Abstract
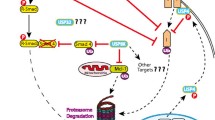
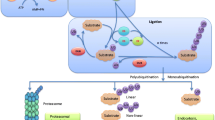
Turnover of several regulatory proteins results from targeted destruction via ubiquitination and subsequent degradation through the proteosome. The timely and irreversible degradation of critical regulators is essential for normal cellular function. The precise biochemical mechanisms that are involved in protein turnover by ubiquitin-mediated degradation have been elucidated using in vitro assays and cell culture systems. However, pathways that lead to ubiquitination of critical regulatory proteins in vivo are more complex, and have both temporal and tissue-specific differences. In vivo models will allow identification of substrates and enzymes of the ubiquitin–proteosome pathway that play important roles in selected tissues and diseases. In addition, assessment of the therapeutic efficacy of drugs designed to inhibit or enhance protein turnover by ubiquitination requires in vivo models. In the present review we describe selected examples of transgenic and knockout models of proteins that are known either to be regulated by ubiquitin-mediated degradation or to have a catalytic function in this process, and to play an important role in breast cancer. We outline the functions of these proteins in vivo and focus on knowledge gained in the comparison of in vivo behavior predicted from cell-free in vitro data or from experiments conducted in cell culture systems.
Similar content being viewed by others
Introduction
Timed degradation of cellular regulatory proteins by the ubiquitin pathway plays a critical role in controlling cellular growth and proliferation. Substrates of this pathway include tumor suppressors, cell cycle proteins, transcription factors, and tyrosine kinase receptors, among others. Proteolysis of many of these regulators is controlled by ubiquitin ligases, the substrate specificity of which is dictated by different F-box proteins that act as substrate recognition factors. Substrates are recognized and bound by the F-box protein subunits only when they are phosphorylated on specific sites.
Because ubiquitination of critical proteins occurs in a tissue-specific and time-regulated manner, the use of animal models becomes critical in the identification of substrates that are involved in cell cycle regulation, apoptosis, and development, which cannot be studied in vitro. An interesting model for the study of general ubiquitination in vivo was recently described [1], in which transgenic mice were made to overexpress a fusion of the human ubiquitin gene (Ubc) and the enhanced green fluorescent protein. The epitope-tagged ubiquitin is first expressed as early as the morula stage in embryonic development, without any effect on viability. In adult mice the transgene is expressed in virtually all tissues. These mice represent a powerful tool for the recovery of as yet unknown substrates that are ubiquitinated in vivo. In contrast, the models described below target proteins that are known to regulate or to be regulated by the ubiquitin–proteosome system, and to play a role in breast carcinomas.
The cyclin-dependent kinase inhibitor p27
The ubiquitin–proteosome pathway plays a major role in the turnover of cell cycle regulatory proteins. Loss of the p27 protein – a cyclin-dependent kinase inhibitor – may contribute to uncontrolled proliferation. In several human cancers, including breast cancer, targeted inactivation of p27 is associated with aggressive behavior (for review [2]). Human Skp1 and the F-box protein Skp2 were originally identified as two proteins that physically interacting with cyclin A, and are therefore designated as S-phase kinase-associated proteins [3]. In both yeast and humans, a protein ubiquitin ligase system known as Skp1/Cul1/F-box (SCF) complex targets a number of proteins for ubiquitin-mediated proteolysis in a phosphorylation-dependent manner. In this complex, the F-box protein determines the substrate specificity. Skp2 is the F-box protein required for the ubiquitination and consequent degradation of phosphorylated p27 [4–6].
