Abstract
Introduction
Recent publications suggest potential benefits from statins as a preventive or adjuvant therapy in sepsis. Whether ongoing statin therapy should be continued or discontinued in patients admitted in the intensive care unit (ICU) for sepsis is open to question.
Methods
We retrospectively compared patients with severe sepsis and septic shock in whom statin therapy had been discontinued or continued. The primary endpoint was the number of organ failure-free days at day 14. Secondary end-points included hospital mortality and safety. The association of statin continuation with outcome was evaluated for crude analysis and after propensity score matching and adjustment. We also measured plasma atorvastatin concentrations in a separate set of ICU septic patients continuing the drug.
Results
Patients in whom statin therapy had been continued in the ICU (n = 44) had significantly more organ failure-free days (11 [6–14] vs. 6 [0-12], mean difference of 2.34, 95%CI from 0.47 to 5.21, P = 0.03) as compared to others (n = 32). However, there were important imbalances between groups, with more hospital-acquired infections, more need for surgery before ICU admission, and a trend towards more septic shock at ICU admission in the discontinuation group. The significant association of statin continuation with organ failure free days found in the crude analysis did not persist after propensity-matching or multivariable adjustment: beta coefficients [95% CI] of 2.37 [-0.96 to 5.70] (P = 0.20) and 2.24 [-0.43 to 4.91] (P = 0.11) respectively. We found particularly high pre-dose and post-dose atorvastatin concentrations in ICU septic patients continuing the drug.
Conclusions
Continuing statin therapy in ICU septic patients was not associated with reduction in the severity of organ failure after matching and adjustment. In addition, the very high plasma concentrations achieved during continuation of statin treatment advocates some caution.
Similar content being viewed by others
Introduction
Statins are effective lipid-lowering agents that have been shown to improve survival in the primary and secondary prevention of atherosclerosis in several large randomized clinical trials [1]. Many experimental models have also shown pleiotropic activity of statins (including anti-inflammatory, anti-oxidative, and immunomodulatory effects) that may account for a potential beneficial impact during sepsis [2, 3]. A recent systematic review and meta-analysis of 20 clinical studies suggests that statins may have a positive impact on the outcome of patients with infection or sepsis [4]. Since January 2006 [2], we encouraged continuation of ongoing statin therapy whenever possible in patients chronically treated with statins who were admitted to our ICU with severe sepsis, although current prescribing guidelines still suggest caution in the continued use of statins in patients hospitalized for acute illness because of concern of serious side effects [5].
The aim of this preliminary report was: to evaluate the effectiveness and safety of statin therapy continuation on the incidence of organ failure in septic patients (compared with patients in whom statins were routinely stopped); and to assess atorvastatin plasma concentrations during its continuation in a subset of ICU septic patients.
Materials and methods
The study was approved by the institutional ethics committee of the "Société de Réanimation de Langue Française". Informed consent was waived and written and oral information about the study was given to the families.
Patients
We conducted a retrospective cohort study among patients admitted between January 2005 and August 2007 for severe sepsis and septic shock in our ICU and with ongoing statin therapy (initiated at least one month before ICU admission and continued with no interruption until ICU admission). Severe sepsis or septic shock was defined according to the ACCP/SCCM (American College of Chest Physicians/Society of Critical Care Medicine) Consensus Conference [6]. Non-inclusion criteria included a moribund state, an anticipated ICU stay of less than 24 hours, a contraindication to enteral statin therapy administration (intolerance to enteral feeding with vomiting), liver dysfunction with aminotransferase enzymes (either aspartate or alanine) more than three times the upper limit of normal (ULN), rhabdomyolysis with creatine phosphokinase (CPK) levels above five ULN, myopathy, status epilepticus, concomitant administration of azole derivatives, delavirdine, or telithromycin.
Discontinuation and continuation groups
In the first period (January to December 2005), routine discontinuation of ongoing statin therapy at admission of septic patients to our ICU was recommended. The second period (January to December 2006) was an overlap period during which the decision to continue or discontinue ongoing statin therapy was left to the clinician in charge of the patient. During the third period (January to August 2007), routine continuation of ongoing statin therapy was encouraged. Consecutive patients with severe sepsis and septic shock were retrospectively identified from January 2005 to August 2007 using the unit computerized database and data collected by manual chart review. Two groups of septic patients were distinguished: the discontinuation group (patients in whom statins were stopped at ICU admission); and continuation group (patients in whom statins were continued whenever possible). Patients in both groups were managed in the ICU according to current recommendations [7].
