside currently in development, has activity against isolate-producing KPC enzymes and does not seem susceptible to the same resistance mechanisms present in older aminoglycosides, although it does not have activity against strains that harbor 16S ribosomal methylases. It has completed phase 2 trials [60]. Biapenem/RPX7009 (Carbavance; Rempex Pharmaceuticals, Inc., San Diego, CA, USA), a carbapenem combined with a novel boronate inhibitor, currently in phase 1 trials, appears active in vitro against KPC-producing organisms and other class A carbapenemases, including resistant Pseudomonas and Acinetobacter strains, although it is not active against class B and D carbapenemases [6, 61]. Eravacycline is a novel tetracycline that is not susceptible to efflux resistance mechanisms or to the protection of the ribosomal target that renders older tetracyclines ineffective. It has in vitro activity against KPC-producing bacteria but not against non-fermenters [62].
Conclusions
None of the antimicrobials currently in development has activity against the entire spectrum of carbapenemase-producing Gram-negative bacteria. The mortality associated with the failure rates from the current salvage therapies highlighted above is disconcerting. The treatment of serious infections with CRE remains an enormous challenge. A concerted global commitment to the intelligent use of antimicrobials, better antibiotic stewardship, the implementation of effective infection control strategies, and the development of more effective therapies are desperately needed.
Note
This article is part of a series on Antibiotic resistance in the ICU, edited by Steven Opal. Other articles in this series can be found at http://ccforum.com/series/antibioticresistance.
Abbreviations
- CRE:
-
Carbapenem-resistant Enterobacteriaceae
- FDA:
-
US Food and Drug Administration
- IV:
-
Intravenous
- KPC:
-
Klebsiella pneumoniae carbapenemase
- MIC:
-
Minimum inhibitory concentration
- UTI:
-
Urinary tract infection.
References
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL: Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012, 18: 268-281. 10.1111/j.1469-0691.2011.03570.x
Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP: Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008, 29: 1099-1106. 10.1086/592412
Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA: Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 2012, 4: 148ra116.
Evans HL, Lefrak SN, Lyman J, Smith RL, Chong TW, McElearney ST, Schulman AR, Hughes MG, Raymond DP, Pruett TL, Sawyer RG: Cost of Gram-negative resistance. Crit Care Med 2007, 35: 89-95. 10.1097/01.CCM.0000251496.61520.75
Queenan AM, Bush K: Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 2007, 20: 440-458. table of contents 10.1128/CMR.00001-07
Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP: Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013, 13: 785-796. 10.1016/S1473-3099(13)70190-7
Bratu S, Tolaney P, Karumudi U, Quale J, Mooty M, Nichani S, Landman D: Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J Antimicrob Chemother 2005, 56: 128-132. 10.1093/jac/dki175
Nordmann P, Naas T, Poirel L: Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011, 17: 1791-1798. 10.3201/eid1710.110655
Centers for Disease Control and Prevention (CDC): Notes from the Field: New Delhi metallo-β-lactamase-producing Escherichia coli associated with endoscopic retrograde cholangiopancreatography - Illinois, 2013. MMWR Morb Mortal Wkly Rep 2014, 62: 1051.
Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL: Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012, 25: 682-707. 10.1128/CMR.05035-11
Zavascki AP, Barth AL, Gaspareto PB, Goncalves AL, Moro AL, Fernandes JF, Goldani LZ: Risk factors for nosocomial infections due to Pseudomonas aeruginosa producing metallo-beta-lactamase in two tertiary-care teaching hospitals. J Antimicrob Chemother 2006, 58: 882-885. 10.1093/jac/dkl327
Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N: What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents 2011, 37: 415-419. 10.1016/j.ijantimicag.2011.01.012
Newton BA: The properties and mode of action of the polymyxins. Bacteriol Rev 1956, 20: 14-27.
