Abstract
This experimental investigation is the first to generate a surface iron-tantalum (Fe/Ta) alloy as a sublayer-layer using a plasma focus device. Examining how ion beams from a plasma focus device alloy iron and tantalum with varying melting points is one of the key objectives of this study. Fe/Ta thin film nanostructure and surface morphology were also examined. The distance from the tip anode and the varied number of shots are the experimental variables. Although tantalum's melting point (3020 \(^\circ{\rm C}\)) is generally known to be near to that of iron (2862 \(^\circ{\rm C}\)), it is possible that iron vaporizes and partial alloying of iron with tantalum occurs before tantalum reaches its melting point. Fe/Ta thin film identification techniques include scanning electron microscopy, mapping of cross-section, energy dispersive X-ray spectroscopy, and X-ray diffraction pattern. Additionally, the composition of multilayer structures is examined using EDS. In conclusion, the results of the X-ray diffraction pattern showed that the number of shots had a significant impact on the residual strain degree of the thin films that were deposited. Furthermore, structures made of FeTa and Fe2Ta were produced. Additionally, photos from scanning electron microscopy and cross-section mapping verify that the sample with five shots at an 8 cm distance from the tip anode formed a uniform Fe/Ta alloy structure. The sample with five shots at a distance of 4 cm from the tip anode formed micro-island structures, as seen by scanning electron microscopy, with decreasing distance. Furthermore, depth elemental distribution revealed that the optimal depth of penetration in a homogenous material to develop alloying is best determined by number of PF shots.
Highlights
-
Selected parameters of experiment impact on the lattice parameters, as well as the fabricating Fe2Tahexagonal and FeTarhombohedral structures.
-
The degree of residual strain in the crystal lattice of the thin films, remarkably dependent on the number of shots.
-
Homogenous alloy only observed in one sample.
-
The creation of black-lines and micro-structures is attributed to enhance of O2 ratio.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
The technique of creating a film by depositing a thin layer of material onto a substrate is known as thin film deposition. There are several ways to accomplish this. Thin films are used in coatings, electronics, and optics to modify characteristics including adhesion, reflectance, and conductivity. The procedure plays a key role in the production of solar cells, semiconductors, and other cutting-edge technologies, as well as in the advancement of a variety of high-performance materials. Often called nanoparticles, tiny particles, or nanomaterials, they have become an essential component of contemporary science and technology. These structures are a focus in chemistry, physics, materials science, biology, and other sciences because of their unique behaviors, qualities, and interactions that distinguish them from bulk materials.
Iron atoms make up the majority of a subgroup of nanoscale objects known as iron nanoparticles (Fe NPs), or 3d valence orbitals. Their distinct magnetic feature has attracted a lot of interest from scientists working to remove contaminated sites from the environment [1,2,3,4]. Pure iron (Fe) corrodes quickly in aqueous solutions to produce Fe (OH)x, which covers the surface and reduces the reactivity of the nanocomposite (NCs) with impurities [5]. To address these problems, iron nanoparticles (Fe NPs) can be combined with another metal to create bimetallic nanoparticles (BNPs) [6, 7]. While one metal acts as an electron donor to reduce the impurities in these BNPs, another metal with a negative redox potential acts as a catalyst to boost the reactivity of the Fe NPs [3].
Fe NPs can be produced in a variety of ways. To develop NCs with improved mechanical, electrical, or magnetic properties, Fe NPs can be placed in BNPs, polymer matrices, or other materials. Important components of using BNPs and NCs are their synthesis and functionalization, which enable researchers to tailor their properties for particular uses. Furthermore, safe Fe NP deployment requires an understanding of their toxicity and environmental impact [8]. Fe NPs are an intriguing family of nanomaterials with a broad range of uses, including, and environmental remediation [9, 10], medicine [11], electronics [12] and photocatalysis [13, 14].
Tantalum nanoparticles, or Ta NPs, are highly promising for use in a wide range of scientific and technological applications since they are mostly made of tiny tantalum atoms. Ta NPs have been investigated in the medical field for their potential as contrast agents in imaging procedures including computed tomography (CT) scans [15, 16]. Ta NPs can be used as catalysts in a variety of chemical reactions, such as the processes of hydrogenation and dehydrogenation [17,18,19]. Pure tantalum metal had the lowest recorded current density (Ta). The persistent oxide layer of the base metal explains this by preventing electron exchange and redox reactions as a result [20]. Ta NPs are used in the semiconductor business, mostly as capacitors. Tantalum capacitors are crucial parts of electronic devices because of their great capacitance, stability, and dependability [21,22,23]. Ta is a refractory metal that has special qualities when reduced to NPs, which is why it is a topic of great interest for researchers in many different fields. Ta NPs are added to cutting-edge materials to improve their mechanical and corrosion-resistant qualities, such as NCs [24, 25], coatings to enhance mechanical properties [24, 26], and corrosion resistance [27,28,29,30,31].
A class of sophisticated materials known as Fe/Ta NCs combines Fe and Ta at the nanoscale. In general, the electron configurations of Fe and Ta as d-clock elements are (n−1) d1−10 ns2, with a few exceptional exceptions where electrons are rearranged to form half-full or filled d subshells. The transition metals are filling in their five d orbitals. Although many transition metals can lose two or three electrons, producing cations with charges of 2 + or 3 + , some can also produce charges as high as 1 + . These hybrid structures combine the special qualities of the two components, providing a broad range of uses in various scientific and industrial fields. Fe/Ta NCs are good for structural and permanent magnet applications because they frequently show improved mechanical properties over the large spin–orbit coupling [32,33,34]. Improved mechanical stability is a result of Fe's strength [35] and Ta's high melting point [36]. Additionally, Fe/Ta NCs may be electrically conductive depending on their composition, which is helpful in applications requiring both structural and electrical qualities [37,38,39]. When paired with Fe, Ta's natural corrosion resistance can increase the NCs' overall corrosion resistance [40,41,42].
Fe/Ta NCs may be able to maintain the magnetic characteristics of the Fe component, opening up new possibilities for data storage, lithium ion batteries (LIB), and magnetic sensing [4, 43]. Fe/Ta NCs may find use in water treatment systems [44], where photocatalysis plays a crucial role in eliminating pollutants from the environment. These NCs can be applied to electronic devices and components that need to be electrically conductible and mechanically resilient [45, 46]. Furthermore, because of their biocompatibility and ability to release drugs under controlled conditions, Fe/Ta NCs may find application in medical implants [47]. Fe/Ta NCs are appropriate for protective coatings in harsh settings due to their resistance to corrosion. Owing to their intriguing properties, Fe/Ta NC synthesis and research have drawn a lot of interest.
Explosive welding (EW) [48], ultrasonic spray drying [49], rapid quenching [43, 50, 51], anodizing of sputter-deposited material [52], RF diode sputtering [53], Ion beam sputtering [33, 54], Ion implantation [55, 56], arc melting [57, 58], and chemical reduction [59, 60] are some of the methods that can be used to prepare Fe/Ta NCs.
Using a plasma focus (PF) apparatus, thin film deposition with PF entails producing a concentrated, high-energy (1025–1026 m–3) and high temperature (1–2 keV) plasma beam in a little amount of time (10–7 s) [61]. After that, a thin film is created on a substrate by the deposition of thin atoms. PF devices provide powerful, brief energy pulses that offer high energy density circumstances ideal for effective material synthesis. In addition, the brief length of the plasma pulse permits fast heating and cooling, which facilitates the formation of NCs with homogeneous and finely structured microstructures. This method is applied in several fields, including optics and microelectronics, where accurate and regulated thin films are necessary for the operation of devices. By acting as a neutron generator, the PF device [62, 63] facilitates effective and focused deposition [64, 65], as well as the irradiation of relativistic electrons [66] and energetic ions [67,68,69] to aid in the creation of thin films [70]. Additionally, the PF device is a promising option for both soft and hard X-rays [69, 71, 72].
The parameters (number of shots and distance from the anode tip of the PF system) that we have selected for our current investigation have an effect on the structure of thin films as well as the alloying process. Under the same processing circumstances, Hosseinzadeh et al.'s experiment [73] produced separate pores with varying diameters, and the thickness of the coatings decreased as the distance between the substrate and the anode tip increased. Furthermore, the quantity of NPs in the deposited coating increased together with the coating's thickness. The XRD spectra shown in the Shirazi et al. [74] study clearly illustrate that for thin films deposited with additional shots, the peaks intensities rise and suggest improved crystal quality. It is reasonable to surmise that a higher crystalline material thickness should be obtained with a higher number of shots. Siddiqui et al. [75] speculate that an Ion energy drop farther away from the anode tip may be the cause of the peak shifting caused by an increase in d-spacing. The number of shots and distances selected were meant to investigate the potential for electron penetration to cause lattice stresses.