Statin continuation protocol
"Continuation" patients received their usual statin therapy orally (or via an oro-gastric tube, after crushing the tablets) in the same dosage as used during ambulatory care, while conforming to several other precautions as follows: i) the association with certain medications was avoided (oral anticoagulants, fibrates and cyclosporine for all statins, erythromycin, clarithromycin, or verapamil for atorvastatin and simvastatin); ii) if the patient received enteral feeding via an oro-gastric tube, gastric residue evaluations were performed at least six hours after drug administration. Possible side effects of statins were monitored, by reviewing daily blood chemistry as well as aminotransferase and CPK levels when available. Statin therapy was interrupted in the following cases: food intolerance with vomiting, aminotransferase elevation above three ULN, increase in serum CPK levels to above five ULN. In case of interruption of statin therapy because of food intolerance, the treatment was reintroduced after 24 hours of resumption of enteral feeding without vomiting. The analysis was performed on an intention-to-treat basis (i.e., patients in the continuation group remained in that group even if statins were secondarily discontinued during ICU stay).
Study of atorvastatin plasma concentrations
We prospectively assessed atorvastatin pharmacokinetics during its continuation (in accordance with the above mentioned statin continuation protocol) in nine ICU patients admitted for severe sepsis or septic shock during the year 2008 (these patients are not included in the above mentioned retrospective cohort analysis). A total of 11 daily administrations of 40 mg of atorvastatin were assessed for plasma concentrations (two patients were assessed twice). Blood samples were collected pre-dose and at 90 minutes post-dose. Demographic data and information on concurrent medications that might interact with the cytochrome P450 3A4 enzyme system were collected. After centrifugation, plasma was stored frozen at -80°C until analysis. Samples were analysed by High Performance Liquid Chromatography (ThermoFisher Scientific, Waltham, USA) using ultraviolet detection at 245 nm (Waters, Saint-Quentin, France) [8]. The mobile phase was NaH2PO4 10 mM pH5.5/ACN (67/33, v/v), and thiopental was used as internal standard. Standard calibration curves for atorvastatin were linear over concentrations ranging from 20 to 200 ng/mL (average r2 of 0.99). The limit of quantification was 20 ng/mL. Intraday precision was good with a coefficient of variation of 15.3%, 6.0%, and 3.2% for three levels of control 25, 75, and 150 ng/mL. Interday precision was also acceptable with coefficient of variation of 18%, 7.1%, and 5.7%, respectively. Accuracy was correct with error percentages of 5.1%, 2.0%, and 2.5%, respectively, for the three controls.
Endpoints
The primary endpoint was the number of organ failure-free days up to day 14. Secondary end-points included the number of hemodynamic failure-free days and organ dysfunction-free days up to day 14, ICU and hospital survival, and evaluation of treatment safety, assessed as the proportion of patients with serum CPK above three ULN and of patients with a transaminases level above two ULN. Organ dysfunction and organ failure were defined by a Sequential Organ Failure Assessment (SOFA) score for the appropriate function above one and above two, respectively [9]. Hemodynamic failure was defined by a cardiovascular SOFA score above two (dopamine > 5 μg/kg/min, norepinephrine regardless of the dose and adrenaline regardless of the dose) [10]. Organ failure-free days were defined as the number of days between ICU admission (day 1) and day 14 with the patient alive without any organ failure. Organ failure-free days were considered equal to zero in case of ICU death before day 14. Patients with unavailable SOFA score (because of ICU discharge before day 14) were considered free from organ failure after ICU discharge. We also assessed the pre-dose and post-dose plasma atorvastatin concentrations during its continuation.
Statistical analysis
Statistical analysis was performed using SPSS Base 17.0 package (SPSS Inc, Chicago, IL, USA) and the nonrandom package 1.1 using R 2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria) [11]. Dichotomous variables are reported as percentage and compared using the Chi-square test or exact Fisher test (when the expected count was < 5). Quantitative variables are reported as median (1st quartile to 3rd quartile) and compared using the nonparametric Mann-Whitney test. In addition, we used standardized differences to estimate the balance in measured variables between continuation and discontinuation groups, independently of the sample size and variable unit [12].