Whereas p27 knockout mice develop generalized hyperplasia and spontaneous pituitary tumors [7–9], Skp2-deficient mice grow slower than do littermate controls and have smaller organs, with all tissues containing decreased numbers of cells [10]. Interestingly, all cellular and histopathologic abnormalities observed in Skp2-deficient mice are abolished in Skp2/p27 double knockout mice, indicating that p27 is a primary substrate of Skp2 in vivo (K Nakayama, personal communication). Several in vitro studies indicated an inverse functional relationship between p27 and Skp2, and in vivo data in part confirm those findings. Skp2 transgenic mice targeted to the T-lymphoid lineage demonstrated a cooperative oncogenic effect when crossed with activated N-ras transgenic mice [11]. These double transgenic mice developed tumors with shorter latency and higher penetrance as compared with N-ras transgenic animals. Interestingly, no change in p27 phosphorylation was observed in transgenic mice, suggesting that some other component may be a limiting factor for p27 destruction. That study demonstrated the oncogenic potential of Skp2 in vivo and provided a unique tool for the evaluation of functional interactions of this ubiquitin ligase with other proto-oncogenes.
We recently demonstrated that Skp2-positive cells in human breast carcinomas represent a subpopulation of proliferating tumor cells [12]. However, approximately one-third of breast carcinomas with low proliferative rates display low p27 levels despite the absence of Skp2. These data suggest that an alternative mechanism leading to proteosomal degradation of p27 may be operative in this subset of breast carcinomas with low proliferative rates. Malek et al. [13] utilized an interesting approach to investigate the role of p27 ubiquitination in vivo. Those investigators genetically engineered a 'knock in' of a non-phosphorylatable mutant of p27, in which the critical threonine residue (the phosphorylation of which is required for substrate recognition by Skp2) is mutated to alanine (p27T187A). The surprising finding was that there is a proteolytic pathway that controls p27 degradation in G1, before the activation of the cyclin E-cyclin dependent kinase 2 complex, whereas p27T187A was stable in S-phase, with a half-life similar to that in quiescent cells. Findings in that in vivo model confirmed the in vitro data [14] and pointed to the fact that p27 inactivation via degradation appears to switch from being mitogen dependent in G1 to mitogen independent in the DNA replication phase of the cell cycle. Malek et al. also proposed the existence of phosphorylation sites other than those that may mediate Skp2–p27 interaction. More recently, the ubiquitin ligase responsible for the G0/G1 degradation of p27 has been identified. This protein, namely G1-phase Kip1 ligase (GKL) 1/2, does not appear to require phosphorylation either on T187 or S10 (N Nakayama, personal communication) to accomplish the degradation of p27. It remains to be proven whether, in human breast cancer, these two mechanisms of p27 inactivation are operative in tumor cells that do and in those that do not express Skp2. This would have considerable therapeutic implications.
Androgen regulation of p27 levels has been suggested by experiments conducted in cell culture systems. Although both p27 and p21 have been shown to be degraded by the proteosome, in a rat model of prostate castration and testosterone-mediated regeneration we determined that ubiquitin-mediated degradation of p27, but not that of p21, is under androgen control [15]. Androgen induction of p21 occurred at the transcriptional level, with no change in ubiquitin-mediated degradation. In addition, peak epithelial cell proliferation and maximal p27 protein levels were unexpectedly achieved simultaneously during regeneration. Utilizing this in vivo model, we determined that androgen action was both differentiating (with stabilization of p27 protein through inhibition of proteosomal degradation in the majority of prostate epithelial cells) and proliferating (via the induction of p27 degradation in proliferating epithelial cells). That study provided evidence of a previously unrecognized level of complexity in the in vivo regulation of critical cyclin-dependent kinase inhibitors (CKIs) by androgens. We recently demonstrated that Skp2 and p27 are modulated by the proliferative action induced by estrogens in breast cancer cells [12], and it would therefore be interesting to test the hypothesis that the same mechanisms elucidated in the prostate are operative in breast epithelium as well, utilizing models of breast regeneration. In addition, because Skp2 inhibitors may soon be ready for testing, animal models such as the ones described above represent ideal tools to determine the specificity and efficacy of such compounds.