Gales AC, Reis AO, Jones RN: Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J Clin Microbiol 2001, 39: 183-190. 10.1128/JCM.39.1.183-190.2001
Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, Kapaskelis A, Nikita D, Michalopoulos A: Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents 2010, 35: 194-199. 10.1016/j.ijantimicag.2009.10.005
Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhão RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP: Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 2013, 57: 524-531. 10.1093/cid/cit334
Dalfino L, Puntillo F, Mosca A, Monno R, Spada ML, Coppolecchia S, Miragliotta G, Bruno F, Brienza N: High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012, 54: 1720-1726. 10.1093/cid/cis286
Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL: Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011, 55: 3284-3294. 10.1128/AAC.01733-10
Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H: Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 2009, 53: 3430-3436. 10.1128/AAC.01361-08
Roberts JA, Lipman J: Editorial commentary: Closing the loop - a colistin clinical study to confirm dosing recommendations from PK/PD modeling. Clin Infect Dis 2012, 54: 1727-1729. 10.1093/cid/cis311
Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A: Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011, 17: 1798-1803. 10.1111/j.1469-0691.2011.03514.x
Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y: Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012, 56: 2108-2113. 10.1128/AAC.06268-11
Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M: Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012, 55: 943-950. 10.1093/cid/cis588
Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G: Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis 2009, 48: 1724-1728. 10.1086/599225
Bogdanovich T, Adams-Haduch JM, Tian GB, Nguyen MH, Kwak EJ, Muto CA, Doi Y: Colistin-resistant, Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae belonging to the international epidemic clone ST258. Clin Infect Dis 2011, 53: 373-376. 10.1093/cid/cir401
Kontopoulou K, Protonotariou E, Vasilakos K, Kriti M, Koteli A, Antoniadou E, Sofianou D: Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 beta-lactamase resistant to colistin. J Hosp Infect 2010, 76: 70-73. 10.1016/j.jhin.2010.03.021
Landman D, Georgescu C, Martin DA, Quale J: Polymyxins revisited. Clin Microbiol Rev 2008, 21: 449-465. 10.1128/CMR.00006-08
Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J: Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis 2008, 47: 1298-1304. 10.1086/592577
Bergamasco MD, Barroso Barbosa M, de Oliveira Garcia D, Cipullo R, Moreira JC, Baia C, Barbosa V, Abboud CS: Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in solid organ transplantation. Transpl Infect Dis 2012, 14: 198-205. 10.1111/j.1399-3062.2011.00688.x
Lee J, Patel G, Huprikar S, Calfee DP, Jenkins SG: Decreased susceptibility to polymyxin B during treatment for carbapenem-resistant Klebsiella pneumoniae infection. J Clin Microbiol 2009, 47: 1611-1612. 10.1128/JCM.02466-08
Daikos GL, Markogiannakis A: Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011, 17: 1135-1141. 10.1111/j.1469-0691.2011.03553.x
Levy Hara G, Gould I, Endimiani A, Pardo PR, Daikos G, Hsueh PR, Mehtar S, Petrikkos G, Casellas JM, Daciuk L, Paciel D, Novelli A, Saginur R, Pryluka D, Medina J, Savio E: Detection, treatment, and prevention of carbapenemase-producing Enterobacteriaceae: recommendations from an International Working Group. J Chemother 2013, 25: 129-140. 10.1179/1973947812Y.0000000062
Sbrana F, Malacarne P, Viaggi B, Costanzo S, Leonetti P, Leonildi A, Casini B, Tascini C, Menichetti F: Carbapenem-sparing antibiotic regimens for infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in intensive care unit. Clin Infect Dis 2013, 56: 697-700. 10.1093/cid/cis969
Michalopoulos A, Virtzili S, Rafailidis P, Chalevelakis G, Damala M, Falagas ME: Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin Microbiol Infect 2010, 16: 184-186. 10.1111/j.1469-0691.2009.02921.x
Pontikis K, Karaiskos I, Bastani S, Dimopoulos G, Kalogirou M, Katsiari M, Oikonomou A, Poulakou G, Roilides E, Giamarellou H: Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents 2014, 43: 52-59. 10.1016/j.ijantimicag.2013.09.010
Alexander BT, Marschall J, Tibbetts RJ, Neuner EA, Dunne WM Jr, Ritchie DJ: Treatment and clinical outcomes of urinary tract infections caused by KPC-producing Enterobacteriaceae in a retrospective cohort. Clin Ther 2012, 34: 1314-1323. 10.1016/j.clinthera.2012.05.002
Satlin MJ, Kubin CJ, Blumenthal JS, Cohen AB, Furuya EY, Wilson SJ, Jenkins SG, Calfee DP: Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011, 55: 5893-5899. 10.1128/AAC.00387-11
Bulik CC, Nicolau DP: Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011, 55: 3002-3004. 10.1128/AAC.01420-10
