Abstract
A 2.2-kJ Mather type plasma focus device charged at 18 kV was used to deposit titanium nitride on 304 type stainless steel substrates. The plasma focus device is fitted with solid titanium anode and operated with nitrogen as the filling gas. The process of deposition was done at room temperature, and samples were deposited at a constant distance and at different angles with respect to the anode axis. X-ray diffractometry (XRD), atomic force microscopy (AFM), scanning electron microscopy (SEM), and energy dispersive X-ray analysis (EDX) were employed to characterize crystalline structure, morphology, nanostructure, distribution, and elemental composition of deposited films, respectively. As the ion flux and energy of the ions change with angular position from the head of the anode (titanium), it is observed that these changes directly affect both surface morphology and the nanostructure of the films. XRD patterns show the growth of polycrystalline titanium nitride thin films of different phases. AFM and SEM images show that the grain size is affected by the energy of ions that reached the surface. Grain size, average roughness, and root mean square decreased by increasing the angle with respect to the anode axis. EDX mapping verifies the elemental distributions of titanium nitride on the surface. In this work we have shown the possibility of production of titanium nitride thin films of different phases, using a Mather type plasma focus system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The major properties of TiN thin films which cause them to be extensively used in industrial applications mainly in tribology is their excellent wear and corrosion resistance[1]. TiN coatings have been one of the most essential material in a wide range of applications due to their high hardness[2, 3], high melting point, low friction coefficient, thermal stability[4, 5], chemical stability[6], and biocompatibility[7]. Various methods such as chemical vapor deposition[8], sputtering deposition[9], arc evaporation[10], and pulsed laser deposition[11] have been used for depositing titanium nitride thin films.
Plasma focus device is a simple pulsed plasma system in which the electrical energy of a capacitor bank istransferred to two coaxial electrodes by means of a spark gap and a thin current sheath is produced, which travels along the inner electrode by the J × B force (J × B force is the Lorentz force which acts on the current density by magnetic field). When the current sheath reaches at the top of the electrodes, the dense plasma column called focus is formed. This plasma is short-lived and unstable. Moreover, due to instabilities the plasma column breaks up and emits X-rays, energetic ions, relativistic electrons, and neutrons[12–14].
The use of plasma focus as an efficient source of energetic ions and relativistic electrons has recently received much attention. It has been successfully used in thin film deposition and surface processing[15–18]. Therefore, considering the interesting properties of TiN and both the high energy and high density of the plasma focus system, it was decided to benefit from these and produce titanium nitride films using plasma focus as the source for deposition of Ti in the plasma environment of nitrogen ions.
Experimental details
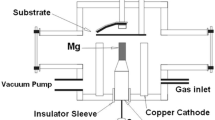
The deposition process was performed in a Mather type plasma focus device energized by an 11 μF, 25 kV capacitor bank with storage energy of 2.2 kJ. The schematic arrangement of the experimental set-up is given in Figure1. The coaxial electrode system consists of a copper rod having 10 mm diameter and 100 mm long serving as anode surrounded by 12 copper rods each of diameter 9 mm serving as a cathode. A Ti rod (50 mm in length and 10 mm in diameter) was fixed at the top of copper anode. The chamber and cathode were kept at ground potential. The chamber was evacuated to 102 mbar and then filled with nitrogen as a working gas. The chamber was purged with nitrogen gas several times to make sure that no residual gas is remained in the chamber. The pressure of nitrogen was kept at 1 mbar. The charging voltage was 18 kV. A steep current dip in the current signal observed on the oscilloscope indicates strong focusing action.
The substrates used in this experiment were 304 type stainless steel of 1 mm thickness and they are cut into 1 cm× 1 cm. A heated ultrasonic bath was used to clean the substrates, sequentially with acetone, alcohol and deionized water for 10 min each. Then they were mounted axially above the anode at a distance of 9 cm at angular positions of 0°, 15°, and 30° with respect to the anode axis on a designed holder. The holder was covered by a metallic shutter. It was used to prevent the exposure of substrates to the weak focusing shots at the beginning of the process before achieving strong focusing action. The number of focused shots used for these substrates was 25, and the time period between each shot was 1 min. After every five shots, the discharged chamber was evacuated, and the fresh nitrogen gas was filled in order to minimize the impurities.