Wnt-1/β-catenin pathway
β-Catenin is an important cellular regulator that is involved in the control of growth and development, as well as cell-cell adhesion (for review [16]). A pool of β-catenin is present in the cytoplasm bound to Apc, the product of the tumor suppressor gene adenomatous polyposis coli [17], which facilitates phosphorylation of β-catenin and its subsequent ubiquitination by an SCF complex. Thus, β-catenin is constitutively degraded in the cytoplasm, but in response to activation of the Wnt pathway β-catenin phosphorylation is inhibited and degradation of β-catenin decreases. Consequently, β-catenin accumulates and migrates into the nucleus, where it binds to a transcription factor of the Lef-1/Tcf-1 family to induce the expression of target genes [18, 19]. Importantly, two of those target genes encode proto-oncoproteins, namely c-Myc and cyclin D1[20–22]. Genetic mutations or altered protein expression of β-catenin and Apc have been implicated in human cancers, and all result in increased β-catenin levels, which in turn lead to increased Lef-1/Tcf-1 transcriptional activity and deregulated proliferation [23]. In several malignancies, increased levels of β-catenin have been shown to be due to β-catenin mutations that abolish phosphorylation sites essential for its degradation [16, 24]. Furthermore, Wnt-1, which induces stabilization of β-catenin, was first identified as a proto-oncogene frequently activated by retroviral insertion of mouse mammary tumor virus (MMTV) into the Wnt-1 locus in mouse mammary tumors. Wnt is a positive regulator of β-catenin, leading to stabilization of β-catenin and allowing its migration from the cytoplasm to the nucleus. Thus, wild-type and mutated forms of β-catenin acquire oncogenic properties when they accumulate because of a defect in degradation. The F-box protein that determines substrate specificity for the E3 ligase involved in β-catenin ubiquitination is β-TrCP [25].
Transgenic mouse models show that β-catenin leads to hair follicle tumors when overexpressed in epidermal cells [26] and to colon adenomas when overexpressed in the intestines [27]. Attractive animal models have been engineered to investigate the in vivo functions of the β-catenin pathway in the mouse mammary gland. In one of these, namely ΔN89 β-catenin transgene, which cannot undergo degradation and accumulates in the cytoplasm, is driven by the MMTV long terminal repeat to luminal cells of mammary and salivary glands [28]. MMTV–ΔN89 β-catenin transgenic mice, even at an early stage of puberty, develop lobular-alveolar hyperplasia, which is normally associated with hormonal stimulation in late pregnancy. Importantly, MMTV–ΔN89 β-catenin transgenic mice develop breast cancer with 100% penetrance. In contrast, the phenotype of mammary gland in MMTV–Wnt-1 transgenic mice is characterized by ductal hyperplasia with a feathery, hyperbranched pattern, which is reminiscent of the morphologic features of the mammary gland during early pregnancy [28, 29]. In addition, mammary adenocarcinomas arise in approximately 50% of female transgenic mice by 6 months of age [29].
The influence of estrogenic hormones on β-catenin/Wnt-1-induced tumors is also of interest. Because breeding females of MMTV–ΔN89 β-catenin and MMTV–Wnt-1 transgenic mice develop tumors slightly earlier than do virgin ones, it has been proposed that estrogen may increase the oncogenicity of both Wnt-1 and β-catenin. However, it is not possible to exclude that the acceleration of tumor formation in the breeding females may depend on the increased mass of the mammary gland. Interestingly, in MMTV–Wnt-1 transgenic/estrogen receptor (ER) knockout mice and in ovariectomized MMTV–Wnt-1 transgenic mice, ductal hyperplasia and tumors continue to form, albeit with delayed onset, suggesting that the Wnt-1 transgene does not require estrogen to induce mammary hyperplasia and tumors [29]. These compelling models support the hypothesis that a fraction of ER-negative estrogen-independent breast cancers may originate directly from ER-α-negative cells, rather than from ER-α-positive cells, which later undergo ER loss.