We used propensity score analyzes to better scrutinize the association of statin continuation with the primary outcome (organ failure free days). The rationale and methods underlying the use of propensity scores for proposed causal exposure variables have been previously described [13, 14]. The selection of covariates included in the multivariable logistic regression model used to estimate the propensity score for statin continuation was guided by clinical significance and imbalances between continuation and discontinuation groups, as estimated by an absolute standardized difference above 20% and/or a relative effect above five (relative effect is a measure describing the extent to which a covariate is confounding the effect of statin continuation on outcome). The final propensity score model included the following covariates: simplified acute physiology score (SAPS) II score at ICU admission, SOFA score at ICU admission, prior statin therapy duration, surgery treatment before ICU admission, septic shock at ICU admission, infection site, causative organism, type of infection (community acquired vs. hospital acquired), low-dose corticosteroid treatment, and fiscal year of ICU admission (in order to consider potential ongoing temporal changes in sepsis-related outcomes in our unit). We matched patients with statin continuation to those with statin discontinuation, using a greedy-matching algorithm with a calliper width of 0.2 standard deviations of the log odds of the estimated propensity score and sampling without replacement [15]. We used a graphical representation with standardized differences to check the balance of covariates in the matched sample [14]. In addition to propensity score matching, we performed a direct adjustment for confounding using a traditional linear regression model, with the same items selected for the propensity score as covariates and organ failure free days as the dependent variable [16]. A P value less than 0.05 was considered significant in bilateral analysis.
Results
Patients
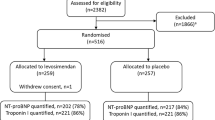
During the study-period, 81 patients receiving chronic statin therapy were hospitalized for severe sepsis or septic shock in our ICU. Five patients were excluded from the study because of non-inclusion criteria at ICU admission (vomiting in two patients and rhabdomyolysis in two others) or insufficient data (one patient; Figure 1). Statins were discontinued upon admission to the ICU in 32 patients (17 in 2005 and 15 in 2006, none in 2007) and continued in 44 patients (7 in 2005, 23 in 2006, and 14 in 2007). Table 1 and Table 2, respectively, display patients' and infection characteristics at ICU admission. Patients in the discontinuation group had significantly more hospital-acquired infections, more need for surgery before ICU admission, and a trend towards more septic shock at ICU admission as compared with the continuation group.
Outcomes
Patient outcomes in the entire cohort are reported in Table 3. The numbers of organ failure-free, hemodynamic failure-free, and organ dysfunction-free days were significantly higher in the continuation group as compared with the discontinuation group. The need for invasive mechanical ventilation and prevalence of acute respiratory distress syndrome were also significantly higher in the discontinuation group as compared with the continuation group. There was a trend towards increased hospital mortality and hospital length of stay in the discontinuation group as compared with the continuation group.
Of the 44 patients who continued statin therapy, 43% (19) were matched using the propensity score to a similar patient in whom statins were discontinued. The covariate balance between the continuation and discontinuation groups improved substantially through propensity-score matching (Figure 2). The association of statin continuation with organ failure-free days was not significant with the propensity-score matching (Table 4 and Figure 3) or with the linear regression adjustment (Table 5 and Figure 3).
Graphical representation of absolute standardized differences before and after propensity score matching comparing covariate values. Imbalance for all variables was substantially reduced after matching. The 20% cut-off was used to select variables included in the propensity score. SAPS, simplified acute physiology score; SOFA, sequential organ failure assessment.
Beta regression coefficients with 95% confidence intervals for the association between statin continuation and organ failure free days in models with crude analysis (2.84 (0.51 to 5.17), P = 0.02), propensity matching (2.37 (-0.96 to 5.70), P = 0.20) or multivariable adjustment (2.24 (-0.43 to 4.91), P = 0.11). The beta regression coefficient indicates the difference in mean number of days between continuation and discontinuation arms.
Safety of statin continuation
Two patients in the continuation group required cessation of enteral diet and statin administration for 48 hours because of food intolerance with vomiting. Multiple blood concentrations of CPK and aminotransferases were available in 55 (72%) and 63 (83%) patients, respectively (representing 27% and 43% of patient-days in ICU for the continuation group, respectively). The proportion of patients with rhabdomyolysis (CPK levels increase above five ULN) or increase of liver enzymes (aminotransferases increase above three ULN) did not differ between the discontinuation and continuation groups: 3 (14%) vs. 1 (3%), P = 0.15; and 6 (25%) vs. 7 (18%), P = 0.54, respectively.
