Abstract
Background
Metabolic syndrome is an obesity dependent disorder with a worldwide high prevalence. Regarding the high prevalence of Metabolic syndrome in Iran we analyzed the influence of -1131T>C (rs662799) and c.56C>G (S19W, rs3135506) polymorphisms of the novel apolipoprotein gene, ApoA5, on some Metabolic Syndrome indicators in population from north of Iran.
Methods
199 volunteers from Babol city-Iran were divided in two groups of low (N = 99, TG ≤ 103 mg/dl) and high (N = 100, TG ≥ 150 mg/dl) serum levels of Triglycerides (TG). We amplified the gene fragments containing -1131T>C and c.56C>G polymorphisms by PCR method and revealed the polymorphisms by RFLP analysis.
Results
We found a significant association (p = 0.016, Independent t-test) between high levels of TG and -1131T>C polymorphism but not between this polymorphism and serum HDL-C concentrations. Carriers of the C allele had a 1.97 times higher odds ratio to be in the high-TG group than those of the TT genotype (95%, CI = 1.05-3.68). We observed no association between -1131T>C polymorphism with either Waist-to-Hip Ratio (WHR) or Body-Mass-Index (BMI). In the case of c.56C>G polymorphism, although it showed a significant relationship with WHR (p = 0/040, Independent t-test), but failed to correlate with either levels of TG (p = 0.594) or HDL-C (p = 0.640) in serum.
Conclusion
Our study confirms that ApoA5 gene polymorphisms, -1131T>C and c.56C>G are associated with the two criteria of Metabolic Syndrome, TG and WHR, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Metabolic syndrome (MetS) is a combination of clinical and paraclinical signs characterized by abdominal obesity, dyslipidemia (high triglyceride (TG), low HDL-cholesterol (HDL-C)), hypertension and hyperglycemia (3 out of 5). Several definitions have been presented to diagnose MetS, with the most earliest being the WHO definition, which declares high TG and waist-to-hip ratio (WHR) as the basic criteria [1]. Recent studies presented hypertriglyceridemia as a hallmark of MetS [2]. Also, Hypertriglyceridemia can subsequently result in serum HDL-C reduction, another hallmark of MetS [2, 3].
MetS has been known as a risk factor for cardiovascular disease (CVD) and diabetes mellitus [4] and has an increasing rate of prevalence globally [5, 6]. The prevalence of MetS based on National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) in Iran varies from 21% in southeast [7] to 23.7% in the west [8] and 29.9% in the center [9] with higher rate in women than men. It is worth mentioning that the prevalence of MetS is 31% among women in the north [10].
The exact pathological mechanism of MetS has not yet been clarified but it has been established that both environmental and genetic factors are involve [11–13]. ApolipoproteinA5 (ApoA5) gene which is located on chromosome 11q23 (NC_000011.9) on APOA1/C3/A4/A5 gene cluster has been recently discovered [14]. Despite its low plasma concentration, ApoA5 has been shown to have great effect on the plasma TG concentration [15, 16]. These effects are partially attributed to the role of ApoA5 on TG metabolism (Reviewed in [17]) as well as to its influence on food intake [18].
Molecular mechanism of ApoA5 effect on TG metabolism is not completely understood. ApoA5 is suggested to impede the second step of VLDL gathering [19]. In vivo studies on human ApoA5 transgenic mice demonstrated that ApoA5 could interact with lipoprotein lipase and significantly increase its activity [20]. These results suggest that ApoA5 might induce a decrease in VLDL associated TG levels by both decreasing hepatic VLDL synthesis and increasing VLDL clearance. Besides, a recent study showed that ApoA5 may be absorbed by human adipocytes and may play a role in TG storage regulation [21].
Two polymorphisms of ApoA5, -1131T>C (rs662799) and c.56C>G (rs3135506) have been shown to be associated with TG level and dyslipidemia in different ethnics [22–25]. ApoA5 -1131T>C polymorphism is located in the promoter of ApoA5 gene; therefore, it is expected to affect gene transcription and consequently serum ApoA5 levels. Relationship between ApoA5 level and TG has been reported in some research [26].
c.56C>G polymorphism of APOA5 gene has been identified as a functional variant which tryptophan is substituted with serine in signal peptide. This polymorphism results in decrease in ApoA5 secretion and 3 fold decline in plasma protein level with subsequent increase in plasma TG [27].
