Abstract
Biohydrogen production could be generated from organic wastes - food and beverage processing wastewater, restaurant food waste, and raw starch waste. Fermentative hydrogen production from food and beverage processing wastewater by sewage microflora was optimized in terms of pH (4.5 to 7.0), mesophilic condition (35°C ±2°C), and thermophilic condition (50°C ±2°C). Low initial pH (6.5) and mesophilic condition favored hydrogen production (0.28 L/L) indicating that such parameters along with the wastewater characteristics were crucial to dark-fermentative hydrogen production. Pretreatment methods (methanogenic inhibitor, sterilization, sonication, and acidification) on restaurant food waste and raw starch waste to enhance biohydrogen production were also investigated in this study. Maximum hydrogen yields of 3.48 ml H2/g COD and 2.18 ml H2/g COD were observed in sterilization of pretreated restaurant food and raw starch wastes, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The dependence of global energy requirements on fossil fuels may eventually lead to their depletion. It is imperative to seek for an alternative energy resource as the possible successor. Hydrogen (H2) is a clean fuel with no carbon dioxide (CO2) emissions, in case sustainably produced, could be the ideal fuel of the future to replace the fossil fuels [1]. Biological hydrogen production (biohydrogen production) is less energy intensive and environmental friendly. Dark fermentation is a biohydrogen production process that applies anaerobic bacteria to decompose the organic materials. Moreover, organic waste such as wastewater from municipalities or industries, agricultural waste, dung, food waste, etc. is used as substrate for biohydrogen production and can reduce a quantity of waste to environment [2]. H2 is produced by bacteria through bio-process under ambient temperature and pH regimes, and the yield can be enhanced by the manipulation of other environmental conditions. The initial pH and temperature are the important factors that influence H2 production by bacteria. However, pretreatment methods - methanogenic inhibitor, sterilization, sonication, acidification, freezing, and thawing of organic waste - are one strategy to promote the hydrolysis and disintegration of organic compounds and augment the fermentation to enhance H2 generation [3]. Although, the several studies demonstrated the role of those conditions on fermentative hydrogen production, there still exist different values with regard to optimum of initial pH, temperature, and pretreatment method [3, 4].
Therefore, the main objectives of this study were to look for the impacts of fermentative hydrogen production from food and beverage processing wastewater in terms of initial pH (4.5 to 7.0), mesophilic (35°C ±2°C), and thermophilic (50°C ±2°C) conditions and to investigate the impacts of pretreatment method on organic wastes for enhancing the biohydrogen production.
Methods
Substrates and seed sludge
Wastewater was collected from six industrial factories in Thailand by a water sampler (grab sampling method). Food and beverage processing wastewater was used as substrate for fermentative hydrogen production. Juice processing wastewater was obtained from coconut milk industry (Ci) and juice industry (Ji). Food processing wastewater was obtained from the starch and rice noodle industry (Sti) and snack industry (Sni). Winery and brewery processing wastewater was from winery industry (Wi) and brewery industry (Bi), respectively. Restaurant food waste collected from the central cafeteria at Mahidol University (Salaya campus, Thailand) and raw starch waste collected from a sludge thickness tank of a noodle processing plant were use as substrates. Both waste types were grinded using a blender and were mixed in a container, as well as were sieved with a 5-mm screen. Then, they were mixed with distilled water with a volume ratio of waste to distilled water of 3:1. The physical and chemical characteristics of organic wastes - pH, Total Suspended Solid (TSS), Total Dissolved Solid (TDS), Volatile Suspended Solid (VSS), Chemical Oxygen Demand (COD), Biochemical Oxygen Demand (BOD), Total Kjeldahl Nitrogen (TKN), and Fat oil and Grease (FOG) - were analyzed [5]. Table 1 shows the physical and chemical characteristics of organic wastes.
Anaerobic sludge was taken from the Bio-fertilizer plant, Nonthaburi province, Thailand. Seed sludge was screened with sieve (2.00 mm) to eliminate the large particulate matter. Seed sludge was heated (90°C, 10 min) to eliminate the hydrogen-consuming bacteria and facilitated growth of spore-former bacteria [6].