Atorvastatin plasma concentrations during treatment continuation
We found very high pre-dose and post-dose atorvastatin concentrations during treatment continuation (up to day 4), with median values of 66 (29-101) and 142 (96-237) ng/mL, respectively. Six of the nine patients explored were receiving known cytochrome P450 3A4 inhibitors (including midazolam, hydrocortisone, amiodarone, and tacrolimus; Table 6). These patients exhibited higher atorvastatin concentrations as compared with those not receiving such inhibitors: 70 (57-105) vs. 29 (27-35) ng/mL for pre-dose concentration (P = 0.05) and 199 (134-255) vs. 96 (80-99) ng/mL for post-dose concentration (P = 0.04), respectively (Figure 4).
Atorvastatin plasma concentrations before and 90 minutes after receiving a 40 mg dose in nine critically ill septic patients continuing this drug while under cytochrome P450 3A4 inhibitors (in red) or not (in black). Two patients were assessed twice (circles). The residual concentrations reported in healthy volunteers after a single 20 mg dose are close to 3 ng/mL [26].
Discussion
Our study suggests that the lesser morbidity associated with continuation of ongoing statin therapy (as compared with systematic discontinuation) in patients with severe sepsis or septic shock may be influenced by confounders. We did not find clear evidence of poor clinical tolerance of statins, but the plasma concentrations achieved during continuation of atorvastatin were particularly high.
A potential beneficial effect of statins during sepsis has been suggested by several studies reporting both a preventive effect on the risk of severe sepsis, as well as a reduction of morbidity and mortality associated with sepsis [4, 17–19], but with significant heterogeneity among studies and potential publication bias [4]. The potential effect of the introduction of statins in sepsis will be resolved by currently ongoing clinical trials (NCT00528580, NCT00676897, NCT00452608, NCT00979121, NCT00450840 and NCT00357123) [20]. However, few publications have studied the effect of the continuation or discontinuation of statins during severe sepsis in patients chronically treated with statins.
In our study, patients in whom statins were continued seemed to have a better outcome compared with the discontinuation group after crude analysis. Kruger et al previously reported a particularly high mortality in bacteriemic patients in whom chronic statin therapy had been interrupted at time of the septic episode [21]. In these patients, the poorer outcomes in the discontinuation group could be due to a possible rebound effect of statins interruption on inflammatory response [22–24], but many potential sources of bias not addressed may confound the interpretation of these results [21].
In our study, there were significant imbalances between groups that may explain the differences in outcomes. In particular, patients in whom statins were discontinued had a higher prevalence of hospital-acquired infections and septic shock at ICU admission as compared with others. It is possible that more severely ill patients, and those with more complex presentation, might have been less likely to have their statins continued because their physicians were more focused on treating immediately life-threatening problems or implementing complex diagnostic procedures. After controlling for these selection bias via propensity matching and multivariable adjustment, there was no significant association between statin continuation and the main outcome (organ failure-free days). These negative findings are in line with a recent randomized trial that did not provide evidence of any beneficial role of continuing pre-existing statin therapy on sepsis progression and inflammatory parameters [25].
The absorption and metabolism of statins may vary widely in ICU patients, especially in those with sepsis, because of frequent alterations of the digestive tract function. However, only two patients in our study had treatment interrupted due to gastric intolerance. We found very high atorvastatin concentrations in patients continuing this drug, with a nearly 20-fold increase in pre-dose concentrations as compared with residual concentrations reported in healthy volunteers [26]. These results are in accordance with those of Kruger et al [26] who recently reported similarly high plasma concentrations of atorvastatin in ICU septic patients. In the later report, the peak and residual statin concentrations averaged 84 and 23 ng/mL, respectively, in septic ICU patients after a single 20 mg atorvastatin dose. Our report demonstrates even higher residual concentrations (up to 100 ng/mL) after several days of statin continuation in septic ICU patients. These high concentrations could be explained by specific pharmacokinetic and pharmacodynamic considerations in septic conditions, including increased capillary permeability, changes in plasma protein binding, and altered liver metabolism by cytochrome systems. Concomitant treatments by cytochrome P450 3A4 inhibitors may also impair atorvastatin metabolism [27, 28]. Accordingly, we found significantly higher atorvastatin concentrations in patients receiving such inhibitors as compared with others.