With the aim of addressing the role of APOA5 polymorphisms in the high prevalence of MetS in the north of Iran, we investigated the association of the two common polymorphisms of ApoA5 gene, -1131T>C and c.56C>G, with the MetS criteria.
Subjects, material and methods
Ethical consideration
This cross-sectional study was performed from August 2008 to August 2009 in Babol, Iran. This study was done in agreement with the Helsinki Declaration, following approval by the ethical committee of the Medical school. Written informed permission was obtained from all subjects after ensuring them about safety of the procedure and security of their information.
Subjects
The study subjects were 199 men and woman with the age of 30 to 73 years old. Volunteers had referred to Pars laboratory in Babol-Iran from 2008-08-01 to 2009-08-17. We obtained written, informed consent from the subjects. Their personal data including age, living place, cigarette and alcohol consumption, past medical history and drugs consumption were collected through an interview. Those who had diabetes, any thyroid disorders or being treated for these disorders or who had an abnormal FBS or TSH serum levels were excluded. Everyone with serum cholesterol more than 300 mg/dl or treating with any drugs that influence on lipid metabolism was omitted. Besides, people with alcohol consumption or severe weight loss during the previous two weeks were excluded too. Anthropometric characteristics of the included individuals such as: height, weight, hip and waist circumferences were measured by a trained person, and then BMI and WHR were calculated. The subjects based on serum TG level were divided into two groups: low TG (TG ≤ 103 mg/dl) including 49 men and 50 women and high TG (TG ≥ 150 mg/dl) including 50 men and 50 women.
Biochemical and DNA Analyses
10 ml of blood sample was taken from each individual after overnight fasting, 5 ml for biochemical and 5 ml for DNA extraction. Serum was separated immediately after clotting and serum FBS, TG, total cholesterol, HDL-C and LDL-cholesterol (LDL-C) levels were measured by BS-300 MINDRAY (Shenzhen Mindray Bio-medical Electronics Co., China) by DIASIS kits (Germany). Serum TSH was measured by (AWARENESE, Stat Fax-200 model), using a commercially available ELISA DIAPLUS, INC (Q1) kits (America). For quality control purpose TruCal U Lot: 10929 and TruCal HDL/LDL, Lot: 10502 were used to calibrate the biochemical tests and TruLab N, P Lot: 11382 and TruLab Lipid, Lot: 10501 were applied to check the accuracy of biochemical tests. To control the quality of TSH tests Dia Plus EIA, RIA, CLIA Control Serum, Lot: MC1A5 was used.
Genomic DNA was extracted by the salting out method from the peripheral blood. Genes of interest were amplified by 200 mM dNTP, 2U SmarTaq (Cinagen, Iran), 1× smarTaq buffer, 200 nM each primer 1.5 mM MgCl2 and 1 ul of DNA were mixed and the volume was adjusted to 25 ul with water. PCR was performed by PRIMUS (MWG-Biotech) thermal cycler. -1131T>C polymorphism was amplified at 95°C for 12 min followed by 30 cycles of 94°C for 15 s, 55°C for 30s, and 72°C for 30s and a final extension at 72°C for 3 min to produce 187 bp fragments. Products were digested with MseI (Tru1I) and resolved on 3% agarose gels post-stained with ethidium bromide and imaged with SYNGENE transilluminator and INGENIUS (SYNGENE BIO IMAGING) Gel Duct system. The resulted segments in the presence of common allele T were 167 and 20 bp versus non digested 187 bp segment in the presence of rare allele C.
For c.56C>G polymorphism fragment of interest was amplified at 95°C for 10 min followed by 35 cycles of 95°C for 35 s, 58°C for 25 s and 72°C for 30s and a final extension at 72°C for 2 min to produce 292 bp fragment. PCR products were digested with Sau96I (Cfr13I) enzyme. After digestion we had 210 and 82 bp segments in the presence of common allele C and 150, 82 and 60 bp segments in the presence of rare allele G. Genotypes were determined two times using blind reader method. Sequences of used primer for each polymorphism have been demonstrated in Table 1.