Experimental procedures
The nutrient solution for bacterial growth contained 10 g C6H12O6 (D-Glucose), 5,240 mg NH4HCO3, 6,720 mg NaHCO3, 125 mg K2HPO4, 100 mg MgCl2 · H2O, 25 mg FeSO4 · 7H2O, 15 mg MnSO4 · 6H2O, 4.37 mg CuSO4 · 5H2O, 0.125 mg CoCl2 · 5H2O in 1,000 ml distilled water [4]. Regarding food and beverage processing wastewater as the substrate, a batch reactor of 500-ml serum bottle was added with 20 ml seed sludge, 50 ml nutrient solution, and 250 ml wastewater. On the other hand, batch reactor of 250-ml serum bottle consisted of 150 ml the substrate and 10 ml seed sludge as well as 40 ml of nutrient solution was set up for biohydrogen production from restaurant food or raw starch waste. The mixed liquor was purged with N2 for 1 min to ensure anaerobic condition prior to each run and clogged with silicone rubber stoppers to avoid the gas leakage from the bottles.
The experiment was conducted to compare the biohydrogen production at uncontrolled pH at room temperature in case of food and beverage processing wastewater as the substrate. Subsequently, biohydrogen production was monitored at initial pH of 4.5, 5.0, 5.5, 6.0, 6.5, or 7.0 end under mesophilic (35°C ±2°C) and thermophilic (50°C ±2°C) conditions. In order to find out the proper pretreatment method of waste for enhancing biohydrogen production, restaurant food and raw starch wastes using as substrates were pretreated by four pretreatment methods of methanogenic inhibitor by 1 M Sodium 2-bromoethanesulfonate (BESA: C2H4BrO3SNa, 0.2/l), sterilization by autoclave at 121°C for 20 min, sonication by an ultrasonic bath for 20 min and acidification by HClO4 at initial pH 3. All bottle reactors were placed in a shaking water bath with speed 120 ± 1 (rpm) at different temperature setup. Each batch experiment was conducted in triplicate.
Analytical methods
The volume of biogas production was measured by a plunger displacement of gas-tight syringes [7]. The hydrogen concentration was determined by gas chromatography (Varian STAR 3400, Palo Alto, CA, USA) which equipped with a thermal conductivity detector (TCD) and packed stainless-steel column (Alltech Molesieve 5A 80/100 10′ × 1/8″, Nicholasville, KY, USA). Argon was used as the carrier gas for analysis [8]. The temperatures of injector, detector, and column were maintained at 80°C, 90°C, and 50°C, respectively.
Hydrogen gas production was calculated from headspace measurements of gas composition and total volume of biogas produced at each time interval by using a following equation (Equation 1) [9]:
where VH,i and VH,i-1 are cumulative hydrogen gas volumes at the current (i) and previous (i - 1) time intervals, VG,i and VG,i-1 are the total gas volumes in the current and previous time interval, CH,i and CH,i-1 are the fraction of hydrogen gas in the headspace of the bottle measured using gas chromatography in the current and previous intervals, and VH is total volume of headspace in the reactor.
A modified Gompertz equation (Equation 2) was used to calculate cumulative hydrogen data depict.
where H (ml) is the cumulative hydrogen production, P (ml) is the hydrogen production, Rm (ml/h) is the maximum hydrogen production rate, λ (h) is the lag phase time, t (h) is the incubation time, and e = 2.71828.