Animal models have been also used to explore β-catenin downstream targets. Wild-type mice do not express significant levels of cyclin D 1 mRNA until mid-pregnancy, whereas northern blot analysis reveals high levels of cyclin D 1 as well as c-Myc mRNA in virgin MMTV–ΔN89 β-catenin transgenic mice [28]. Moreover, cyclin D1-null mice are characterized by hypoplastic lobular-alveolar structures, with a normal side branching pattern [30], whereas MMTV–cyclin D 1 transgenic mice and MMTV–c-myc trangenic mice develop lobular-alveolar hyperplasia in perfect concordance with the early phenotype of MMTV–ΔN89 β-catenin transgenic mice [31]. Interestingly, these transgenic mice develop mammary gland carcinomas with slower kinetics than do MMTV–ΔN89 β-catenin transgenic mice, providing evidence that β-catenin concurrently elevates both cyclin D1 and c-myc proto-oncogenes.
Although the activation of the Wnt-1/β-catenin pathway is well established in a subset of human breast cancers, increased susceptibility to mammary neoplasia has not been reported to date in the human syndrome associated with mutations in the APC gene. In contrast, 20% of mice carrying Apc Min (Min), a nonsense mutation of Apc, develop mammary tumors, as well as intestinal adenocarcinomas [32]. Because the life-span of these transgenic mice is very short, study of the effects of chemical carcinogens on Min/+ mice and the use of transplants from Min/+ treated mice have better highlighted the increased susceptibility of these mice to mammary tumors. Although Apc mutations appear to have an additional effect on breast epithelial transformation in mice, which is in contrast to humans, the possibility that the high background rate of mammary tumors in the population may hide the increased risk for breast cancer in human carriers of APC mutations cannot be ruled out.
The protein kinase CK2 promotes Wnt signaling [33]. Histologic abnormalities in the mammary gland, such as retardation of development, incomplete involution after lactation, and dysplastic squamous and alveolar lesions, have been found in half of MMTV–CK2α transgenic mice [34]. Moreover, over a period of 2 years 30% of these transgenic mice developed mammary tumors, specifically glandular, adenosquamous, scirrous, and sarcomatoid carcinomas. The long latency suggests a multistep pathway in CK2α-induced tumorigenesis, whereas the wide spectrum of tumor histotypes supports the existence of several cooperating CK2α downstream targets. Furthermore, high protein levels of β-catenin and c-Myc are detected in these tumors, confirming the emerging role of CK2α as a positive regulator of the Wnt-1/β-catenin pathway [33].
The ubiquitin ligase MDM2
Originally cloned from a tumorigenic mouse cell line, which contains amplified DNA sequences in the form of double minutes [35], MDM2 is amplified and/or overex-pressed most frequently in sarcomas [36] but also in other tumors, including breast carcinomas [37]. Mdm2 gene transforms immortalized mouse NIH3T3 cells and rat embryo fibroblasts when transfected alone and cotransfected with activated ras gene, respectively [35, 38]. Its oncogenicity is mainly attributed to its interaction with p53, a transcription factor with known tumor suppressor functions. MDM2 binds to the activation domain of p53, with consequent inhibition of its transcriptional activity, and exports p53 into the cytoplasm and targets it for proteosome-mediated degradation through its well known E3 ligase activity [36]. Because MDM2 is itself a transcriptional target of p53, MDM2 and p53 are coordinately modulated and ensure proper protection from DNA damage. MDM2 is also negatively regulated by the tumor suppressor protein p14ARF. Arf directly associates with MDM2 and blocks its ability to interact with p53. Because human MDM2 is overexpressed in 5–10% of human tumors and ARF is silenced in many others, disruption of the ARF-Mdm2-p53 axis is common in cancers [39]. The hypothesis that the MDM2-mediated negative regulation of p53 accounts for MDM2 oncogenicity is also supported by the evidence that in most human sarcomas either a p53 mutation or MDM2 amplification is detected [40]. However, splice variants of MDM2 that lack the p53-binding site maintain their ability to transform NIH3T3 cells [41], suggesting the existence of other p53-independent MDM2 oncogenic pathways. These may involve other cell cycle regulators that have been shown to bind to MDM2, such as E2F1, pRb, and p107 [36].