Statistical analyses
Statistical analyses were done using SPSS 10 and Microsoft excel. Allele frequencies in low and high TG groups were accessed by χ2 test. Associations between genotypes, lipids, and anthropometric parameters were evaluated using independent t-test and One-way ANOVA. Lipid levels were expressed in mg/dl and all values were reported as means ± SD. P < 0.05 (two-tailed) was considered significant. Odds ratio was analyzed through logistic regression.
Results
The groups were included 99 low TG (50 male age: 43.30 ± 12.15 and 49 female age: 46.53 ± 10.90 years) and 100 high TG (50 male age; 45.78 ± 11.20 and 50 female 48.40 ± 9.25 years) volunteers. The human APOA5 gene map with polymorphism positions indicated in Figure 1.
Maps of the human APOA5 gene with polymorphism positions indicated. (A) Map of the human APOA5 gene. Exons 1–4 are numbered and represented by solid boxes. (B) Position of the single nucleotide polymorphism within the core promoter of the APOA5 gene, a T/C polymorphism at position -1131 and a C/G polymorphism at position 56.
-1131T>C polymorphism
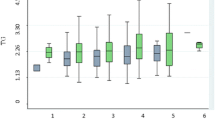
Allele frequencies of this polymorphism were in Hardy–Weinberg equilibrium in both groups of high and low TG. Genotypes in the high (TG ≥150 mg/dl) and low (TG ≤103 mg/dl) TG groups have been shown in Table 2. Both CC and TC genotypes were significantly associated with the high TG group (P = 0.02, χ2 test). The frequencies of the rare allele C in the high and low TG groups were 0.21 and 0.11 respectively. We found a higher number of CC homozygotes in the high TG group as compared with the low TG group. Likewise, the number of TC heterozygotes in the high TG group was higher than those in the low TG group.
We also considered CC and TC genotypes in one group (allele C carriers) and used independent t-test to compare the biochemical and anthropometric parameters of subjects in this group with those of the volunteers with the TT genotype. Thus, a significant mean difference was observed only between the levels of TG in the two above mentioned groups (Table 3).
When the subjects were classified according to sex, differences between the TG levels of men with the TT genotype and those with CC and TC genotypes remained statistically significant (Table 4). This difference did not achieve significance with respect to women.
Results of logistic regression analysis also showed that the allele C carriers were associated with a high TG with an odds ratio of 1.97 (95%, CI = 1.05-3.68) when compared with subjects with TT genotype (Table 5). When odds ratio was adjusted with BMI, it remained two fold but not significant yet.
c.56C>G polymorphism
The frequencies of allele G in the two groups of high and low TG were respectively 0.055 and 0.045 that were not significantly different from one another. Allele G frequency in combined high and low TG groups was 0.05 with the genotype distribution being within Hardy-Weinberg equilibrium. We found a significant association between allele G frequency and WHR but we failed to find any significant association between serum TG (P = 0.594) and HDL-C (P = 0.640) with c.56C>G polymorphism (Table 6).
Discussion
The results of our cross-sectional study show that ApoA5 -1131T>C polymorphism is associated with TG levels in a cohort of subjects from Babol-Iran. These data add to those of studies performed on other human populations [28–30]. Our finding that allele C carriers had a higher chance (two folds) of having a high TG than people with TT genotype is in agreement with haplotype analyses conducted on Taiwanese population [31].
Allele C frequency of this polymorphism was 21% in the high TG population (TG ≥150 mg/dl), 11% in the low TG population (TG ≤103 mg/dl) and 16% in total population. These values are close to allele C frequency reported for other Caucasian populations, such as the Brazilians 16% [32] Hispanics 13-16% and Turks 13% [23] but differ from other ethnic population like East Asians 27-37% [29, 33].
Subgroup analysis revealed a significant association between the rare allele frequency and high TG just in men but not in women. These results are consistent with that reported for a Chinese population [34].