It is evident from a review of the Ta and Fe research that PF has not yet looked at Fe/Ta NCs. Only the reports on Fe and Ta individually have been examined in the sample below: In the investigation by Siddiqui et al. [76], ion radiation and a combination of (O + N) reactive gases were used to generate Ta-N–O thin films. These results showed that when O2 levels in the samples rise, the likelihood of a spherical shape appearing increases. Moreover, the roughness of the samples has improved due to the quantity of accessible O2. Additional research by Rawat et al. [77] demonstrates that a 3.3 kJ PF device was used to deposit nano-structured Fe thin films, with a range of shot counts at two distinct angle positions. Furthermore, Kotb et al. [78] report that a 3.3 kJ PF device was used to deposit a nano-structure Fe-Si thin layer at various distances from the top of the central electrode on a glass substrate.
We investigated the application of high-energy PF devices for Fe/Ta thin-film deposition on an aluminum substrate in this hands-on investigation. This article will investigate Fe/Ta thin films deposition, one of the essential d-block components. This work aimed to investigate the effects of the number of shots and distance from the tip anode of the high-energy PF device on the morphological and nano-structural properties of the deposited Fe/Ta thin films.
In the following section, we will look at materials and methods such as preparation and characterization. The results and comments are presented in Sect. 3. The conclusion and summary are presented in Sect. 4. The last portions contain the acknowledgement and author.
2 Materials and methods
2.1 Preparation
Samples should be mounted after being first cut with a cutting machine. Using abrasive paper with No. 240, 320, 600, 800, 1000, 2000, and 3000, polished samples in the shape of discs with a diameter of 2 cm and a thickness of 0.5 cm are then made, as shown in Fig. 1. Following the abrasion process, the samples are cleaned, dried, and then quickly moved to the ultrasonic phase of the procedure. The samples are placed in different acetone and alcohol-filled containers and left in an ultrasonic bath for 15 min each. The samples are then dried by exposing them to air after being cleaned with distilled water (DIW). Following the preparation stages, Fig. 2 shows images of the prepared substrates (s1–s3). The polarized optical microscope (POM) characterizations revealed that during polishing, numerous domains with multi-layered structures and distinct orientations may be formed, as well as contrast in the pictures.
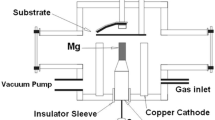
Furthermore, direct current (DC) magnetron sputtering is used to deposit the prepared samples once they have been created. The aluminum (Al) substrates in the device's anode section are where the Fe samples are positioned. Relatively smooth surfaces are provided by the anodized aluminum substrates [52]. The cathode portion contains the Ta target layer. The cathode has a circular design with a 5.30 cm diameter and a 0.53 mm thickness. The diffusion pump was then turned on, raising the chamber pressure to 5 × 10–6 mbar. After then, the chamber pressure was increased to 1.2 × 10–2 mbar by adding argon (Ar) as a carrier gas. The voltage and input current are adjusted to 250 V and 50 to 52 mA, respectively.
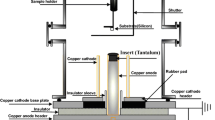
Subsequently, a varied series of shots from the plasma focus (PF) device are applied to the deposition samples from the preceding phase. The samples were first placed in the sample holder at a position of 0° from the anode axis after the chamber had been cleaned with alcohol, and the shutter was adjusted in front of them. A rotating pump creates vacuum pressure in the device's chamber, which holds the electrode system and samples, raising the pressure to 4.4 × 10–2 mbar. Subsequently, an extremely pure nitrogen (N) gas is introduced into the chamber as a reactive background gas in order to raise the ideal pressure to 1 mbar and deposit significant nitrides structures [70]. The chamber and cathode rods are connected to the earth. A 10 mF capacitor in the PF utilized in this experiment is charged to generate energy up to an 18 kV potential differential. Table 1 displays the PF device's experimental settings as well as the deposited samples. Samples s1-s3 are exposed to 1, 5, and 10 shots from the PF device at a distance of 8 cm from the anode tip in order to assess the impact of radiant radiation on the samples that were deposited in the same manner in the preceding phase. Every sample is coated for one hour and ten minutes. Figure 3 displays the PF device's general schematics.
A manual shutter was positioned over the substrate holder at the beginning of the experiment to stop weak shots from coating the substrates. Once strong focusing had established, usually after firing 2–3 shots, the shutter was removed and the Fe/Ta thin films were deposited with varied numbers of shots (0, 1, 5, and 10 shots). After three shots for s1, two shots for s2, and three shots for s3, a strong plasma focusing was achieved, and the shutter was removed from the front of the substrates. The new sample was positioned 4 cm away from the tip anode in the following phase to test the impact of distance. Once intense focus was achieved, the shutter was taken off, and deposition was carried out. A four-channel digital oscilloscope (model MHz 500 B 304 Tektronix TDS) acquired an extreme voltage peak in the voltage probe signal, which represents the desired focusing (Fig. 4).
An outline of the PF device's synthesis procedure is as follows: A PF device is a pulsed power system that quickly discharges electrical energy across electrodes to produce very high-energy plasma. An anode tip and a cathode submerged in a gas medium are the standard components of this device. The PF device is filled with a certain gas, such as Ar/N2, to start the process. The properties of the plasma can be affected by the gas selection [79, 80]. The electrical discharge produces a short-lived but incredibly powerful burst of plasma when it starts. This process can release energy in the range of kilo-joules to mega-joules. The Fe and Ta source materials are vaporized and ablated by the high-energy plasma produced in the PF chamber. Thin films are one appropriate structure for these materials. In the plasma environment, the vaporized Fe and Ta species cool and condense quickly, forming nanoscale particles. By varying the plasma settings and the starting materials, it is possible to regulate the size, composition, and structure of Fe/Ta NCs to some extent.
2.2 Characteristics
After the synthesis process, the Fe/Ta NCs are characterized using various techniques. The X-ray diffraction (XRD, STAD MPJ-\(\lambda\)=0.154 nm—UK) provide insights into the size distribution, composition, crystallinity, and other properties of the NCs. For XRD measurement, concentrated solutions are placed onto a Si substrate and allowed to dry for 10 days. The scanning electron microscopy (SEM, HITACHI S-4160 instrument—Japan), investigate the surface morphology, micro/nano structures and the cross-section of the irradiated samples. In order to do this experiment, a drop of the focused suspension was applied to a grid made of Cu Mesh 300 carbon that has been kept fully dry and at room temperature. The energy dispersive X-ray spectroscopy (EDS, 4009-3MST-LV-T—USA) analysis, checked the percentage of elements and thickness in thin films. For EDS analysis, we mount the prepared NPs on stainless steel. Changes in valence states and surface functional groups samples were detected using a high-performance X-ray photoelectron spectrometer. The PF and DC magnetron sputtering machines are made by the Islamic Azad University (SRBIAU) in Tehran, Iran's plasma engineering division. The design, building, and operation of the PF system were all managed by the plasma physics research center (PPRC). The project's completion resulted in the submission of multiple national and international patents as well as the publication of numerous articles.
3 Results and discussion
3.1 X-ray diffraction (XRD) studies
An effective method for examining the crystalline structure of materials, such as Fe/Ta NCs, is X-ray diffraction (XRD). Furthermore, XRD analysis can be particularly helpful in examining potential peak shifts/broadening, lattice distortion, and intrinsic elastic residual stress when deposition is carried out at a high temperature. As seen in Fig. 5, the crystal structure, lattice parameters, and phase composition of the Fe/Ta NCs are determined by comparing the observed diffraction pattern with databases of expert highscore software and the ICDD PDF-2 database. Furthermore, some crystal planes in the samples correspond to peaks in the diffraction pattern. It is possible to ascertain the crystallographic characteristics of the NCs structures by locating these peaks and quantifying their positions and intensities.
Fe NPs' lattice parameter may vary depending on the size and crystal structure, among other things. The most prevalent crystal structure for bulk Fe is bcc, with a lattice value of roughly 2.866 Å. However, the lattice parameter in NPs may differ from the bulk value, particularly if they are very small or have unique forms. Regarding Fe nanoparticles, the pattern (ref. No. 01-087-0722) might exhibit three prominent peaks that align with the Fe lattice's crystallographic (110), (200), and (211) planes. These planes connected to the bcc structure's 2ϴ = 44.65°, 65.43°, and 82.47°. The size and arrangement of the NPs determines the locations and intensities of these peaks. Size effects may cause broader peaks to appear in smaller NPs [81, 82].