Several studies conducted in animal models, summarized in Table 1[42–46], have investigated the interaction between MDM2 and p53 in vivo and other possible p53-independent MDM2 pathways. Specifically, studies in Mdm2-null mice point to the importance of MDM2 in tumorigenesis, mainly as a p53-negative regulator. Mdm2-null mice die early in development, whereas double homozygous Mdm2/p53 mutant mice are viable, providing evidence that, in early mouse development, MDM2 is required to inhibit p53-mediated cell cycle arrest and apoptosis [42].
In contrast, experiments conducted in transgenic mice with overexpression of MDM2 not confined to particular tissues support the hypothesis of a p53-independent function of MDM2. Because constitutively high levels of MDM2 affect early embryonic development, transgenic chimeras were generated from a stem cell line expressing low levels of transgenic Mdm2 transcript [43]. Interestingly, these Mdm2-transgenic mice, when compared with p53-null mice, develop tumors at a slower rate and show a somewhat different histologic spectrum, with an increase in the number of sarcomas. This peculiar tumor spectrum, which is also retained in a p53-null background, suggests that the MDM2 pathway is at least partly p53 independent.
Additional in vivo studies have been focused on tissue-specific expression of MDM2. The Mdm2-transgene, driven by the β-lactoglobulin promoter, is directed exclusively at the pregnant and lactating mammary gland [44, 45]. These transgenic mice display lactation defects, with a decreased number of lobules paradoxically accompanied by ductal hyperplasia with atypical epithelial cells with multiple, large hyperchromatic nuclei. MDM2 induces cyclin A overexpression in these cells, leading to repetitive rounds of DNA replication not followed by mitosis, with consequent cellular polyploidy. In addition, after a long period of latency, a small subset of these Mdm2-transgenic mice develop ductal carcinomas of the breast. The atrophic/dysplastic breast phenotype is not suppressed when Mdm2-transgenic mice are crossed with p53-null [44] or E2F1-null mice [45] and is not enhanced when Mdm2-trangenics are crossed with E2F1-trangenic mice [45]. These findings suggest a p53- and E2F1-independent function of MDM2 in breast.
Targeted MDM2 overexpression in the basal layer of the epidermidis, using a human cytokeratin K14 promoter, produces an early and transient phenotype, characterized by altered expression of selected cytokeratins, high level of proliferation, and increased apoptosis [46]. Paradoxally, this phenotype is abolished in a p53-null background. In addition, in the Mdm2-transgenic mice the induction of p53 and p21 after ultraviolet exposure is decreased. Interestingly, later in life, one-third of Mdm2-transgenic mice develop hyperplastic/dysplastic skin lesions and, in a small percentage, skin carcinomas. These data point to a complex interaction between MDM2 and p53 in the skin that is different from that which occurs in breast epithelium.
MDM2 inactivation in vivo results in tumor-suppressor activity in a dose-dependent manner in nude mice bearing MCF-7 or MDA-MB-468 breast carcinoma xenografts [47]. In both of these in vivo models, synergistic or additive therapeutic effects of MDM2 inhibition with several clinically used chemotherapeutic agents were observed, suggesting that MDM2 inhibitors may have a broad spectrum of tumor suppressor activities in human breast cancers, regardless of p53 status.
Thus, these animal models appear to confirm the oncogenicity of MDM2 in vivo and suggest the existence of diverse mechanisms of action that appear to be tissue/organ specific.
The ubiquitin ligase E6-associated protein
Originally discovered because of its ability to target p53 for degradation by the proteosome in association with the human papillomavirus E6 protein [48], E6-associated protein (E6-AP) was later found to be a nuclear hormone receptor coactivator [49]. The genomic locus that encodes E6-AP is mutated in Angelman syndrome, a neurologic human disorder that is characterized by motor dysfunction and mental retardation [50]. Consistent with this finding, heterozygous E6-AP-null mice show neurologic defects and decreased expression levels of E6-AP in the hippocampal neurons and in the Purkinje cells of the cerebellum, with consequent increase in p53 levels [51]. These data suggest that it is the E3-ligase function of E6-AP that is involved in the pathogenesis of Angelman syndrome, caused by uniparental disomy of chromosome 15, and characterized by mental retardation, ataxia, seizures, and inappropriate laughter.