Giamarellou H, Galani L, Baziaka F, Karaiskos I: Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother 2013, 57: 2388-2390. 10.1128/AAC.02399-12
Ceccarelli G, Falcone M, Giordano A, Mezzatesta ML, Caio C, Stefani S, Venditti M: Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2013, 57: 2900-2901. 10.1128/AAC.00188-13
Karaiskos I, Masgala A, Galani L, Baziaka F, Giamarellou H: Double carbapenems regimens for infections in humans due to carbapenemase-producing Klebsiella pneumonia (CPKP). Denver, CO, USA: Paper presented at 53rd Interscience Conference in Antimicrobial Agents and Chemotherapy (ICAAC);
Sader HS, Farrell DJ, Jones RN: Tigecycline activity tested against multidrug-resistant Enterobacteriaceae and Acinetobacter spp. isolated in US medical centers (2005–2009). Diagn Microbiol Infect Dis 2011, 69: 223-227. 10.1016/j.diagmicrobio.2010.10.020
Guner R, Hasanoglu I, Keske S, Kalem AK, Tasyaran MA: Outcomes in patients infected with carbapenem-resistant Acinetobacter baumannii and treated with tigecycline alone or in combination therapy. Infection 2011, 39: 515-518. 10.1007/s15010-011-0161-1
Lee GC, Burgess DS: Treatment of Klebsiella pneumoniae carbapenemase (KPC) infections: a review of published case series and case reports. Ann Clin Microbiol Antimicrob 2012, 11: 32. 10.1186/1476-0711-11-32
Lee YT, Tsao SM, Hsueh PR: Clinical outcomes of tigecycline alone or in combination with other antimicrobial agents for the treatment of patients with healthcare-associated multidrug-resistant Acinetobacter baumannii infections. Eur J Clin Microbiol Infect Dis 2013, 32: 1211-1220. 10.1007/s10096-013-1870-4
Kontopidou F, Giamarellou H, Katerelos P, Maragos A, Kioumis I, Trikka-Graphakos E, Valakis C, Maltezou HC: Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin Microbiol Infect 2014, 20: O117-O123. 10.1111/1469-0691.12341
Cai Y, Wang R, Liang B, Bai N, Liu Y: Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011, 55: 1162-1172. 10.1128/AAC.01402-10
Prasad P, Sun J, Danner RL, Natanson C: Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012, 54: 1699-1709. 10.1093/cid/cis270
Tasina E, Haidich AB, Kokkali S, Arvanitidou M: Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011, 11: 834-844. 10.1016/S1473-3099(11)70177-3
Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, Chuang YC, Maroko RT, Dukart G, Cooper CA, Korth-Bradley JM, Dartois N, Gandjini H, 311 Study Group: Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis 2010, 68: 140-151. 10.1016/j.diagmicrobio.2010.05.012
Cunha BA: Pharmacokinetic considerations regarding tigecycline for multidrug-resistant (MDR) Klebsiella pneumoniae or MDR Acinetobacter baumannii urosepsis. J Clin Microbiol 2009, 47: 1613. 10.1128/JCM.00404-09
Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC: Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 2013, 57: 1756-1762. 10.1128/AAC.01232-12
FDA Drug Safety Communication: Increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. [http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm] []
FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new Boxed Warning. [http://www.fda.gov/drugs/drugsafety/ucm369580.htm] []
Endimiani A, Patel G, Hujer KM, Swaminathan M, Perez F, Rice LB, Jacobs MR, Bonomo RA: In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010, 54: 526-529. 10.1128/AAC.01235-09
Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME: Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents 2009, 34: 506-515. 10.1016/j.ijantimicag.2009.08.013
Michalopoulos AS, Livaditis IG, Gougoutas V: The revival of fosfomycin. Int J Infect Dis 2011, 15: e732-e739. 10.1016/j.ijid.2011.07.007
Karageorgopoulos DE, Miriagou V, Tzouvelekis LS, Spyridopoulou K, Daikos GL: Emergence of resistance to fosfomycin used as adjunct therapy in KPC Klebsiella pneumoniae bacteraemia: report of three cases. J Antimicrob Chemother 2012, 67: 2777-2779. 10.1093/jac/dks270
Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D: 10 x ′20 Progress - development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 2013, 56: 1685-1694.
Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N: Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother 2011, 66: 48-53. 10.1093/jac/dkq408
Livermore DM, Mushtaq S: Activity of biapenem (RPX2003) combined with the boronate beta-lactamase inhibitor RPX7009 against carbapenem-resistant Enterobacteriaceae. J Antimicrob Chemother 2013, 68: 1825-1831. 10.1093/jac/dkt118
Sutcliffe JA, O’Brien W, Fyfe C, Grossman TH: Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother 2013, 57: 5548-5558. 10.1128/AAC.01288-13
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yamamoto, M., Pop-Vicas, A.E. Treatment for infections with carbapenem-resistant Enterobacteriaceae: what options do we still have?. Crit Care 18, 229 (2014). https://doi.org/10.1186/cc13949
Published:
DOI: https://doi.org/10.1186/cc13949