Ta NPs' lattice parameter can be related to elements like crystalline size, structure, and processing circumstances. Ta can crystallize in a variety of crystal forms, the most prevalent of which are bcc and hcp. According to our research, three peaks for each spectrum -corresponding to 2ϴ = 36.73°, 42.78°, and 62.11° with cubic structure- are assigned to diffraction from (111), (200), and (220) (ref. No. 88-2338). Ta NPs' lattice parameter may vary as a result of surface and size effects. Furthermore, using the PF approach may result in a different lattice parameter. Furthermore, details on the NPs' orientation and crystalline structure would be disclosed by the particular diffraction pattern.
It would take specific methods and tools, like XRD, to identify the crystallographic planes of an NC, like Fe/Ta, produced by a PF experiment. The crystal structures, manufacturing circumstances, and particular composition all affect the Fe/Ta NCs' crystallographic planes. It would normally require knowledge of the crystal structures of both the Fe and Ta phases in the NCs in order to ascertain the crystallographic structure of Fe/Ta NCs. The Ta-Ta connection always shows repulsive interaction when Ta NPs are embedded in Fe NPs [83]. However, there is a local energy minimum for Ta NPs at the third distance from nearest neighbor. Thus, Ta NPs will be dispersed equally across Fe NPs. The repulsive energy for the two nearest neighbors Ta is 0.47 eV [84]. In Ta atoms, Fe atoms create interstitial sites with lower symmetry defects. The extra doublet positions of Fe in Ta appear to be related to the localization of Fe atoms at dislocation borders or the formation of Fe vacancies in clusters [56]. Fe atoms are found to form two main positions in Ta, referred to as the doublet position, according to Mössbauer research. The hyperfine electric and magnetic fields on Fe nuclei are defined by the local atomic environment and can be quantified using the Mössbauer effect. This makes it possible to identify various crystallographic locations of other Fe atoms or distinct phases in which Fe atoms are confined [56].
As for the formation of multiple types of steel, the Fe phases at atmospheric pressure are important. Above 1392, there is a transition in the Fe structure from fcc to bcc, which is also known as the δ (Delta-ferrite) form. Additionally, the transformation adheres to the underlying elastic residual stress field and lattice distortion [85]. Along with the change, a significant drop in energy is seen, even for the large-sized nanoparticles [86]. According to recent study, atomic spin-orbital interaction, interface tension, the stress field produced by the additional volume in grain borders, and changes in electron density at the fermi level are the main causes of lattice deformation [46, 87]. The fundamental presumption that the significant lattice distortion will cause the diffraction peak intensity to decrease. Despite experimental evidence of lattice distortion in Fe/Ta NCs, diffraction peak intensities did decrease with increasing constituent element count [85, 88, 89]. The atom size change results in the formation of a local volumetric strain field [89, 90]. Grain boundary changes are also seen in the results of the SEM and EDS studies.
In bcc and fcc frameworks, Fe and Ta NPs form in relation to temperature and medium pressure, respectively. The Ta-fcc structure vanished in all cases that have been reported as the substrate temperature rose or the film thickness increased [91,92,93,94]. Because of the large contribution of surface forces and the inclusion of surface impurities, the fcc structure could only be present in very thin (10 nm or thinner) Ta films created using the PF technique [95]. The Ta-fcc granules disappeared at thicknesses greater than 25 nm [96]. Check out EDS analysis to learn a little bit more about thickness. Furthermore, around about 45°, exceptionally strong peaks associated with bcc Ta are seen in all samples.
Fe and Ta NPs have a cubic structure in the current research, based on the databases that are available. However, the Fe/Ta alloy structure that was generated is based on non-cubic uniaxial structures, specifically the FeTarhombohedral with ref. no. 001-021-0433 and the Fe2Tahexagonal with ref. no. 03-065-1999. The peaks located at 2ϴ = 34.58° and 45.01° are ascribed to the FeTa alloy NCs' Bragg reflections (110) and (021). Furthermore, diffraction from (101), (110) and (112) is attributed to two peaks, which correspond to 2ϴ = 25.26°, 37.59°, and 45.12° for the Fe2Ta alloy structure. In our investigation, the bandwidth increases in the FeTa alloy structures (s1 and s3) near the 35° as the number of shots increases. When the number of shots around the 45° increases, the intensity of the peaks increases (s0–s3). In addition, there were no peaks in the area of 25.26° on the Fe2Ta alloy structure of s0. For the other samples, however, a broad bandwidth peak is observed (s1–s3). On the other hand, the peaks (s0–s3) that are seen at the 45° have a very high intensity.
The synthesized NCs' large magneto-crystalline anisotropy energy (MAE) is significantly increased by these discovered structures (FeTa and Fe2Ta) [32]. Furthermore, it has been discovered that the effects of ferromagnetism in NCs structures are more connected with the concentration of Fe NPs in the samples than with the lattice parameter [4]. Either a Fe or Ta excess can induce a ferromagnetic ordering at low temperatures (150 K) in the Fe2Ta structure, a paramagnet [97]. Permanent magnets (PMs) can be made using the Fe-bcc and Fe2Ta structures [98]. The magnetic moment of the non-cubic Fe/Ta NCs increases with alloying Ta because of differences in the valence electrons and atomic radius between those solute atoms (Fe and Ta). The mechanical and chemical impacts on the bond structure of Fe and Fe/Ta are characterized by plots of spin alignments and deformation electron density, respectively [46].
According to a recent study, impurities such as iron(ɪɪɪ) nitride (FeN cubic, ref. No. 01-075-2127), iron oxide (Ta2O, ref. No. 25-922), and tantalum nitride (TaN hexagonal, ref. No. 00-039-1485) can appear as extra peaks in the diffraction pattern that do not correspond to the material's main phase. Determining the presence and kind of impurities in XRD samples is essential to determining the material's purity, composition, and quality. The position, strength, and form of these extra peaks can be examined to learn more about the degree of crystallinity.
When the number of shots is zero (s0, n = 0), it is clear from comparing sample s0 with other samples that Ta2O is not produced. At 2ϴ = 26.59° and 28.98°, the peaks that correspond with it become visible at the start of the photos (s1, s2, and s3). Only in s2 and s3, in proximity to the angles of 43.83° and 52.23°, is the FeN structure discernible. However, it has been shown that as the number of shots at 64.90° in the Fe2N structure increases, the peak's height increases in all samples (s0-s3). Additionally, only s2 and s3 show the Fe2N structure in the vicinity of 43.86°. It is evident that the height intensity is incredibly low when compared to other peaks. As the number of shots rises, it is observed that the Full width at half maximum (FWHM) associated with the s0-s3 in the TaN structure between the 35.21° and 37.56° grows. It is apparent that when additional images are taken, the intensity of the Fe–N peaks rises as well, suggesting that there are more Fe–N bonds in the samples when more N atoms are added. Conversely, the strength of Ta-N peaks decreases with increasing shot count [70]. The mole ratios of Ta-N and Fe–N in the thin films formed with increasing shot counts were computed by integrating the regions under the different peaks/FWHM. The absence of a Ta-N bond in a large number of shots is the reason for the electronegativity (EN) of NEN = 3.04 and FeEN = 1.83 compared to TaEN = 1.5.
The N2 and O2 vacuum medium would evaporate and break down when the Fe/Ta NCs were placed at a high temperature. The XRD data indicate that the presence of impurities in the high vacuum deposition chamber is not critical to the production of non-cubic structures, even though the nucleation and growth of the previously mentioned bcc phase depend on the presence of a piece of N2 and O2 near the substrate surface [91, 95]. The PF technique utilized in our work to produce the NCs had an impact on the crystallographic planes that were produced. Certain facets of the crystal lattice can be linked to distinct crystallographic planes. The atomic configuration and Fe/Ta ratio in the NCs, as well as their impact on the lattice parameters and possible interfacial planes, are discussed next.