Although other investigators have reported a significant positive relationship between serum levels of total cholesterol and LDL-C with -1131T>C genotype [35], but our data failed to show such an association. The present data indicate that subjects with the TT genotype tended to show higher mean and minimum levels total cholesterol in their sera as compared to those with CC genotype. This trend was not statistically significant, but regarding the role of total cholesterol and LDL-C levels in the occurrence of CVD, it is worthy to examine if the trend will achieve significance with a larger number of volunteers.
Hypertriglyceridemia is a hallmark of MetS [2]. Regarding the great impact of -1131T>C polymorphism on the TG level, the association of this polymorphism with MetS has been the subject of many population genetic studies. Thus, the association of -1131T>C polymorphism with MetS has been shown in Korean [36] and Chinese populations [37]. A meta-analysis also showed the significant association of this polymorphism with MetS in Asian, but not in white people [38].
Association of this allele with a spectrum of cardiac diseases has been shown. Lima et al. reported this association with atheromatosis in Coronary Artery Disease [39] and Ding et al. with Acute Coronary Syndrome [40]. Besides, a meta-analysis has indicated the association of this variant with risk of Ischemic Stroke [41]. Also, the association of -1131T>C polymorphism with CVD has been shown in studies done on various populations, including Chinese [42], Indian [43] and Korean [44].
With respect to the location of -1131T>C polymorphism in the promoter of ApoA5 gene, its effect on gene transcription and consequently serum ApoA5 levels sounds reasonable. It seems that presence of allele C, by reducing ApoA5 level, results in an increase in TG level [44]. Although, relationship between this variant with ApoA5 level and TG has been reported in some studies [26], this association was independent of ApoA5 levels in some others [45]. Therefore, it has been hypothesized that this association might be because of the strong relationship between -1131T>C polymorphism with disequilibrium of polymorphisms of ApoC3 gene [46].
The frequency of the G Allele of ApoA5 c.56C>G polymorphism in our population (5%) is close to the corresponding values reported for Caucasians such as Danish 6% [24], Turks 6% [47] and British 6% [48], as well as populations like Brazilians 7% [32], but it is less than that obtained for Costaricans 10.2% [49]. The reason for similarities between results we obtained for our Iranian population and those obtained for Caucasians can be attributed to the close ethnic relationship between the two populations. Despite the low frequency of the G allele of the c.56C>G polymorphism in our population, we detected a significant association between this minor allele with WHR, a finding that points to the strong influence of this polymorphism on anthropometric parameters. The interaction of G Allele of ApoA5 c.56C>G polymorphism with LPL polymorphism to heighten the genetic susceptibility to obesity have also been reported [50]. However, to our knowledge, there is no data on the independent effect of ApoA5 c.56C>G polymorphism on the anthropometric parameters in the literature.
WHR is shown to be a better predictor for MetS and some other diseases [51–53]. Our present data indicate that G allele of ApoA5 c.56C>G polymorphism had a great impact on WHR. Therefore it is of interest to investigate other ethnic groups to confirm the influence of this ApoA5 polymorphism on the WHR.
Results of a number of studies have shown an association between the G allele of ApoA5 c.56C>G polymorphism and levels of TG, both in the general population [45, 54] and in subgroups of patients, like diabetics [55] and type III hyperlipidemias [56]. Although, the mean TG level in our subjects who were heterozygotes for allele G was higher than that of non-carriers but possibly due to the small sample size, the difference was not statistically significant. However, in consistent with our data, a study on 1703 Costarican subjects didn’t find any association between this variant and TG level [49]. Likewise, data of another study on 1020 Puerto Rican subjects, showed that c.56C>G polymorphism was associated with levels of HDL-C but not TG in the sera of their subjects [57]. These inconsistencies may be due to ethnic differences or linkage disequilibrium with other genetic factors.
Association of the minor allele of c.56C>G polymorphism of ApoA5 with decreased HDL-C [25, 49, 57] and increased LDL-C and total cholesterol [47] has been reported. However, our data didn’t show this association, maybe because of aforementioned reason.