The process responsible for the formation of titanium nitride may be explained as follows: the accelerated nitrogen ions on their incidence on the substrate transfer a high energy to the substrate surface and cause etching and cleaning of the surface prior to the deposition. Then the electrons from the cathode are accelerated towards the anode and ablate the titanium atoms from the top layer of the anode. These Ti atoms interact with nitrogen ions of the same shot and form titanium nitride that are deposited on the substrate surface.
The deposited films are characterized for their crystalline structure, surface morphology, and elemental distribution using various techniques.
The crystalline structure analysis of the films was performed using a Siemens D5005 X-ray diffractometer (Siemens AG, Munich, Germany) with Cu-Kα source. Atomic force microscopy (NT-MDT scanning probe microscope model BL022, NT-MDT Zelenograd, Moscow, Russia) and scanning electron microscopy (Philips XL30, Amsterdam, The Netherlands) were used to investigate the morphology of thin films. Identification of elemental compositions of the films and their distribution on the surface was carried out using energy dispersive X-ray (EDX) spectroscopy attached to scanning electron microscopy (SEM).
Results and discussion
X-ray diffraction analysis
X-ray diffraction analysis (XRD) patterns of the deposited films at different angular positions at a constant distance from the top of the anode are shown in Figure2. The diffraction peaks related to different crystalline planes of titanium nitride are observed, and there is no diffraction peaks related to the stainless steel 304, which confirms the successful growth of titanium nitride on the stainless steel 304 substrates. Titanium nitride diffraction peaks are in agreement with the Joint Committee for Powder Diffraction Standards (JCPDS) standard data. The crystalline structures of titanium nitride observed are the following:
-
1.
ε-TiN(110) with respect to JCPDS card number 08–0418
-
2.
TiN(111) with respect to JCPDS card number 38–1420
-
3.
Ti2N(200) with respect to JCPDS card number 23–1455
-
4.
Ti2N(211) with respect to JCPDS card number 17–0386
-
5.
ζ-Ti4N3−x(1112) with respect to JCPDS card number 39–1015
-
6.
TiN0.26(104) with respect to JCPDS card number 44–1095
The crystalline structure of ε-TiN(110) is formed only in the sample located at 0° and it is vanished by increasing the angle with respect to the axis of the anode. The diffraction peak corresponding to TiN(111) is reduced significantly with the increase in the angle and vanished at 30°. The diffraction peak corresponding to Ti2N(200) does not vary a lot by increasing the incident angle to 15°, but a small reduction is observed at 30°. The diffraction peaks related to Ti2N(211), ζ-Ti4N3−x(1112), and TiN0.26(104) are increased; while diffraction peaks related to ε-TiN(110) and TiN(111) vanished, and Ti2N(200) reduced with increasing the angle.
The reduction of the intensity of diffraction peaks with increasing the angle is due to the fact that the emission of accelerated ions towards the substrates is fountain-like and consequently, the flux of energetic ions decreases with increasing the angle. By reducing the flux of energetic ions, formation of titanium nitride changes and in general, the growth of crystalline structure of titanium nitride declines. The average crystal size of titanium nitride is calculated for about 30 nm.
Atomic force microscopy
Surface morphology of thin films was analyzed by atomic force microscopy (AFM). 2D and 3D AFM images of samples are shown in Figure3. All images are taken in 3μm × 3 μm scales. According to the images in Figure3, it can be observed that the average grain size is reduced by increasing the angle.
Average grain sizes were calculated using the J-MicroVision software (J Microvision, Geneva, Switzerland) and are given in Table1. Due to the fact that the emission of energetic ions from the focus region is fountain-like, a sample located at 0° is encountered by high flux of ions and according to this; large grains are formed on the substrate.
By increasing the deposition angle and decreasing the amount of ion flux and their energy, the size of grains were decreased significantly. As shown it can be deduced from Table1 that the grain sizes were decreased with increasing the deposition angle. The formation of small grains between the larger grains is largely because of different crystallographic phases of titanium nitride which were grown during the deposition process. It is exactly in agreement with XRD patterns in which increasing the angle was accompanied by significant decline in the amount of energetic ions and therefore, crystalline structures of titanium nitride were changed both in intensities and types.