High temperatures, which are frequently needed for the synthesis of Fe/Ta NCs, particularly those containing refractory metals like Ta NPs, can be reached by PF devices. Conversely, the creation of nanoscale Fe/Ta NCs is made possible by the high energy environment, which also allows for control over particle size and dispersion. XRD examination of the generated material would be required to determine the crystalline size of Fe/Ta NCs. Lastly, it can be carried out in the next study, the findings will reveal details regarding the dimensions, distribution, and existence of strain in the crystal lattice of the NCs. The tension within the crystal structure is indicated by the peaks' displacement. Lattice strain can be evaluated by analyzing the peak shifts/broadening [81, 82, 85]. Furthermore, XRD testing can determine whether crystallites in the NCs have a preferred orientation [81, 82], which is significant for comprehending mechanical characteristics. Furthermore, the crystal structure shifts from Fe2Tahexagonal to FeTarhombohedral upon repositioning the peaks (Table 2).
Nanoscale materials are frequently present in smaller crystallite sizes. Comparable results from the Witusiewicz et al. investigation [57] show that the alloy has been seen to display several phases. While the phase has been observed at 37° and 39°, it first seemed nearer to 37 and 40. In the δ phase, Ta is most soluble in Fe around 1440 °C [51]. The average crystallite size is determined using the Debye–Scherrer equation (Eq. 1) where D is the average crystallite size, K is the shape factor (0.94), λ is the X-ray wavelength, β is the FWHM of the principal surface peak, and θ is the diffraction angle:
Peak intensity and FWHM for transit metals are influenced by inter-particle spacing, morphology, and particle size [99]. It is discovered that the average crystal diameters of s0-s4 are 31 nm, 18 nm, 13 nm, and 20 nm, in that order. The crystal diameters of s1 and s3 increase up to the optical limiting activity [100]. Even though Ta's melting point (3020 °C) is typically close to Fe's boiling point (2862 °C), Fe may evaporate and partial Fe/Ta alloying may happen before Ta reaches its melting point [51]. As a result, s2 has greater Ta solubility in Fe. The crystalline size in s2 is lower than in s1 and s3. The cross-section SEM image shows the same outcome. Table 2 displays the average crystallite size of the deposited samples.
The strong heat and pressure produced by PF can help Fe and Ta form an alloy, giving NCs the appropriate characteristics. Furthermore, the PF device operates in a low-pressure or vacuum environment, which lowers the possibility of contamination during synthesis from ambient gases. However, the creation of an impurity-filled NCs structure is caused by the activity of Fe above its low percentage/solubility in Fe/Ta NCs [57]. The fraction of Fe NPs in the generated NCs structure is acceptable only in the s2. You can use EDS analysis to verify this. The spin-flipping and bond structure alterations demonstrate that the electron redistributions regulate the spin state modification of Fe/Ta NCs at different shots. This phenomenon clarifies how Fe/Ta NCs and Fe-bcc NPs' ferromagnetism is impacted by lattice distortion [46].
Ultimately, the XRD analysis results can be summed up as follows: Because of the high energy environment's ability to control particle size and dispersion, nanoscale Fe/Ta NCs are generated. On the other hand, peak displacement indicates the tension inside the crystal structure, and peak shifts/broadening analysis subsequently allows for the evaluation of lattice strain. The significant lattice distortion will cause the diffraction peak intensity to decrease, and the atom size shift will cause a local volumetric strain field to form. Different crystallographic planes can be associated with specific crystal lattice facets. The primary causes of lattice distortion are the interface tension, the stress field created by the extra volume at the grain boundaries, the atomic spin-orbital interaction, and the fluctuation in electron density at the fermi level. It is evident that as the number of shots grows, so does the bandwidth of the Fe/Ta NCs and the intensity of the Fe–N peaks. After then, as the number of shot increases, the power of Ta-N peaks decreases. The Ta film with fcc structure is produced as a result of the presence of surface impurities and the significant contribution of surface forces. Consequently, the diffraction peak intensities did in fact decrease as the number of constituent elements rose. The Ta-fcc structure disappeared when the substrate temperature climbed or the layer thickness grew.
3.2 Energy dispersive X-ray spectroscopy (EDS)
In order to ascertain the elemental composition of various locations inside the NCs and validate the existence and distribution of Fe and Ta, energy dispersive X-ray spectroscopy (EDS) can be used in conjunction with scanning electron microscopy (SEM). To help comprehend the homogeneity of the material, EDS can also offer elemental mapping, which displays the spatial distribution of elements across the sample. The EDS analysis is used to determine whether elements are present in the layers that have developed and how different shots affect the quantity of elements (Fig. 6).
More impurities are seen in the structures (s1–s3) when the number of shots is increased at the same distance from the anode tip (8 cm) (Fig. 6b, c, and d). It is evident that there are no impurities in the structure at 4 cm from the anode tip by comparing s2 and s4, which were deposited with the same number of shots but different distances. In actuality, the structure appears more contaminated the farther one looks. Tables 3, 4, 5, 6, and 7 display the atomic percentage of the elements based on the EDS analysis results of elements s0-s4.
Tables 3 and 4 demonstrate that the atomic percentage of Fe in the deposited layer increases and the atomic ratio of Ta decreases as the number of shots increases from n = 1 to n = 5. Additionally, by increasing the number of shots to n = 5, a homogenous composite structure of Fe/Ta NCs is produced at a greater distance from the surface. Only in the s2, where the Ta and Fe percentages are somewhat close to one another, is the best alloy formation discernible (Fig. 6c). Other samples (s0, s1, and s3) do not exhibit the ferromagnetic property due to the high Ta ratio and low Fe content (Fig. 6a, b, and d). Additionally, a shorter distance (4 cm) has led to a reduced Ta content and enhanced alloy formation, as shown by the EDS analysis of s4.
The Ta2O on the surface is thought to have been created by the interaction between O2 and Ta either during or after deposition. First, oxygen diffusion may occur with free radicals during the synthesis process due to non-ultra-high vacuum conditions. Second, Ta and O2 in the air can react once the deposited samples are taken out of the PF apparatus [55, 101]. Because of the tiny corrosion potential (Ecorr), the creation of Ta2O is a significant contributing factor to the rapid corrosion, according to the XRD results [55]. Through galvanic corrosion, the Ta and Fe's differing Ecorr values could accelerate the pace of corrosion. Additionally, corrosion cells and corrosion rate tend to develop on a rougher surface [55, 102]. The formation of crack-like flaws enhances the Ecorr of Fe/Ta NCs, per the SEM investigation [42, 55]. It is advantageous to add more Fe to the NCs in order to increase compactness [42]. Following Ta deposition with increasing incident doses, the corrosion current density of the s0, s1, s3, and s4 is reduced. The combined impacts of surface roughness and microstructures are responsible for the highest rate of corrosion [55].
Fe and Ta are densely distributed close to the grain boundary, despite the fact that they disperse unevenly throughout the grain, as shown by the EDS mapping (Fig. 6e). The more Fe-rich phases there are in the mixture, the more energy is needed for deposition. The samples with lower Fe content should, however, be at a lower temperature. Ferromagnetic ordering grows at sharply raised temperature at Fe/Ta NCs. By increasing the electron spin resonance with the thermal enhancement, it is shown that aberrant thermal expansion occurs in tandem with the creation of ferromagnetic ordering at Fe/Ta sites [42].
Ultimately, based on the images and elemental percentages (Fe and Ta) derived from the EDS analysis results of the samples with varying shots, it can be concluded that the alloying process has occurred with a low probability because of the low percentage of Fe in the deposited layer (Fes1 = 13.57% and Fes3 = 13.44%). It is likely that the phase structure of Ta2O will form as a result of the high atomic percentage of O2 in s1 to s3. However, the likelihood of nitride phases (NOx) forming also rises as a result of the presence of N2. Ultimately, the existence of Ta is shown by the degree of color change in the vicinity of the coated surface, as shown in Fig. 6c, which is connected to the sample exposed to n = 5 Ion beam shoots from the PF device (s2). Furthermore, this sample (s2) has undergone the alloying process, and it is anticipated that the XRD study will corroborate these findings.
3.3 Characterization of morphology and structure
3.3.1 Scanning electron microscopy (SEM) studies
Fe/Ta NCs produced by a PF device can yield important details regarding the material's microstructure, morphology, and elemental distribution when analyzed using scanning electron microscopy (SEM). SEM can provide information about the size, shape, and distribution of particles or other features within the material, as well as the surface morphology of the NCs. In order to ascertain whether the NCs are in more than one phase, the following SEM can be utilized for phase identification. Additionally, surface roughness can be obtained via SEM, which is useful for applications involving adhesion or friction. It can disclose surface topography, such as the existence of cracks, defects, or other characteristics that might impair the performance of the material. The SEM picture of samples (s0 to s4) is displayed in Fig. 7a–d.