Results and discussion
Feasibility of biohydrogen production from organic wastes
The cumulative hydrogen production from all kinds of wastewater showed that the highest production was approximately 0.53 l H2/l from snack wastewater, 0.52 l H2/l from coconut milk wastewater, 0.31 l H2/l from juice wastewater, and 0.08 l H2/1 from starch and rice noodle wastewater and the least (approximately 0.0003 l H2/l) from winery wastewater. However, H2 production from brewery wastewater was not observed (Figure 1). Among the six kinds of wastewater, coconut milk wastewater was best suited for continuous biohydrogen production. However, snack wastewater supported maximum cumulative hydrogen production, but it could not be used as the representative substrate because of the discontinuous biohydrogen production. As the snack wastewater had a much higher suspended solid than others, these solid remains might be non-degradable. Cumulative hydrogen production in snack wastewater and coconut milk wastewater is higher than that of other wastewaters. It is associated with the high-carbohydrate wastewater and is significantly related to the much of COD and BOD values in wastewater [10]. Presence of carbohydrate content as carbon source has positive effect on the hydrogen production in the metabolic reactions involving molecular hydrogen generation [11]. Thus, carbohydrate rich food and beverage processing wastewater may be further processed to convert the carbohydrate content to organic acids and then to hydrogen gas by dark fermentation [12]. The results are similar to other researches [10] as overall H2 conversions were 0.7 to 0.9 l H2/l for the apple wastewater, 0.1 l H2/l for confectioner-A, 0.4 to 2.0 l H2/l for confectioner B, and 2.1 to 2.8 l H2/l for the potato wastewater. Moreover, cumulative hydrogen production from wastes increased in early experiments and then slowly decreased until out of biogas production in the batch reactor. Consequently, hydrogen yield of restaurant food waste (2.82 ml H2/g COD) was higher than raw starch waste (2.02 ml H2/g COD). These results were similar to other researches that hydrogen yield could be produced by food waste (121.6 ml H2/g carbohydrate-COD), 250 ml H2/g VS and 62.6 ml H2/g VS and industrial wasted sludge (32.6 ml H2/g carbohydrate-COD) [13, 14].
Hence, it could imply that H2 production depended on the different organic components of the wastes. Organic wastes - food and beverage processing wastewater, restaurant food, and raw starch wastes - have high COD and BOD values and are therefore suitable for fermentative hydrogen production [10].
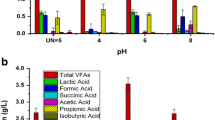
Impacts of initial pH and temperature on biohydrogen production from food and beverage processing wastewater
Biohydrogen production from coconut milk wastewater as the substrate at the initial pH from 4.5, 5.0, 5.5, 6.0, 6.5, and 7.0 as the anaerobic dark-fermentation at room temperature revealed that lag phase was about 8 h. The lag phase was greatly affected by the initial pH. The results are different from those of others as the lag time was 74 h (at initial pH 5.0) and 41 h (at initial pH 6.0), respectively, and the lag time was longer than 19 to 28 h for initial pH in the range of 7.0 to 10.0 [15]. At the initial pH ranges of 5.5, 6.0, 6.5, 7.0, biohydrogen production was quite high only initially, and it gradually declined to the steady state of biogas production. Regarding cumulative hydrogen production, the hydrogen yield was quite low at initial pH 4.5, 5.0, 5.5, and 6.0, which produced about 0.02 l H2/l, 0.01 l H2/l, 0.08 l H2/l, and 0.15 l H2/l, respectively. However, the maximum cumulative hydrogen production was approximately 0.28 l H2/l at the initial pH of 6.5 but was slightly decreased at initial pH 7.0 (Figure 2). Consequently, the initial pH 6.5 is the best pH value for hydrogen production because fermentative conversion of substrate to hydrogen can be increased by maintaining operating pH in and around six compared to neutral pH [1]. Moreover, the initial cultivation pHs were adjusted to 6.5 to 7.0, the pH would further decrease to a suitable value (around 6.0) for hydrogen production [16]. Nevertheless, pH control could stimulate microorganisms to achieve maximum hydrogen production ability because the activity of hydrogenase was inhibited by low or high pH in fermentation [1]. Results are consistent with those of other cites, regarding biohydrogen from starch in wastewater through anaerobic fermentation at the optimal pH of 6.5 and starch concentration at 5 g/l (37°C) with a hydrogen yield reaching 186 ml H2/g starch [17], while achieved the conversion of organics in wastewater to H2 in batch experiments, the highest rate of H2 production (74.7 ml H2/l.h) at pH 5.5 the COD of 7.5 g/l corresponding