Interestingly, E6-AP is overexpressed in spontaneous mouse mammary tumors and its expression is inversely related to ER and progesterone receptor (PR) expression [52]. These tumors, in fact, do not express ER and PR. Moreover, tissue extracts from these tumors show that E6-AP maintains its catalytic ability to ubiquitinate an artificial substrate, although the levels of its well known substrate p53 do not inversely correlate with E6-AP levels. More recently, it has been shown that mammary gland growth in response to estrogen and progesterone administration is not decreased in homozygous E6-AP-null mice [53]. Taken together, these data suggest that the ER and PR coactivator function of E6-AP is not required to mediate estrogen and progesterone action on mammary gland. In contrast, prostate growth induced by testosterone administration and uterine growth induced by estradiol administration were attenuated, and gonad size was reduced in these homozygous E6-AP-null mice. The authors speculated the compelling possibility that the role of E6-AP in the mouse mammary gland tumorigenesis may depend on its E3-ligase activity, involve the turnover of ER and PR, and play an important role in the acquisition of hormone independency.
Conclusion
The ubiquitin–proteosome pathway is an important mechanism for irreversible elimination of critical cell regulatory proteins. A great deal of information on its function and interaction has been gained from yeast and mammalian cell systems. Mouse models are greatly expanding our knowledge of tissue-specific and temporal mechanisms of action of this pathway in physiologic states, disease, and development. These models will provide invaluable insights into the involvement of the ubiquitin–proteosome pathway in oncogenesis, and constitute models for testing novel therapeutics directed at enhancing or inhibiting ubiquitination of critical proteins involved in cancer.
Abbreviations
- Apc:
-
= adenomatous polyposis coli
- E6-AP:
-
= E6-associated protein
- ER:
-
= estrogen receptor
- MDM:
-
= murine double minutes
- MMTV:
-
= mouse mammary tumor virus
- PR:
-
= progesterone receptor
- Skp:
-
= S-phase kinase-associated protein.
References
Tsirigotis M, Thurig S, Dube M, Vanderhyden BC, Zhang M, Gray DA: Analysis of ubiquitination in vivo using a transgenic mouse model. Biotechniques. 2001, 31: 120-130.
Slingerland J, Pagano M: Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000, 183: 10-17. 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.3.CO;2-9.
Zhang H, Kobayashi R, Galactionov K, Beach D: p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995, 82: 915-925.
Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H: p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999, 9: 661-664. 10.1016/S0960-9822(99)80290-5.
Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W: p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999, 1: 207-214. 10.1038/12027.
Carrano AC, Eytan E, Hershko A, Pagano M: SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999, 1: 193-199. 10.1038/12013.
Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY: Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996, 85: 707-720.
Kiyokawa H, Kineman R, Manova-Todorova K, Soares V, Hoffman E, Onoi M, Hayday A, Frohman D, Koff A: Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996, 85: 721-732.
Fero M, Rivkin M, Tasch M, Porter P, Carow C, Firpo E, Tsai L, Broudy V, Permutter R, Kaushansky K, Roberts J: A syndrome of muti-organ hyperplasia with features of gigantism, tumorigenesis and female sterility in p27Kip1-deficient mice. Cell. 1996, 85: 733-744.
Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamici I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, Kigagawa M, Hatakeyama S: Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. Embo J. 2000, 19: 2069-2081. 10.1093/emboj/19.9.2069.
Latres E, Chiarle R, Schulman BA, Pavletich NP, Pellicer A, Inghirami G, Pagano M: Role of the F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci USA. 2001, 98: 2515-2520. 10.1073/pnas.041475098.