The agglomeration of Ta NPs can be seen in the SEM-related images by increasing the number of exposures. A similar article [27] has observed the phenomena of clustering. Ta atoms migrate toward the Fe-rich grain border during deposition, resulting in an even dispersion of the elements. It should be noted that some ditches, which correspond to the crack-like defects and the black lines in Fig. 7e, persist after the grain boundaries have been disrupted. The impact of the PF device's ion beams has caused damage to certain areas of the s4. Subsequently, there have been discernible modifications to the morphological surfaces of the S4, as the sample distance from the anode tip has decreased from 8 to 4 cm. Additionally, micrometer-scale zones have been generated, which may cause a shift in the fraction of surface elements.
A closer look at the samples' cross-sectional area in Sect. 3.3.b reveals more about the degree of uniformity. It's possible that the grain boundaries become more corrosion-resistant. It illustrates how, as the distance from the anode tip decreases, black lines and crack-like flaws may form suddenly. The increase in the O2 ratio in s4 is responsible for the formation of microstructures and black lines. Similar findings can be found in research conducted by Cen et al. [42]. According to the same investigation by Rassell et al., Fe/Ta NCs with an allotriomorph grain border melted at 1400 °C. In the δ phase, Ta is most soluble in Fe around 1440 °C. Regardless of Ta's solubility decreasing with temperature in the same research by Fe, the low Ta solubility inhibits age hardening. The δ phase field was used in the solution treatment as a result of this constraint. As it cooled, the material surrounding the polygon-shaped borders transitioned from δ → γ to γ → α again [51].
A recent study utilizing EDS analysis shows that the contribution of Fe atoms to the alloy structure is higher in s2. Additionally, according to the findings published by Su et al. [9], the captured microscopic image of Fe NPs is identical to s2 and shows the effective existence of Fe atoms. The total amount of O2 in each sample causes the color to change from light gray to dark gray. The more O2 there is during the process, the lighter gray the samples' hues become. The alloy NPs in s0 seem dark gray [76]. In s1-s3, the hue of the alloy structures changes from light gray to dark gray. As shown by XRD analysis and later EDS, the creation of a thin layer suggests the presence of various compounds, such as oxide and nitride structures, in the samples. In fact, a separate alloy thickness forms on the substrate as a result of the aforementioned structures competing with one another during deposition [76, 103, 104].
The amount of O2 in the reaction has a direct impact on how spherical structures form in alloy samples [105]. The more O2 there is when alloy structures are formed, the more likely it is that a structure will form. The s0 and s4 exhibit a structure that indicates a considerable O2 content, as shown in Fig. 7. The proportion of O2 is shown using EDS analysis. Detectable microstructures arise when the O2 concentration in the thin layer is higher than the threshold [76, 106]. Siddiqui et al.'s comparable study [76] yields the same results. In s4, where the concentration of O2 is very high, the microstructures that are formed are easily visible. Their surface roughness increases together with the O2 concentrations in s0-s4. Additionally, there has been a discernible rise in surface roughness, especially in s4, which contains island microstructures. The surface roughness in s4 has increased due to the significant presence of chemicals like TaN and Ta2O in the structure [104, 107, 108].
3.3.2 SEM image and EDS mapping of cross-section studies
Samples were placed vertically in the SEM device after the metallography process in order to examine the morphology produced by different shots and gauge the thickness of the formed layers. The results of the SEM image cross-section analysis for samples s1 through s3 are shown in Fig. 8a, b, and c.
Figure 8a illustrates that the thickness of the coated layer on the substrate in s1 is around 2.29 μm. The atomic proportion of Ta and Fe NPs is equivalent at 3.5 μm from the surface of the s1. The overall length of the scanned linear cross-section is roughly 7.27 μm. The results of the cross-sectional examination of s1 are shown in Table 8.
A glance at the Fig. 8b provided reveals the scanned linear cross-section's overall length is around 8 μm, and the coated layer's thickness in s2 is close to 2.03 μm. Also noteworthy is the fact that the relative percentage of Fe/Ta NCs has reached equilibrium at a distance of approximately 2.7 μm from the surface of the s2. Thus, it is reasonable to surmise that the Table 9 presents the findings from the cross-sectional examination of s2.
Given is Fig. 8c illustrating the coated layer thickness in s3 is close to 2.99 μm. In stark contrast, however, the relative atomic percentage of Fe/Ta NCs is the same at a distance of approximately 3.4 μm from the surface of s3, as indicated by the scanning along the cross-section with an approximate size of 7.42 μm (Table 10). Also evident is the fact that the synthetic composite structure is uniform at this distance from the surface. The cross-sectional statistics for the samples are shown in Table 11. In conclusion, it can be shown that as the number of shots increases, the coated layer's thickness decreases.
The cross-sectional EDS mapping is displayed in Fig. 9a, b, and c. Ta and Fe are seen to be unequally distributed in the grain in s1 and s3, and the grain border is rich in Ta but deficient in Fe, according to the EDS mapping. From the information supplied it is apparent that, the distribution of elements is almost same in the s2 grain boundary.
Given are three Fig. 9a, b, and c providing a comparison of move Ta atoms into the Fe-rich grain boundary during deposition, resulting in a uniform distribution of elements. Grain composition in s1 is measured to be Fe13.57 Ta86.43 (n = 1), but after n = 5 shots in s2, it changes to Fe61.56 Ta38.44. After n = 10 shots, the grain composition in s3 changes to Fe13.44 Ta86.56. During deposition, the Fe-concentration fluctuates irregularly [42].
Ta NPs melt greater duration than Fe NPs because of their higher melting point (3020 \(^\circ{\rm C}\)) compare to boiling point of Fe (2862 \(^\circ{\rm C}\)) [48], and not all samples will produce a homogenous alloy structure with the same distribution of NPs. The corresponding melting point suggested by Paul and Swartzendruber [57, 109] is the next. In compared to s1 and s3, Ta and Fe NPs in s2 have actually produced an NC with a superior elements distribution. For more likely, the plasma focus coating device can rapidly increase the surface temperature of the layer (Ta) and the substrate (Fe) with n = 5 shots simultaneously, before evaporating Fe atoms. Conclude, with the surface melting operation, Ta as a layer in the molten phase penetrates deeper than Fe as a substrate. This was followed by, the layer and the substrate are cooled and a uniform and homogeneous alloy is formed due to the phenomenon of convection.
Two intermetallic phases exhibit an extended homogeneity range with increasing temperature in the binary alloy system Fe/Ta NCs: the λ phase, which is based on the Ta/Fe NCs stoichiometry, melts congruently near 1857 \(^\circ{\rm C}\), while the μ phase, which is based on the Ta/Fe NCs stoichiometry, forms through the reaction at 1786 \(^\circ{\rm C}\) [57].
One particularly interesting fact highlighted by the reports is that Fe/Ta thin film structures are superimposed with favorable wetting angle (~ 0°) due to the vortex at the NCs explosively welded interface [34, 48]. Moreover, Chen et.al [41] conducted that bonding strength of NCs structure over the obvious atomic reconstruction is remarkable. For Fe/Ta surfaces, the covalent bond is the predominant interfacial connection [41]. However, it is evident that the structure of the produced Fe/Ta NCs exhibits a noteworthy catalytic property.
3.3.3 Depth elemental distribution
Using EDS, the composition of the thin films was ascertained as follows. It showed that the samples were composed of Ta and Fe phases [110].
The coated layer of the s1 is primarily made up of 86.43% Ta with a trace quantity of 13.57% Fe, as seen in Fig. 10a. The concentration of Ta drops abruptly to around 4% when the depth is increased to 8 μm, while the concentration of Fe increases linearly to approximately 99%. The Ta and Fe roughly form a Gaussian distribution with a peak concentration of 48% at a depth of 3.5 μm. As the sputtering depth increases over 6 μm (i.e., reaching the substrate), only the element Fe can be identified. For the s2 and s3, similar elemental distributions are seen. Figure 10b shows that the s2 coated layer has a maximum Ta concentration of 38.44% and is approximately 2.03 μm in size. As seen in Fig. 10c, the s3 exhibits a thicker coated layer of 2.99 μm and a higher maximum Ta concentration of 86.56%. It may be inferred that when the number of shots rises, the coated layer thickness increases from 2.29 to 2.99 μm. In a different work by Wang et al. [55], coated layers of Fe/Ta NCs had inverted results.