In the end it is very important to consider the strengths and weakness of the current study. One of the highlights of our study is age and gender matched groups, which significantly decrease the effects of these factors on the outcomes of the study. On the other hand, there are some limitation in this work including low sample size, especially for c.56C>G polymorphism that have lower frequency. Finally, we couldn’t evaluate the association of all MetS indicators with these polymorphisms that would be the target of our future studies.
Conclusion
Our results indicate the association of two common polymorphism of ApoA5 gene, -1131T>C and c.56C>G, with respectively TG and WHR, both of which are indicators of MetS. It could be concluded that modification of dietary intake together with losing weight, increase the physical activity and decrease environmental stress are essential factors that might decrease MetS frequency.
Authors’ information
SH Department of Biochemistry and Biophysics, FJ Department of Cardiology, KH Department of Social Medicine, School of Medicine, Babol University of Medical Sciences, Babol, Iran. SS Department of Biochemistry, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
Abbreviations
- ApoA5:
-
Apolipoprotein A5
- BMI:
-
Body-Mass-Index
- CVD:
-
Cardiovascular Disease
- HDL-C:
-
HDL-Cholesterol
- LDL-C:
-
LDL-Cholesterol
- MetS:
-
Metabolic syndrome
- TG:
-
Triglycerides
- WHR:
-
Waist-to-Hip Ratio.
References
Alberti KG, Zimmet PZ: Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998, 15: 539–553. 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
Cifuentes-Goches JC, Gomez-Lopez Jde D, Hernandez-Ancheyta L, Flores-Fuentes SE, Inchaustegui-Arias JL, Canas-Urbina AO: [Hypertriglyceridemia and low HDL cholesterol as high impact factors for metabolic syndrome diagnosis in apparently healthy adults]. Rev Med Inst Mex Seguro Soc 2012, 50: 301–306.
Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J: Harrison's Principles of Internal Medicine. 18th edition. McGraw Hill Professional; 2012.
Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB: Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112: 3066–3072. 10.1161/CIRCULATIONAHA.105.539528
Liu M, Wang J, Jiang B, Sun D, Wu L, Yang S, Wang Y, Li X, He Y: Increasing prevalence of metabolic syndrome in a chinese elderly population: 2001–2010. PLoS One 2013, 8: e66233. 10.1371/journal.pone.0066233
Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S: Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol 2013, 62: 697–703. 10.1016/j.jacc.2013.05.064
Kaykhaei M, Hashemi M, Narouie B, Shikhzadeh A, Jahantigh M, Shirzaei E, Rezazehi B, Hoseinian M, Yousefi S, Masoudian S, Emamdadi A, Alavi S, Mashhadi R, Ansari H: Prevalence of metabolic syndrome in adult population from zahedan, southeast iran. Iran J Public Health 2012, 41: 70–76.
Sharifi F, Mousavinasab SN, Saeini M, Dinmohammadi M: Prevalence of metabolic syndrome in an adult urban population of the west of Iran. Exp Diabetes Res 2009, 2009: 136501.
Fakhrzadeh H, Ebrahimpour P, Pourebrahim R, Heshmat R, Larijani B: Metabolic syndrome and its associated risk factors in healthy adults: a population-based study in iran. Metab Syndr Relat Disord 2006, 4: 28–34. 10.1089/met.2006.4.28
Delavar MA, Lye MS, Khor GL, Hanachi P, Hassan ST: Prevalence of metabolic syndrome among middle aged women in Babol, Iran. Southeast Asian J Trop Med Public Health 2009, 40: 612–628.
Hashemi M, Rezaei H, Kaykhaei MA, Taheri M: A 45-bp insertion/deletion polymorphism of UCP2 gene is associated with metabolic syndrome. J Diabetes Metab Disord 2014, 13: 12. 10.1186/2251-6581-13-12
Hashemi M, Rezaei H, Eskandari-Nasab E, Kaykhaei MA, Taheri M: Association of promoter methylation and 32-bp deletion of the PTEN gene with susceptibility to metabolic syndrome. Mol Med Rep 2013, 7: 342–346.