Signoretti S, di Marcotullio L, Richardson A, Ramaswamy S, Carrano A, Isaac B, Rue M, Monti F, Ravaioli A, Loda M, Pagano M: Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Invest. 2002, 110: 633-641. 10.1172/JCI200215795.
Malek NP, Sundberg H, McGrew S, Nakayama K, Kyriakides TR, Roberts JM, Kyriakidis TR: A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature. 2001, 413: 323-327. 10.1038/35095083.
Hara T, Kamura T, Nakayama K, Oshikawa K, Hatakeyama S: Degradation of p27(Kip1) at the G(0)-G(1) transition mediated by a Skp2-independent ubiquitination pathway. J Biol Chem. 2001, 276: 48937-48943. 10.1074/jbc.M107274200.
Waltregny D, Leav I, Signoretti S, Soung P, Lin D, Merk F, Adams JY, Bhattacharya N, Cirenei N, Loda M: Androgen-driven prostate epithelial cell proliferation and differentiation in vivo involve the regulation of p27. Mol Endocrinol. 2001, 15: 765-782. 10.1210/me.15.5.765.
Peifer M: Beta-catenin as oncogene: the smoking gun. Science. 1997, 275: 1752-1753. 10.1126/science.275.5307.1752.
Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P: Association of the APC gene product with beta-catenin. Science. 1993, 262: 1731-1734.
Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W: Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996, 382: 638-642. 10.1038/382638a0.
Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H: XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996, 86: 391-399.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW: Identification of c-MYC as a target of the APC pathway. Science. 1998, 281: 1509-1512. 10.1126/science.281.5382.1509.
Tetsu O, McCormick F: Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999, 398: 422-426. 10.1038/18884.
Pennisi E: How a growth control path takes a wrong turn to cancer. Science. 1998, 281: 1438-1439. 10.1126/science.281.5382.1438.
Polakis P: Wnt signaling and cancer. Genes Dev. 2000, 14: 1837-1851.
Peifer M, Polakis P: Wnt signaling in oncogenesis and embryogenesis – a look outside the nucleus. Science. 2000, 287: 1606-1609. 10.1126/science.287.5458.1606.
Latres E, Chiaur DS, Pagano M: The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene. 1999, 18: 849-854. 10.1038/sj.onc.1202653.
Gat U, DasGupta R, Degenstein L, Fuchs E: De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998, 95: 605-614. 10.1016/S0092-8674(00)81631-1.
Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM: Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999, 18: 5931-5942. 10.1093/emboj/18.21.5931.
Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P: Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol. 2001, 153: 555-568. 10.1083/jcb.153.3.555.
Li Y, Hively WP, Varmus HE: Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000, 19: 1002-1009. 10.1038/sj.onc.1203273.
Fantl V, Edwards PA, Steel JH, Vonderhaar BK, Dickson C: Impaired mammary gland development in Cyl-1(-/-) mice during pregnancy and lactation is epithelial cell autonomous. Dev Biol. 1999, 212: 1-11. 10.1006/dbio.1999.9329.
Stepanova L, Finegold M, DeMayo F, Schmidt EV, Harper J: The oncoprotein kinase chaperone CDC37 functions as an oncogene in mice and collaborates with both c-myc and cyclin D1 in transformation of multiple tissues. Mol Cell Biol. 2000, 20: 4462-4473. 10.1128/MCB.20.12.4462-4473.2000.
Moser AR, Mattes EM, Dove WF, Lindstrom MJ, Haag JD, Gould MN: ApcMin, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc Natl Acad Sci USA. 1993, 90: 8977-8981.
Song DH, Sussman DJ, Seldin DC: Endogenous protein kinase CK2 participates in Wnt signaling in mammary epithelial cells. J Biol Chem. 2000, 275: 23790-23797. 10.1074/jbc.M909107199.
Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC: Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001, 20: 3247-3257. 10.1038/sj.onc.1204411.
Fakharzadeh SS, Trusko SP, George DL: Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. Embo J. 1991, 10: 1565-1569.