4 Summary and conclusion
In this research, alloying process occurs in such a way that initially thin layers of Ta are deposited on Fe substrates using a PF system under the specific conditions. The effects of the number of shots, the distance between anode tip and samples and, alloy quality (for Fe and Ta with different melting points) have been investigated in this study, in order to test the hypothesis. One of the other objectives of this research is to determine the best conditions for the synthesis of homogenous thin films. PF device play an important role as heat transfer sources to the surface due to their high temperature and energy density. Moreover, high controllability during the necessary time in the occurrence of physical and chemical reactions on the surface is another characteristic of PF. Then, the prepared samples are subjected to 0, 1, 5 and 10 shots by the PF device with an energy of 18 keV at the different distances from anode tip.
According to the results obtained from XRD analysis, it can be seen that the number of shots and changes the distance between anode tip and samples, impacts on the lattice parameters and possible interfacial planes, as well as the atomic arrangement, ratio of Fe and Ta within the NCs and fabricating Fe2Ta hexagonal and FeTa rhombohedral structures. The phase structure of Fe2Ta hexagonal and FeTa rhombohedral indicates that the alloying process took place with different percentage due to the variety intensity of the peak associated with Fe. In order to study the structure and morphological characteristics of the samples, we have used SEM. The color of each sample alters from light to dark gray, based on the total amount of O2 present in each sample. Moreover, spherical morphology is the fundamental shape in samples with high O2 percentage. EDS analysis shoes that because of the low ratio of Fe NPs in s1, s3, and s4, the alloying process has happened with little probability. On the other hand, Fe activity's in light of low percentage/solubility, lead to the formation of structures with impurities. It has been seen that the δ phase Ta is most soluble in Fe; then, homogeneous alloys are only found in s2. Elemental depth profile also suggests the formation of a structure with almost the same relative distribution of Fe and Ta NPs in s2.
In conclusion, Fe/Ta NCs represent a fascinating convergence of two distinct materials, offering a unique combination of properties that can be tailored for specific applications. In the end, it can be stated that changes in distance from anode tip and number of shots, can be effective in the alloying process by PF. In order to investigate other possible parameters in the formation of Fe/Ta alloy, in addition to the parameters mentioned in this study, effect of electron beams and shift of sample’s location can also be investigated in the next research.
Data availability
The data that support the findings of this study are included in the articles.
Abbreviations
- NPs:
-
Nanoparticles
- BNPs:
-
Bimetallic nanoparticles
- NCs:
-
Nanocomposites
- Ta NPs:
-
Tantalum nanoparticles
- Fe NPs:
-
Iron nanoparticles
- Fe/Ta NCs:
-
Iron/Tantalum nanocomposite
- TaN:
-
Tantalum nitride
- Ta2O:
-
Tantalum dioxide
- FeN:
-
Iron(ɪɪɪ) nitride
- Fe2N:
-
Iron nitride
- PF:
-
Plasma focus
- POM:
-
Polarized optical microscopy
- FWHM:
-
Full width at half maximum
- EN:
-
Electronegativity
- UV:
-
Ultraviolet region
- VIS:
-
Visible region
- XRD:
-
X-ray diffraction pattern
- EDS:
-
Energy dispersive X-ray spectroscopy
- SEM:
-
Scanning electron microscopy
References
Arabi S, Sohrabi MR. Removal of methylene blue, a basic dye, from aqueous solutions using nano-zerovalent iron. Water Sci Technol. 2014;70:1.
Raya PZ, Shipley HJ. Inorganic nano-adsorbents for the removal of heavy metals and arsenic: a review. RSC Adv. 2015;5:29885.
Reddy AVB, Yusop Z, Jaafar J, et al. Recent progress on Fe-based nanoparticles: synthesis, properties, characterization and environmental applications. J Environ Chem Eng. 2016;4:3537–53.
Xu S, Kulathuvayal AS, Xiong L, et al. Effects of ferromagnetism in ab initio calculations of basic structural parameters of Fe-A (A = Mo, Nb, Ta, V, or W) random binary alloys. Eur Phys J B. 2022;95:167.
Zhang LL, Duan S, Yang XL, et al. Insight into cobalt-doping in Li2FeSiO4 cathode material for lithium-ion battery. J Power Sour. 2015;274:194–202.
Shaji S, Mucha NR, Fialkova S, et al. Morphological data on soft ferromagnetic Fe90Ta10 thin film. Data Brief. 2019;27:104714.
Okamoto H. Fe-Ta (Iron-Tantalum). JPEDAV. 2013;34:165–6.
Lei C, Sun Y, Tsang DCW, et al. Environmental transformations and ecological effects of iron-based Nanoparticles. Environ Pollut. 2017;232:1–21.
Su B, Lin J, Owens G, et al. Impact of green synthesized iron oxide nanoparticles on the distribution and transformation of As species in contaminated soil. Environ Pollut. 2020;258:113668.
Al-Khazali ZKM, Alghanmi HA. Environmental toxicity of nano iron oxides (Fe2O3 NPs) on algal growth Klisinema persicum and cellular DNA damage using comet assay. Egypt J Aquatic Biolo Fish. 2023;27(1):431–53.
Guo C, Weber RJM, Buckley A, et al. Environmentally relevant iron oxide nanoparticles produce limited acute pulmonary effects in rats at realistic exposure levels. Int J Mol Sci. 2021;22:556.
Athirah AHN, Chin AB, Hoong WY, et al. Synthesis and characterization of γ-Fe2O3 NPs on silicon substrate for power device application. Mater Res Express. 2018;5:065020.
Roy SD, Das KC, Dhar SS. Conventional to green synthesis of magnetic iron oxide nanoparticles; its application as catalyst, photocatalyst and toxicity: a short review. Inorg Chem Commun. 2021;134:109050.
Huang-Mu L, Devanesan S, Farhat K, et al. Improving the efficiency of metal ions doped Fe2O3 nanoparticles: photocatalyst for removal of organic dye from aqueous media. Chemosphere. 2023;337:139229.
Koshevaya E, Nazarovskaia D, Simakov M, et al. Surfactant-free tantalum oxide nanoparticles: synthesis, colloid properties, and application as a contrast agent for computed tomography. J Mater Chem B. 2020;8(36):8337–45.
Chakravarty S, Hix JML, Wieweor KA, et al. Tantalum oxide nanoparticles as versatile contrast agents for X-ray computed tomography. Nanoscale. 2020;12(14):7720–34.
Sarabadani Tafreshi S, Taghizade N, Sharifian M, et al. A density functional theory study of CO2 hydrogenation on carbon-terminated TaC (111) surface. React Kinet Mech Catal. 2023;136:1945–63.
Savvotin I, Berdonosova E, Korol A, et al. Thermochemical analysis of hydrogenation of Pd-containing composite based on TiZrVNbTa high-entropy alloy. Appl Sci. 2023;13:9052.
Bannenberg LJ, Blom L, Sakaki K, et al. Completely elastic deformation of hydrogenated Ta thin films. ACS Mater Lett. 2023;5:962–9.
Zitter H, Plenk H Jr. The electrochemical behavior of metallic implant materials as an indicator of their biocompatibility. J Biomed Mater Res. 1987;21:881–96.
Martelo LM, Sousa PMS, Silva MAD, et al. A critical updated review of all stages of the tantalum recycling chain from waste of tantalum capacitors. Chem Eng J. 2023;472:144917.
Agrawal M, Singh R, Singh KK. Techno-economic analysis of the sequential recovery of valuable metals from waste tantalum capacitors in Indian context. Process Saf Environ Prot. 2023;179:180–8.
Zhao J, Xu Y, Hou W, et al. An ultra-thin, ultra-high capacitance density tantalum capacitor for 3D packaging. Adv Mater Technol. 2023;8:2201967.
Liguori A, Gino ME, Panzavolta S, et al. Tantalum nanoparticles enhance the osteoinductivity of multiscale composites based on poly(lactide-co-glycolide) electrospun fibers embedded in a gelatin hydrogel. Mater Today Chem. 2022;24:100804.
Zhao M, Ji C, Dai H, et al. Mussel-inspired tantalum nanocomposite hydrogels for in situ oral cancer treatment. ACS Appl Mater Interfaces. 2023;15:4.
Han Q, Wang C, Chen H, et al. Porous tantalum and titanium in orthopedics: a review. ACS Biomater Sci Eng. 2019;5:11.
Zhang L, Haddouti EM, Beckert H, et al. Investigation of cytotoxicity, oxidative stress, and inflammatory responses of tantalum nanoparticles in THP1-derived macrophages. Mediat Inflamm. 2020;3:3824593.