Severe infection is a theoretical contraindication to statin administration, because these drugs might increase the risk of rhabdomyolysis and neuromyopathy [29]. We did not observe significantly more adverse events in the continuation group as compared with the discontinuation group, suggesting an acceptable tolerance of statins in the context of severe sepsis. However, aminotransferases and CPK were not systematically assessed in all patients. In addition, specific evaluation of ICU acquired myopathy was not carried out and a lack of elevated levels of circulating CPK does not rule out structural muscle injury in patients treated with statins [30]. Finally, the very high plasma atorvastatin concentrations during continuation of this drug may raise concern. Dosage regimens specifically adapted to critically ill septic patients, with particular attention to drugs susceptible to metabolic interactions, may need to be studied.
Our study has some limitations. First, the number of patients included was small, a situation that increases the chance of both type 1 and type 2 errors. Secondly, the design was retrospective, and, despite propensity matching and multivariable adjustment, the retrospective cohort design entails a number of residual biases that cannot be controlled for. Finally, although the pleiotropic effects of statins may be observed on longer term [31], our analysis was limited to the short term.
Conclusions
In conclusion, we found that the apparent beneficial effects of continuation of chronic statin therapy in septic ICU patients were driven in part by selection bias and confounders. Although there was no clear clinical evidence of poor tolerance of statins, the very high plasma concentrations achieved during continuation of atorvastatin suggest that caution should prevail if statins are prescribed to septic patients, and their risk/benefit ratio assessed carefully.
Key messages
-
Patients in whom statin therapy had been continued in the ICU during severe sepsis or septic shock had significantly more organ failure-free days as compared with those with statin discontinuation, but this difference did not persist after propensity score matching and multivariable adjustment.
-
The predose and postdose atorvastatin concentrations were particularly high in septic patients continuing the drug in the ICU. These very high concentrations advocate some caution when administering statins to septic patients in the ICU setting.
Abbreviations
- CPK:
-
creatine phosphokinase
- SAPS:
-
simplified acute physiology score
- SOFA:
-
sequential organ failure assessment
- ULN:
-
upper limit of normal.
References
Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists' (CTT) Collaborators: Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366: 1267-1278.
Mekontso-Dessap A, Brun-Buisson C: Statins: the next step in adjuvant therapy for sepsis? Intensive Care Med 2006, 32: 11-14. 10.1007/s00134-005-2860-5
Jacobson JR: Statins in endothelial signaling and activation. Antioxid Redox Signal 2009, 11: 811-821. 10.1089/ars.2008.2284
Janda S, Young A, Fitzgerald JM, Etminan M, Swiston J: The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care 2010, 25: 656. e657-622.
Drug information/Product Labeling/Lipitor/Warning and precautions[http://www.pdr.net/drugpages/productlabeling.aspx?mpcode=62402910#section-5.1]
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G: 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003, 31: 1250-1256. 10.1097/01.CCM.0000050454.01978.3B
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerma JL, Vincent JL, Levy MM: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med 2004, 30: 536-555. 10.1007/s00134-004-2210-z
Bahrami G, Mohammadi B, Mirzaeei S, Kiani A: Determination of atorvastatin in human serum by reversed-phase high-performance liquid chromatography with UV detection. J Chromatogr B Analyt Technol Biomed Life Sci 2005, 826: 41-45. 10.1016/j.jchromb.2005.08.008
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S: Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med 1998, 26: 1793-1800. 10.1097/00003246-199811000-00016
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996, 22: 707-710. 10.1007/BF01709751
The R Foundation[http://www.R-project.org]
Austin PC: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in Medicine 2009, 28: 3083-3107. 10.1002/sim.3697
Austin PC, Mamdani MM: A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Statistics in Medicine 2006, 25: 2084-2106. 10.1002/sim.2328