Columns 3 and 4 of Table1 present the changes in average surface roughness and root mean square surface roughness values of several random areas in all samples with change in the angular positions. These values which have been calculated with Nova software (Chongqing, China) show similar trend to grain size variations. Based on the fact that the grain size was reduced by increasing the angles, a decline in amount of the average surface roughness and root mean square surface roughness was also observed in measured values by increasing the angle. Different average roughness values of different areas for each sample were caused by ions with wide energy spectrum emitted from the focus region. The surface of the samples was encountered by different energies, so surface roughness differed from one place to another.
Scanning electron microscopy and energy dispersive X-ray spectroscopy
Scanning electron microscopy is carried out to investigate the nanostructure of the surface of the samples. Scanning electron images of samples are shown in Figure4.
Figure4c shows the distribution of grains on the surface of the sample which is located at the angle of 30°. The surface is covered by approximately 200 nm grains in size which are distributed evenly over the surface. The surfaces of the samples which are deposited in the lower angles to the anode axis, as shown in Figures4a,and 4b, show the different distribution of grains. The agglomeration of smaller grains can be easily distinguishable on the surface that can be easily observed at higher magnification (Figure5).
High energetic ions flux, which are collided on the surface at angles of 0° and 15°, transfer high energy to the surface. Due to this high energy transfer, the agglomeration of smaller grains is happened in these samples. Agglomeration is the reorganization of the grains. The positions with the angles of 0° and 15° with respect to anode axis are exposed with higher energetic ion flux due to the fountain-like shape of ion emission in the plasma focus device. So as shown in the AFM images (Figure3), surfaces of the samples located at 0° and 15° consist of larger grains than the sample positioned at 30°. It can be concluded that at 30° angle, the surface is exposed with lower energetic ion flux and grains can be formed in smaller sizes. Smoother surface is also corresponding to this sample (Figure4c).
Elemental composition of thin films is identified by energy dispersive X-ray spectroscopy. The results of EDX for films produced in this work are given in Figure6. The presence of titanium is clearly specified in the elemental distribution as shown in the Figure6. The spectra of all samples are identical, but the intensities of the peaks are different. The amount of atomic and weight percentage of titanium decrease by increasing the angle. These graphs verify our argument about the shape of ion emission in plasma focus device. Other peaks in the EDX spectra are related to the elements such as Cr, Fe, Ni, Mn, and Si, which are formed the 304 type stainless steel as used for substrate. So the successful deposition of titanium on the surfaces is confirmed. Based on the presence of only the elements corresponding to film and substrate in the spectra, it is deduced that there are no impurities in the deposition process.
Conclusions
The aim of this work to investigate the feasibility of production of TiN thin films, using a plasma focus device, is achieved successfully. Titanium nitride thin films on 304 type stainless steel substrates are produced at a fixed distance from the anode (source) and different deposition angles. Results are discussed qualitatively. The effect of angular position of samples in the focus chamber according to ion flux and energy is reported by analyzing the crystalline structure (XRD patterns) and surface morphology (AFM and SEM images) of the deposited samples. The observed results confirm ion emission characteristics, and it can be concluded from the XRD, AFM, SEM, and EDX results that the ion emission in this device is fountain-like and the flux and energy of emitted ions decrease by increasing the angle with respect to the anode axis. Polycrystalline structures of titanium nitride in different phases are observed in all samples that prove the possibility of plasma focus devise in material processing and thin film deposition.
References
Panjan P, Navinsek B, Cvelbar A, Zalar A, Milosev I: Oxidation of TiN, ZrN, TiZrN, CrN, TiCrN and TiN/CrN multilayer hard coatings reactively sputtered at low temperature. Thin Sol. Film. 1996, 281: 298.
Constable CP, Yarwood J, Munz WD: Raman microscopic studies of PVD hard coatings. Surf. Coat. Tech. 1999, 116: 155.