Bakhsheshi-Rad HR, Ismail AF, Aziz M, et al. Antibacterial activity and corrosion resistance of Ta2O5 thin film and electrospun PCL/MgO-Ag nanofiber coatings on biodegradable Mg alloy implants. Ceram Int. 2019;45:11883–92.
Ji C, Zhao M, Wang C, et al. Biocompatible tantalum nanoparticles as radiosensitizers for enhancing therapy efficacy in primary tumor and metastatic sentinel lymph nodes. ACS Nano. 2022;16:6.
Li H, Yao Z, Zhang J, et al. The progress on physicochemical properties and biocompatibility of tantalum-based metal bone implants. SN Appl Sci. 2020;2:671.
Jiang Y, Li T, Yang J, et al. Sustained intra-articular reactive oxygen species scavenging and alleviation of osteoarthritis by biocompatible amino-modified tantalum nanoparticles. Front Bioeng Biotechnol. 2023;11:1118850.
Arapan S, Nieves P, Herper HC, et al. Computational screening of Fe-Ta hard magnetic phases. Phys Rev B. 2019;101:014426.
Jafri Y, Sharma G, Gupta A, et al. Thickness dependent magnetic properties of ferromagnetic films (Fe, Co) interfaced with Ta. Thin Solid Films. 2021;719:138490.
Vedel D, Storozhenko M, Mazur P, et al. Wetting and interfacial behavior of Fe Co, Ni on (Ti, Zr, Hf, Nb, Ta)C high entropy ceramics. Open Ceram. 2023;15:100393.
Yuan Z, Kobayashi S. Determination of phase equilibria among -Fe, -Fe and Fe2M phases in Fe-Cr-M (M: Hf, Ta) ternary systems. Metals. 2022;12:102.
Zhang K, Cui C, Deng L, et al. In-situ observations of solidification process of Fe–Al–Ta eutectic alloy. J Phys Chem Solids. 2023;172:111067.
Yang B, Guo C, Li C, et al. Experimental investigation and thermodynamic modelling of the Fe-Ni-Ta system. J Phase Equilib Diffus. 2020;41:500–21.
Grzywa M, Röß-Ohlenroth R, Muschielok C, et al. Cooperative large-hysteresis spin-crossover transition in the iron(II) triazolate [Fe(ta)2] metal−organic framework. Inorg Chem. 2020;59:15.
Zhou X, Chen T, Ma T, et al. CuS@TA-Fe nanoparticle-doped multifunctional hydrogel with peroxide-like properties and photothermal properties for synergistic antimicrobial repair of infected wounds. Adv Healthc Mater. 2023;12:2301206.
Asakuray K, Yamashita O, Yamada T, et al. Effects of TaandNbon microstructures activation ferritic 9cr-2W-0.2V steel and mechanical properties of low tor fusion reactor. ISIJ Int. 1990;30:937–46.
Chen L, Li Y, Zhao Z, et al. First-principles calculation of the adhesion work, fracture toughness and tensile behavior of the Fe/MCs (M = Nb and Ta) interfaces by two different optimization methods. Chem Phys. 2021;547:111193.
Cen D, Wang B, Chu R, et al. Design of (Hf, Ta)Fe2/Fe composite with zero thermal expansion covering room temperature. Scripta Mater. 2020;186:331–5.
Subramania T, Navrotsky A. Thermochemistry of cation disordered Li ion battery cathode materials, Li1+x M′x M′′1−2xO2 (M′ ¼ Nb and Ta, M′′ Mn and Fe). RSC Adv. 2020;10:6540.
Akter J, Hanif MdA, Islam MdA, et al. Visible-light-active novel α-Fe2O3/Ta3N5 photocatalyst designed by band-edge tuning and interfacial charge transfer for effective treatment of hazardous pollutants. J Environ Chem Eng. 2021;9:106831.
Ahmed I, Burman V, Biswas R, et al. Bifunctional electrochemical OER and HER activity of Ta2O5 nanoparticles over Fe2O3 nanoparticles. New J Chem. 2023;47:17284–92.
Wang WY, Shang SL, Wang Y, et al. Lattice distortion induced anomalous ferromagnetism and electronic structure in FCC Fe and Fe-TM (TM = Cr, Ni, Ta and Zr) alloys. Mater Chem Phys. 2015;162:748–56.
Lee MK, Lee H, Park C, et al. Accelerated biodegradation of iron-based implants via tantalum-implanted surface nanostructures. Bioactive Mater. 2022;9:239–50.
Yang M, Xu J, Ma H, et al. Elucidating the formation mechanism of the vortex at the Ta/Fe explosively welded interface using microstructure characterizations and numerical simulations. Metall Mater Trans A. 2022;53:364.
Song N, Yang L, Liu P. Preparation of Ta(Ph)-Fe/AP composite microspheres by ultrasonic spray drying and characterization of their catalytic properties. Propellants Explos Pyrotech. 2020;45:368.
Takayama T, Myeong YW, Ishizawa TN. Effect of magnetic transition on the solubility of alloying elements. Trans Jpn Inst Metals. 1981;22:315–25.
Jones RH, Zackay VF, Parker ER. Laves phase precipitation in Fe-Ta alloys. Metall Trans. 1971;3:1972–2835.
Mato S, Alcala G, Thompson GE, et al. Anodic oxidation of Ta/Fe alloys. Corros Sci. 2003;45:2881–92.
Naoe M, Kodaira M, Hoshi Y, et al. Properties of (Fe, Co)-(Ta, W) amorphous alloy films deposition RF sputtering. IEEE Trans Magn. 1981;17:6.
Grabowski KS, Correll FD, Vozzo FR. Sputtering and migration during Ta implantation of Fe. Nucl Instrum Methods Phys Res B. 1985;7:798.
Wang H, Zheng Y, Jiang C, et al. In vitro corrosion behavior and cytocompatibility of pure Fe implanted with Ta. Surf Coat Technol. 2017;320:201–5.
Andrianov VA, Bedelbekova KA, Ozernoy AN, et al. Mössbauer studies of 57Fe implantation in metal Ta and Mo. Nucl Inst Methods Phys Res B. 2020;475:71–6.
Witusiewicz VT, Bondar AA, Hecht U, et al. Experimental study and thermodynamic re-assessment of the binary FeTa system. Intermetallics. 2011;19:1059–75.
Taxak M, Kumar S, Sheelvantra S, et al. Effect of iron on the solubility of hydrogen in tantalum. J Mater Sci. 2014;49:8471–7.
Morita ZI, Iwanaga Y, Hamada S, et al. Solubility of nitrogen and equilibrium of Ta-nitride forming reaction in liquid Fe-Ta alloys. Tetsu-to-Hagané. 1973;59:214.
Clérac R, Fenske D, Issac I, et al. Syntheses and structures of heterometallic Fe-Ta chalcogenido clusters. J Cluster Sci. 2004;15:2.
Decker G, Wienecke R. Plasma focus devices. Physica. 1976;82C:155–64.
Rawat RS. Dense plasma focus - from alternative fusion source to versatile high energy density plasma source for plasma nanotechnology. J Phys Conf Ser. 2015;591:012021.
Niranjan R, Srivastava R, Joycee J, et al. Development of a portable pulsed fast⩾106 neutron generator based on a flexible miniature plasma focus tube. Plasma Phys Control Fusion. 2023;65:075010.
Chernyshova M, Karpinski L, Scholz M et al. process of thin film deposition using dense plasma focus. 34th EPS conference on plasma phys. ECA. 31f. 2007; 2.071.
Mazières V, Pascaud R, Liard L et al., Time reversalplasmasasaversatilespace-time patterning depositionmethod. 2021 IEEE 16th Nanotechnology Materials and Devices Conference (NMDC). 2022.
Khan MZ, Ling YS, Yaqoob I, et al. Low-energy plasma focus device as an electron beam source. Sci World J. 2014;2014:240729.
Morozova EV, Maslyaeva SA, Demina AS, et al. Deposition of multicomponent filmlike coating on metal substrate using plasma focus device. Inorg Mater Appl Res. 2016;7:5.
Akel M, AL-Hawat S, Ahmad M, et al. Features of pinch plasma, electron, and ion beams that originated in the AECS PF-1 plasma focus device. Plasma. 2022;5:184–95.
Lerner EJ, Hassan SM, Karamitsos-Zivkovic I, et al. Focus fusion: overview of progress towards p-B11 fusion with the dense plasma focus. J Fusion Energy. 2023;42:7.