Gayat E, Pirracchio R, Resche-Rigon M, Mebazaa A, Mary JY, Porcher R: Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med 2010, 36: 1993-2003. 10.1007/s00134-010-1991-5
Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A: Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: a population-based cohort study. Lancet 2008, 372: 562-569. 10.1016/S0140-6736(08)61121-6
Senn S, Graf E, Caputo A: Stratification for the propensity score compared with linear regression techniques to assess the effect of treatment or exposure. Statistics in Medicine 2007, 26: 5529-5544. 10.1002/sim.3133
Kopterides P, Falagas ME: Statins for sepsis: a critical and updated review. Clin Microbiol Infect 2009, 15: 325-334. 10.1111/j.1469-0691.2009.02750.x
Falagas ME, Makris GC, Matthaiou DK, Rafailidis PI: Statins for infection and sepsis: a systematic review of the clinical evidence. J Antimicrob Chemother 2008, 61: 774-785. 10.1093/jac/dkn019
Tleyjeh IM, Kashour T, Hakim FA, Zimmerman VA, Erwin PJ, Sutton AJ, Ibrahim T: Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med 2009, 169: 1658-1667. 10.1001/archinternmed.2009.286
ClinicalTrials.gov[http://clinicaltrials.gov]
Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G: Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med 2006, 32: 75-79. 10.1007/s00134-005-2859-y
van der Harst P, Asselbergs FW, Hillege HL, Bakker SJ, Voors AA, van Veldhuisen DJ, van Gilst WH: Effect of withdrawal of pravastatin therapy on C-reactive protein and low-density lipoprotein cholesterol. Am J Cardiol 2007, 100: 1548-1551. 10.1016/j.amjcard.2007.06.054
Cubeddu LX, Seamon MJ: Statin withdrawal: clinical implications and molecular mechanisms. Pharmacotherapy 2006, 26: 1288-1296. 10.1592/phco.26.9.1288
Li JJ, Li YS, Chen J, Yang JQ: Rebound phenomenon of inflammatory response may be a major mechanism responsible for increased cardiovascular events after abrupt cessation of statin therapy. Med Hypotheses 2006, 66: 1199-1204. 10.1016/j.mehy.2005.06.035
Kruger PS, Harward ML, Jones MA, Joyce CJ, Kostner KM, Roberts MS, Venkatesh B: Continuation of statin therapy in patients with presumed infection: a randomised controlled trial. Am J Respir Crit Care Med 2011,183(6):774-781. 201 10.1164/rccm.201006-0955OC
Kruger PS, Freir NM, Venkatesh B, Robertson TA, Roberts MS, Jones M: A preliminary study of atorvastatin plasma concentrations in critically ill patients with sepsis. Intensive Care Med 2009, 35: 717-721. 10.1007/s00134-008-1358-3
Knopp RH: Drug treatment of lipid disorders. N Engl J Med 1999, 341: 498-511. 10.1056/NEJM199908123410707
Bellosta S, Paoletti R, Corsini A: Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation 2004, 109: III50-57.
Vincent A, Miller JA: Statins for sepsis: a cautionary note. Intensive Care Med 2006, 32: 795. 10.1007/s00134-006-0143-4
Mohaupt MG, Karas RH, Babiychuk EB, Sanchez-Freire V, Monastyrskaya K, Iyer L, Hoppeler H, Breil F, Draeger A: Association between statin-associated myopathy and skeletal muscle damage. CMAJ 2009, 181: E11-18. 10.1503/cmaj.081785
Thomsen RW, Hundborg HH, Johnsen SP, Pedersen L, Sorensen HT, Schonheyder HC, Lervang HH: Statin use and mortality within 180 days after bacteremia: a population-based cohort study. Crit Care Med 2006, 34: 1080-1086. 10.1097/01.CCM.0000207345.92928.E4
McCabe WR, GG J: Gram-negative bacteremia. Etiology and ecology. Arch Intern Med 1963, 110: 847-855.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
AMD participated in the conception and design of the study, helped to perform the statistical analysis, and drafted the manuscript. IO participated in collection of data, helped to perform the statistical analysis, and helped to draft the manuscript. NR and BB participated in collection of data and helped to draft the manuscript. CB and AH carried out atorvastatin pharmacokinetics and helped to draft the manuscript. SK helped to perform the statistical analysis and helped to draft the manuscript. CBB participated in the conception, design and coordination of the study, and helped to draft the manuscript. All authors read and approved the final manuscript.
Armand Mekontso Dessap, Islem Ouanes contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mekontso Dessap, A., Ouanes, I., Rana, N. et al. Effects of discontinuing or continuing ongoing statin therapy in severe sepsis and septic shock: a retrospective cohort study. Crit Care 15, R171 (2011). https://doi.org/10.1186/cc10317
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc10317