to the conversion efficiency of 38.9 ml H2/(g COD/l) [18]. Furthermore, biohydrogen production from coconut milk wastewater under mesophilic (35°C ±2°C) and thermophilic (50°C ±2°C) conditions (initial pH 6.5) showed a lag phase of about 8 h. These results are not similar to others wherein the lag was relatively longer lasting about 11 to 13 h at initial pH 6.0 and 55°C [19], while some researchers reported biohydrogen production at mesophilic condition to start after a lag of approximately 9 h [20]. Cumulative hydrogen production from coconut milk wastewater was about 0.28 l H2/l and 0.16 l H2/l at mesophilic and thermophilic conditions, respectively (Figure 3). It revealed that temperature can affect the activity of hydrogen-producing bacteria by influencing the activity of some essential enzymes such as hydrogenases for fermentative hydrogen production. In an appropriate range, increasing temperature could increase the ability of hydrogen-producing bacteria to produce hydrogen during fermentative hydrogen production, but temperature at much higher levels could decrease it with increasing levels [4]. However, operation at high temperatures is not favorable for energy recovery. Therefore, for a balance between hydrogen production and energy recovery, it seems to be reasonable to biological hydrogen-producing reactors in the mesophilic range [21]. These results are different from others reported that hydrogen production for thermophilic condition at 55°C was higher (134 ml/day) compared to mesophilic condition (67 ml/day) [22]. Another report showed the effect of temperature and pH on fermentative hydrogen production from cattle wastewater by sewage sludge with the maximum hydrogen yield at pH 5.5 and at 45°C with the peak of hydrogen production (368 ml) or yield (319 ml H2/g COD) [23]. It was also observed that the initial pH of 6.5 at temperatures of 35°C ±2°C and 50°C ±2°C dropped finally to pH 4.5 during 7 days of fermentation. The final pH of fermentation was comparable to that (about 4.7) reported by Zhang and Shen [24]. Some possible reasons for this may be that hydrogen production occurs in acidification stage of metabolic process and the hydrogen-producing bacteria has a high conversion rate of carbohydrate to hydrogen, then the high concentrations of metabolites may cause the pH to drop to such low level [4]. Moreover, acid accumulation in the system causes a sharp drop of the pH, thus inhibiting biohydrogen production. The lowering by pH suggests inactivation of hydrogen producers. The bacteria involved could not sustain its metabolic activity at pH values less than 5.0 and complete inhibition was reported in the pH range of 4.0 to 5.0 [11].
Hence, it may be concluded that the initial pH and temperature affected the biohydrogen production from coconut milk wastewater. The optimum of initial pH of 6.5 and mesophilic condition was suggested for biohydrogen production. It is also suggested that the pH control induced the system for hydrogen production more effectively than the uncontrolled sets.
Impacts of pretreatment method on restaurant food and raw starch wastes for biohydrogen production
Figure 4 illustrates the impacts of pretreatment method on fermentative hydrogen production by utilizing restaurant food and raw starch wastes. In case of methanogenic inhibitor (BESA), the cumulative hydrogen productions (hydrogen yields) were 10.50 ml H2 (0.57 ml H2/g COD) and 5.28 ml H2 (0.48 ml H2/g COD) for food and starch wastes, respectively. In sterilization, there were the cumulative hydrogen production (hydrogen yield) of 65.50 ml H2 (3.48 ml H2/g COD) for food waste and 30.28 ml H2 (2.18 ml H2/g COD) for starch waste, respectively. In sonication, the cumulative hydrogen productions (hydrogen yields) were 38.49 (2.09 ml H2/g COD) and 16.28 ml H2 (1.43 ml H2/g COD) for food and starch wastes, respectively. In acidification, the cumulative hydrogen productions (hydrogen yields) of 55.10 (2.96 ml H2/g COD) and 27.50 ml H2 (2.20 ml H2/g COD) were found using food and starch wastes, respectively. However, the maximum hydrogen production was found in sterilization from restaurant food and raw starch wastes that agreed with previous researches [3, 25]. It might explain to this phenomenon that the substrate after sterilization is nearly complete breakdown from polymeric to monomeric molecules until they release to other nutrients. Consequently, hydrogen-producing bacteria of seed sludge could be easily produced hydrogen gas from waste [26]. On the other hand, biohydrogen production using waste pretreated by sonication and methanogenic inhibitor was much lower than other pretreatment methods. It might imply that both pretreatment methods can also suppress the activity of some hydrogen-producing bacteria in fermentation [27].