Hosseinnejad MT, Ettehadi-Abari M, Panahi N. Structural, morphological and mechanical characterisation of molybdenum nitride thin films deposited by a plasma focus device. J Chem Res. 2017;41:699–704.
Dulatov AK, Krapiva PS, Lemeshko BD. The balanced filter method for studying the hard X-ray spectrum of the plasma focus device. Instrum Exp Tech. 2015;58:774.
Gribkov VA, Borovitskaya IV, Demina EV, et al. Application of dense plasma focus devices and lasers in the radiation material sciences for the goals of inertial fusion beyond ignition. Extremes. 2020;5:045403.
Hosseinzadeh A, Nazmabadi M, Vosoughi N. Deposition of metallic molybdenum thin films on 304L steel substrate by SUT-PF. Surf Coat Technol. 2017;309:1052–61.
Shirazi M, Ghasemloo M, Etaati GR, et al. Plasma focus method for growth of molybdenum nitride thin films: synthesis and thin film characterization. J Alloy Compd. 2017;727:978–85.
Siddiqui J, Hussain T, Ahmad R, et al. Effect of deposition parameters on structural and mechanical properties of niobium nitride synthesized by plasma focus device. Chin Phys B. 2015;24:6.
Siddiqui J, Hussain T, Ahmad R, et al. On the structural, morphological and electrical properties of tantalum oxy nitride thin films by varying oxygen percentage in reactive gases plasma. Chin J Phys. 2017;55:1412–22.
Rawat RS, Zhang T, Thomas Gan KS, et al. Nano-structured Fe thin film deposition using plasma focus device. Appl Surf Sci. 2006;253:1611–5.
Kotb M, Saudy AH, Hassaballa S, et al. Nanostructure iron-silicon thin film deposition using plasma focus device. Mod Trends Phys Res. 2013. https://doi.org/10.1142/9789814504898_0012.
Javadi S, Ghoranneviss M, Hojabri A, et al. Deposition of chromium thin films on stainless steel-304 substrates using a low energy plasma focus device. J Fusion Energ. 2011;31:242–8.
Javadi S, Ouyang B, Zhang Z, et al. Effects of fusion relevant transient energetic radiation, plasma and thermal load on PLANSEE double forged tungsten samples in a low-energy plasma focus device. Appl Surf Sci. 2018;443:311–20.
Ye YF, Liu CT, Yang Y. A geometric model for intrinsic residual strain and phase stability in high entropy alloys. Acta Mater. 2015;94:152–61.
Ye YF, Liu XD, Wang S, et al. The general effect of atomic size misfit on glass formation in conventional and high-entropy alloys. Intermetallics. 2016;78:30–41.
Olsson P, Klaver TPC, Domain C. Ab initio study of solute transition-metal interactions with point defects in bcc Fe. Phys Rev B. 2010;81:054102.
Yan JX, Tian ZX, Xiao W, et al. Interaction of He with Cu, V, and Ta in bcc Fe: a first-principles study. J Appl Phys. 2011;110:013508.
He Q, Yang Y. On lattice distortion in high entropy alloys. Front Mater. 2018;5:42.
Li G, Sui X, Qin X, et al. Structural transformation between bcc and fcc in Fe-Ni nanoparticle during heating process. Phys Lett A. 2016;380:42.
Grimvall G, Magyari-Kope B, Ozolin V, et al. Lattice instabilities in metallic elements. Rev Mod Phys. 2012;84:945.
Tsai MH, Yeh JW. High-entropy alloys: a critical review. Mater Res Lett. 2014;2:3.
Yeh JW. Physical metallurgy of high-entropy alloys. JOM. 2015;67:10.
Yeh JW. Alloy design strategies and future trends in high-entropy alloys. JOM. 2013;65:1759–71.
Shiria S, Odeshia A, Chenb N, et al. FCC tantalum thin films deposited by magnetron sputtering. Surf Coat Technol. 2019;358:942–6.
Chopra KL, Randlett MR, Duff RH. Face-centred cubic modification in sputtered films of tantalum, molybdenum, tungsten, rhenium, hafnium and zirconium. Phil Mag. 2006;16:140.
Janish MT, Mook WM, Carter CB. Nucleation of fcc Ta when heating thin films. Scripta Mater. 2015;96:21–4.
Janish MT, Kotula PG, Boyce BL, et al. Observations of fcc and hcp tantalum. J Mater Sci. 2015;50:3706–15.
Marcus RB, Quigley S. Formation of F.C.C., B.C.C. and β-tantanum films by evaporation. Thin Solid Films. 1968;2:467–77.
Denbigh PN, Marcus RB. Structure of very thin tantalum and molybdenum films. J Appl Phys. 1966;37:4325.
Kai K, Nakamichi T, Yamamoto M. magnetic behavior of the laves phase compound in Fe-Ta binary system. J Phys Soc Jpn. 1970;29:1094.
Scheunert G, Heinonen O, Hardeman R, et al. A review of high magnetic moment thin films for microscale and nanotechnology applications. Appl Phys Rev. 2016;3:011301.
Kumar N, Alam F, Dutta V, et al. Deposition of Ag and Au-Ag alloy nanoparticle films by spray pyrolysis technique with tuned plasmonic properties. J Alloy Compd. 2014;585:312–7.
Mohebi E, AdibAmini S, Sari AH, et al. Formation of Agshell/Aucore bimetallic nanoparticles by pulsed laser ablation method: effect of colloidal/solution concentration. Plasmonics. 2023;4:3765.
Zhao T, Li Y, Xiang Y, et al. Surface characteristics, nano-indentation and corrosion behavior of Nb implanted NiTi alloy. Surf Coat Technol. 2011;205:4404–10.
Nguyen TL, Blanquet A, Staiger MP, et al. On the role of surface roughness in the corrosion of pure magnesium in vitro. J Biomed Mater Res Part B. 2012;100B:5.
Östhagen K, Kofstad P. The reaction between tantalum and nitrogen at 800–1300°c. J Less Common Met. 1963;5:1.
Niu B, Liu B, Xu Z, et al. Controllable synthesis of high-efficient TaOxNy and Ta3N5 photocatalysts through vacuum nitriding using melamine as a nitrogen source. J Alloy Compd. 2019;809:151846.
Amikura K, Kimura T, Hamada M, et al. Copper oxide particles produced by laser ablation in water. Appl Surf Sci. 2008;254:21.
Chung CK, Chen TS, Chang NW. Effect of reactive gases flow ratios on the microstructure and electrical resistivity of Ta–N–O thin films by reactive co-sputtering. Thin Solid Films. 2011;519:15.
Kim I, Park G, Na JJ. Experimental study of surface roughness effect on oxygen catalytic recombination. Int J Heat Mass Transf. 2019;138:916–22.
Gaviria-Martinez A, Castro-Ramirez L, Ladera-Castañeda M, et al. Surface roughness and oxygen inhibited layer control in bulk-fill and conventional nanohybrid resin composites with and without polishing: in vitro study. BMC Oral Health. 2022;22:258.
Swartzendruber LJ, Paul E. The Fe−Ta (Iron-Tantalum) system. Bull Alloy Phase Diagr. 1986;7:254–9.
Chen X, Frisch HL, Kaloyeros AE, et al. Low temperature plasma-promoted chemical vapor deposition of tantalum from tantalum pentabromide for copper metallization. J Vac Sci Technol B. 1998;16:2887.
Acknowledgements
The authors would like to thank the Science and Research Branch of Islamic Azad University (SRBIAU) and above all the PF laboratory at the Plasma Physics Research Center (PPRC) for providing all the necessary facilities. We also recognize the Ms. Hanie Shafiei for her meaningful assistance.
Funding
The Authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The authors confirm their contribution to this document as follows: A. H. Sari devised the project, A. H. Sari presented the main conceptual ideas, A. H. Sari encouraged A. EbneRasool to investigate a specific aspect of Fe/Ta NCs and supervised the findings of this work, A. H. Sari designed the experimental framework, A. H. Sari contributed to PF preparation, A. H. Sari verified the analytical methods, A. EbneRasool worked out all of the experiment, S. AdibAmini wrote the manuscript with support from A. H. Sari, all authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
There are no human and animal subjects in this article and informed consent is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
EbneRasool, A., AdibAmini, S. & Sari, A.H. Investigation of effective parameters on Fe/Ta thin films by plasma focus device: number of shots and distance from tip anode. Discov Mater 4, 34 (2024). https://doi.org/10.1007/s43939-024-00102-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43939-024-00102-9