Alarcon-Vargas D, Ronai Z: p53-Mdm2-the affair that never ends. Carcinogenesis. 2002, 23: 541-547. 10.1093/carcin/23.4.541.
Toi M, Saji S, Suzuki A, Yamamoto Y, Tominaga T: MDM2 in breast cancer. Breast Cancer. 1997, 4: 264-268.
Finlay CA: The mdm-2 oncogene can overcome wild-type p53 suppression of transformed cell growth. Mol Cell Biol. 1993, 13: 301-306.
Sherr CJ: The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001, 2: 731-737. 10.1038/35096061.
Leach FS, Tokino T, Meltzer P, Burrell M, Oliner JD, Smith S, Hill DE, Sidransky D, Kinzler KW, Vogelstein B: p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993, 53 (suppl): 2231-2234.
Sigalas I, Calvert AH, Anderson JJ, Neal DE, Lunec J: Alternatively spliced mdm2 transcripts with loss of p53 binding domain sequences: transforming ability and frequent detection in human cancer. Nat Med. 1996, 2: 912-917.
Jones SN, Roe AE, Donehower LA, Bradley A: Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995, 378: 206-208. 10.1038/378206a0.
Jones SN, Hancock AR, Vogel H, Donehower LA, Bradley A: Overexpression of Mdm2 in mice reveals a p53-independent role for Mdm2 in tumorigenesis. Proc Natl Acad Sci USA. 1998, 95: 15608-15612. 10.1073/pnas.95.26.15608.
Lundgren K, Montes de Oca Luna R, McNeill YB, Emerick EP, Spencer B, Barfield CR, Lozano G, Rosenberg MP, Finlay CA: Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes Dev. 1997, 11: 714-725.
Reinke V, Bortner DM, Amelse LL, Lundgren K, Rosenberg MP, Finlay CA, Lozano G: Overproduction of MDM2 in vivo disrupts S phase independent of E2F1. Cell Growth Differ. 1999, 10: 147-154.
Ganguli G, Abecassis J, Wasylyk B: MDM2 induces hyperplasia and premalignant lesions when expressed in the basal layer of the epidermis. Embo J. 2000, 19: 5135-5147. 10.1093/emboj/19.19.5135.
Wang H, Nan L, Yu D, Agrawal S, Zhang R: Antisense anti-MDM2 oligonucleotides as a novel therapeutic approach to human breast cancer: in vitro and in vivo activities and mechanisms. Clin Cancer Res. 2001, 7: 3613-3624.
Huibregtse JM, Scheffner M, Howley PM: Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993, 13: 775-784.
Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, O'Malley BW: The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol. 1999, 19: 1182-1189.
Kishino T, Lalande M, Wagstaff J: UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997, 15: 70-73.
Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaudet AL: Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998, 21: 799-811. 10.1016/S0896-6273(00)80596-6.
Sivaraman L, Nawaz Z, Medina D, Conneely OM, O'Malley BW: The dual function steroid receptor coactivator/ubiquitin protein-ligase integrator E6-AP is overexpressed in mouse mammary tumorigenesis. Breast Cancer Res Treat. 2000, 62: 185-195. 10.1023/A:1006410111706.
Smith CL, DeVera DG, Lamb DJ, Nawaz Z, Jiang YH, Beaudet AL, O'Malley BW: Genetic ablation of the steroid receptor coactivator-ubiquitin ligase, E6-AP, results in tissue-selective steroid hormone resistance and defects in reproduction. Mol Cell Biol. 2002, 22: 525-535. 10.1128/MCB.22.2.525-535.2002.
Acknowledgement
We thank Michele Pagano, Kornelya Polyak, and Sabina Signoretti for critical review of the manuscript. This work was supported by grants from the NIH (RO1CA-81755), a Novartis Investigator grant, CaP CURE and Barr-Weaver awards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossi, S., Loda, M. The role of the ubiquitination-proteasome pathway in breast cancer: Use of mouse models for analyzing ubiquitination processes. Breast Cancer Res 5, 16 (2002). https://doi.org/10.1186/bcr542
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/bcr542