Conclusion
In this study, organic wastes - some kinds of food and beverage processing wastewater and restaurant food and raw starch wastes - supported fermentative hydrogen production. The maximum cumulative hydrogen production from coconut milk wastewater was at initial pH 6.5 under mesophilic condition. It is also concluded that characteristics of wastewater, initial pH, and temperature conditions affected the fermentative biohydrogen production. Among pretreatment methods of restaurant food and raw starch wastes, it revealed that sterilization was the best method and could enhance the maximum hydrogen yield from restaurant food waste.
References
Mohan SV, Babu VL, Sarma PN: Effect of various pretreatment methods on anaerobic mixed microflora to enhance biohydrogen production utilizing dairy wastewater as substrate. Bioresour. Technol. 2008, 99: 59–67. 10.1016/j.biortech.2006.12.004
Wang J, Wan W: Factors influencing fermentative hydrogen production: a review. Int. J. Hydrogen Energy 2009, 34: 799–811. 10.1016/j.ijhydene.2008.11.015
Guo L, Li XM, Bo X, Yang Q, Zeng GM, Liao DX, Liu JJ: Impacts of sterilization, microwave and ultrasonication pretreatment on hydrogen producing using waste sludge. Bioresour. Technol. 2008, 99: 3651–3658. 10.1016/j.biortech.2007.07.026
Wang J, Wan W: Effect of temperature on fermentative hydrogen production by mixed cultures. Int. J. Hydrogen Energy 2008, 33(20):5392–5397. 10.1016/j.ijhydene.2008.07.010
APHA: Standard methods for the examination of water and wastewater. 21st edition. Washington, DC: APHA; 2005.
Valdez-Vazquez I, Poggi-Varaldo HM: Hydrogen production by fermentative consortia. Renewable and Sustainable Energy Rev. 2009, 13: 1000–1013. 10.1016/j.rser.2008.03.003
Owen WF, Stuckey DC, Healy JB, Young LY, Mccarty PL: Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13(6):485–492. 10.1016/0043-1354(79)90043-5
Selembo PA, Merrill MD, Logan BE: The use of stainless steel and nickel alloys as low-cost cathodes in microbial electrolysis cells. J. Power Sources 2009, 190(2):271–278. 10.1016/j.jpowsour.2008.12.144
Sreela-or C, Imai T, Plangklang P, Reungsang A: Optimization of key factors affecting hydrogen production from food waste by anaerobic mixed cultures. Int. J. Hydrogen Energy 2011, 36: 14120–14133. 10.1016/j.ijhydene.2011.04.136
Van Ginkel SW, Oh SE, Logan BE: Biohydrogen gas production from food processing and domestic wastewaters. Int. J. Hydrogen Energy 2005, 30(15):1535–1542. 10.1016/j.ijhydene.2004.09.017
Mohan SV, Bhaskar YV, Krishna PM, Rao NC, Babu VL, Sarma PN: Biohydrogen production from chemical wastewater as substrate by selectively enriched anaerobic mixed consortia: influence of fermentation pH and substrate composition. Int. J. Hydrogen Energy 2007, 30: 2286–2295.
Kapdan IK, Kargi F: Bio-hydrogen production from waste materials. Enzyme Microb. Tech. 2006, 38: 569–582. 10.1016/j.enzmictec.2005.09.015
Kim SH, Han SK, Shin HS: Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J Hydrogen Energy. 2004, 24: 1607–1616.