Sproul WD: Very high rate reactive sputtering of TiN. ZrN and HFN. Thin Sol. Film. 1983, 107: 141. 10.1016/0040-6090(83)90016-0
Honor P, Sanjines R, Levy F: Characterization of sputter-deposited chromium nitride thin films for hard coatings. Surf. Coat. Tech. 1997, 94–95: 398.
Lim JW, Park JS, Kang SW: Kinetic modeling of film growth rates of TiN films in atomic layer deposition. J. Appl. Phys. 2000, 87: 4632. 10.1063/1.373116
Marco JF, Gancedo JR, Auger MA, Sánchez O, Albella JM: Chemical stability of TiN, TiAlN and AlN layers in aggressive SO2 environments. Surf. Interface Anal. 2005, 37: 1082. 10.1002/sia.2083
Hübler R, Cozza A, Marcondes TL, Souza RB, Fiori FF: Wear and corrosion protection of 316-L femoral implants by deposition of thin films. Surf. Coat. Tech. 2001, 142–144: 1078.
Petersson JR: Partial pressure of TiCl4 in CVD of TiN. J. Vac. Sci. Technol. 1974, 11: 715. 10.1116/1.1312741
Hummer E, Perry AJ: Adhesion and hardness of ion-plated TiC and TiN coatings. Thin Sol. Film. 1983, 101: 243. 10.1016/0040-6090(83)90251-1
Coll BF, Chhowalla M: Modelization of reaction kinetics of nitrogen and titanium during TiN arc deposition. Surf. Coat. Tech. 1994, 68–69: 131.
Biunno N, Narayan J, Hofmeister SK, Srivatsa AR, Singh RK: Low-temperature processing of titanium nitride films by laser physical vapor deposition. Appl. Phys. Lett. 1989, 54: 1519. 10.1063/1.101338
Kies W, Decker G, Berntien U, Sidelnikov YV, Glushkov DA, Koshelev KN, Simanovskii DM, Babashev SV: Pinch modes produced in the SPEED2 plasma focus. Plasma Source Sci. Technol. 2000, 9: 279. 10.1088/0963-0252/9/3/305
Sadowski MJ, Gribkov VA, Kubes P, Malinowski K, Skladnik-Sadowska E, Scholz M, Tsarenko A, Zebrowski J: Application of intense plasma-ion streams emitted from powerful PF-type discharges for material engineering. Phys. Scr. 2006,2006(T123):66.
Takao K, Honda T, Kitamura I, Masugata K: Purity of nitrogen ion beams produced in a plasma focus. Plasma Source Sci. Technol. 2003, 12: 407. 10.1088/0963-0252/12/3/315
Rawat RS, Arun P, Videshwar AG, Lee P, Lee S: Effect of energetic ion irradiation on CdI2 films. J. Appl. Phys. 2004, 95: 7725. 10.1063/1.1738538
Hassan M, Rawat RS, Lee P, Hassan SM, Qayyum A, Ahmad R, Murtaza G, Zakaullah M: Synthesis of nanocrystalline multiphase titanium oxycarbide (TiCxOy) thin films by UNU/ICTP and NX2 plasma focus devices. Appl. Phys. A 2008, 90: 669. 10.1007/s00339-007-4335-8
Kelly H, Lepone A, Marquez A, Lamas D, Oviedo C: Coating on metallic samples produced by a small energy plasma focus. Plasma Source Sci. Technol. 1996, 5: 704. 10.1088/0963-0252/5/4/012
Hassan M, Qayyum A, Ahmad R, Murtaza G, Zakaullah M: Nitriding of titanium by using an ion beam delivered by a plasma focus. J. Phys. D. Appl. Phys. 2007, 40: 769. 10.1088/0022-3727/40/3/013
Acknowledgments
This work was carried out with the support of the University of Tehran, Islamic Azad University and the Iran National Science Foundation (INSF). HS is also grateful to the Centre of Excellence for Physics of Structure and Microscopic Properties of Matter, Department of Physics, University of Tehran for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Fani, N., Savaloni, H. *Investigation on the formation of titanium nitride thin films on 304 type stainless steel using plasma focus device. J Theor Appl Phys 6, 30 (2012). https://doi.org/10.1186/2251-7235-6-30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-7235-6-30