Hahsemi M, Rezaei H, Eskandari Nasab E, Kaykhaei MA, Zakeri Z, Taheri M: Association between chemerin rs17173608 and vaspin rs2236242 gene polymorphisms and the metabolic syndrome, a preliminary report. Gene 2012, 510: 113–117. 10.1016/j.gene.2012.08.048
Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM: An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science 2001, 294: 169–173. 10.1126/science.1064852
Lookene A, Beckstead JA, Nilsson S, Olivecrona G, Ryan RO: Apolipoprotein A-V-heparin interactions: implications for plasma lipoprotein metabolism. J Biol Chem 2005, 280: 25383–25387. 10.1074/jbc.M501589200
O'Brien PJ, Alborn WE, Sloan JH, Ulmer M, Boodhoo A, Knierman MD, Schultze AE, Konrad RJ: The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin Chem 2005, 51: 351–359. 10.1373/clinchem.2004.040824
Kluger M, Heeren J, Merkel M: Apoprotein A-V: an important regulator of triglyceride metabolism. J Inherit Metab Dis 2008, 31: 281–288.
van den Berg SA, Heemskerk MM, Geerling JJ, van Klinken JB, Schaap FG, Bijland S, Berbee JF, van Harmelen VJ, Pronk AC, Schreurs M, Havekes LM, Rensen PC, van Dijk KW: Apolipoprotein A5 deficiency aggravates high-fat diet-induced obesity due to impaired central regulation of food intake. FASEB J 2013, 27: 3354–3362. 10.1096/fj.12-225367
Weinberg RB, Cook VR, Beckstead JA, Martin DD, Gallagher JW, Shelness GS, Ryan RO: Structure and interfacial properties of human apolipoprotein A-V. J Biol Chem 2003, 278: 34438–34444. 10.1074/jbc.M303784200
Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J: Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem 2005, 280: 21553–21560. 10.1074/jbc.M411412200
Zheng XY, Zhao SP, Yu BL, Wu CL, Liu L: Apolipoprotein A5 internalized by human adipocytes modulates cellular triglyceride content. Biol Chem 2012, 393: 161–167.
Dallongeville J, Cottel D, Montaye M, Codron V, Amouyel P, Helbecque N: Impact of APOA5/A4/C3 genetic polymorphisms on lipid variables and cardiovascular disease risk in French men. Int J Cardiol 2006, 106: 152–156. 10.1016/j.ijcard.2004.10.065
Hodoglugil U, Tanyolac S, Williamson DW, Huang Y, Mahley RW: Apolipoprotein A-V: a potential modulator of plasma triglyceride levels in Turks. J Lipid Res 2006, 47: 144–153.
Jorgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg-Hansen A: Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J 2013, 34: 1826–1833. 10.1093/eurheartj/ehs431
Komurcu-Bayrak E, Onat A, Poda M, Humphries SE, Palmen J, Guclu F, Can G, Erginel-Unaltuna N: Gender-modulated impact of apolipoprotein A5 gene (APOA5) -1131T>C and c.56C>G polymorphisms on lipids, dyslipidemia and metabolic syndrome in Turkish adults. Clin Chem Lab Med 2008, 46: 778–784.