Zhu H, Parker W, Basnar R, Proracki A, Falletta P, Béland M, Seto P: Biohydrogen production by anaerobic co-digestion of municipal food waste and sewage sludges. Int. J. Hydrogen Energy 2008, 33: 3651–3659. 10.1016/j.ijhydene.2008.04.040
Fang HHP, Liu H, Zhang T: Phototropic hydrogen production from acetate and butyrate in wastewater. Int. J. Hydrogen Energy 2005, 30(7):785–793. 10.1016/j.ijhydene.2004.12.010
Kawano T, Wada K, Li YY, Noike T: Effects of substrate concentration and pH on hydrogen fermentation of mixed substrate by microflora. J. Soc. Water. Environ. 2004, 27(7):473–479. 10.2965/jswe.27.473
Wei J, Liu ZT, Zhang X: Biohydrogen production from starch wastewater and application in fuel cell. Int. J. Hydrogen Energy 2010, 35(7):2949–2952. 10.1016/j.ijhydene.2009.05.035
Van Ginkel SW, Sung S, Lay JJ: Biohydrogen production as a function of pH and substrate concentration. Environ. Sci. Technol. 2001, 35(24):4726–4730. 10.1021/es001979r
Zhang T, Liu H, Fang HHP: Biohydrogen production from starch in wastewater under thermophilic conditions. J. Envi. Manage. 2003, 69(2):149–156. 10.1016/S0301-4797(03)00141-5
Baghchehsaraee B, Nakhla G, Karamanev D, Margaritis A: Fermentative hydrogen production by diverse microflora. Int. J. Hydrogen Energy 2009, 35(10):5021–5027.
Mu Y, Zheng XJ, Yu HQ, Zhu RF: Biological hydrogen production by anaerobic sludge at various temperatures. Int. J. Hydrogen Energy 2006, 31: 780–785. 10.1016/j.ijhydene.2005.06.016
Cheong DY, Hansen CL: Feasibility of hydrogen production in thermophilic mixed fermentation by natural anaerobes. Biores. Technol. 2007, 98: 2229–2239. 10.1016/j.biortech.2006.09.039
Tang GL, Huang J, Zhen JS, Tang Q, Yan C, Liu G: Biohydrogen production from cattle wastewater by enriched anaerobic mixed consortia: influence of fermentation temperature and pH. J. Biosci. Bioeng. 2008, 106(1):80–87. 10.1263/jbb.106.80
Zhang Y, Shen J: Effect of temperature and iron concentration on the growth and hydrogen production of mixed bacteria. Int. J. Hydrogen Energy 2006, 31: 441–446. 10.1016/j.ijhydene.2005.05.006
Wang CC, Chang CW, Chu CP, Lee DJ, Chang BV, Liao CS: Producing hydrogen from wastewater sludge by Clostridium bifermentans . J. Biotechnol. 2003, 102: 83–92. 10.1016/S0168-1656(03)00007-5
Cakir A, Ozmihci S, Kargi F: Comparison of bio-hydrogen production from hydrolyzed wheat starch by mesophilic and thermophilic dark fermentation. Int. J. Hydrogen Energy 2010, 35: 13214–13218. 10.1016/j.ijhydene.2010.09.029
Kim SH, Shin HS: Effects of base-pretreatment on continuous enriched culture for hydrogen production from food waste. Int. J. Hydrogen Energy 2008, 33: 5266–5274. 10.1016/j.ijhydene.2008.05.010
Acknowledgements
This research was supported by Energy Conservation Promotion Fund, Energy Policy and Planning Office, Ministry of Energy, Thailand (MU-IRB 2011/115.2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JW drafted the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wongthanate, J., Chinnacotpong, K. & Khumpong, M. Impacts of pH, temperature, and pretreatment method on biohydrogen production from organic wastes by sewage microflora. Int J Energy Environ Eng 5, 6 (2014). https://doi.org/10.1186/2251-6832-5-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6832-5-6