Vaessen SF, Sierts JA, Kuivenhoven JA, Schaap FG: Efficient lowering of triglyceride levels in mice by human apoAV protein variants associated with hypertriglyceridemia. Biochem Biophys Res Commun 2009, 379: 542–546. 10.1016/j.bbrc.2008.12.115
Ahituv N, Akiyama J, Chapman-Helleboid A, Fruchart J, Pennacchio LA: In vivo characterization of human APOA5 haplotypes. Genomics 2007, 90: 674–679. 10.1016/j.ygeno.2007.08.003
Xian-sheng H, Shui-ping Z, Qian Z, Lin B, Min H: Association of plasma apolipoprotein AV with lipid profiles in patients with acute coronary syndrome. Atherosclerosis 2009, 204: e99-e102. 10.1016/j.atherosclerosis.2008.11.019
Kim JY, Kim OY, Paik JK, Lee SH, Lee JH: Association of apolipoprotein A-V concentration with apolipoprotein A5 gene -1131T>C polymorphism and fasting triglyceride levels. J Clin Lipidol 2013, 7: 94–101. 10.1016/j.jacl.2012.06.002
Li YY, Yin RX, Lai CQ, Li M, Long XJ, Li KL, Liu WY, Zhang L, Wu JZ: Association of apolipoprotein A5 gene polymorphisms and serum lipid levels. Nutr Metab Cardiovasc Dis 2011, 21: 947–956. 10.1016/j.numecd.2010.04.004
Huang MC, Wang TN, Wang HS, Sung YC, Ko YC, Chiang HC: The -1131T>C polymorphism in the apolipoprotein A5 gene is related to hypertriglyceridemia in Taiwanese aborigines. Kaohsiung J Med Sci 2008, 24: 171–179. 10.1016/S1607-551X(08)70114-1
Furuya TK, Chen ES, Ota VK, Mazzotti DR, Ramos LR, Cendoroglo MS, Araujo LQ, Burbano RR, Smith MA: Association of APOA1 and APOA5 polymorphisms and haplotypes with lipid parameters in a Brazilian elderly cohort. Genet Mol Res 2013, 12: 3495–3499. 10.4238/2013.February.28.7
Austin MA, Talmud PJ, Farin FM, Nickerson DA, Edwards KL, Leonetti D, McNeely MJ, Viernes HM, Humphries SE, Fujimoto WY: Association of apolipoprotein A5 variants with LDL particle size and triglyceride in Japanese Americans. Biochim Biophys Acta 2004, 1688: 1–9. 10.1016/j.bbadis.2003.10.003
Baum L, Tomlinson B, Thomas GN: APOA5–1131T>C polymorphism is associated with triglyceride levels in Chinese men. Clin Genet 2003, 63: 377–379. 10.1034/j.1399-0004.2003.00063.x
Vaessen SF, Schaap FG, Kuivenhoven JA, Groen AK, Hutten BA, Boekholdt SM, Hattori H, Sandhu MS, Bingham SA, Luben R, Palmen JA, Wareham NJ, Humphries SE, Kastelein JJ, Talmud PJ, Khaw KT: Apolipoprotein A-V, triglycerides and risk of coronary artery disease: the prospective Epic-Norfolk Population Study. J Lipid Res 2006, 47: 2064–2070. 10.1194/jlr.M600233-JLR200
Song KH, Cha S, Yu SG, Yu H, Oh SA, Kang NS: Association of apolipoprotein A5 gene -1131T>C polymorphism with the risk of metabolic syndrome in Korean subjects. Biomed Res Int 2013, 2013: 585134.
Ong KL, Jiang CQ, Liu B, Jin YL, Tso AW, Tam S, Wong KS, Tomlinson B, Cheung BM, Lin JM, Yue XJ, Lam KS, Lam TH, Thomas GN: Association of a genetic variant in the apolipoprotein A5 gene with the metabolic syndrome in Chinese. Clin Endocrinol (Oxf) 2011, 74: 206–213. 10.1111/j.1365-2265.2010.03899.x
Liu CF, Yang QF, Chen XL, Liu CY: Apolipoprotein a5 gene polymorphism and risk for metabolic syndrome: a meta-analysis. Genet Test Mol Biomarkers 2012, 16: 1241–1245. 10.1089/gtmb.2012.0183
Lima LM, Carvalho MG, Gomes KB, Santos IR, Sousa MO: Association between apolipoprotein A5–1131T>C polymorphism and atheromatosis extent in coronary artery disease. Cardiovasc Res 2012, 93: S120-S120. 10.1093/cvr/cvr267
Ding Y, Zhu MA, Wang ZX, Zhu J, Feng JB, Li DS: Associations of polymorphisms in the apolipoprotein APOA1-C3-A5 gene cluster with acute coronary syndrome. J Biomed Biotechnol 2012, 2012: 509420.
Pi Y, Zhang L, Yang Q, Li B, Guo L, Fang C, Gao C, Wang J, Xiang J, Li J: Apolipoprotein A5 gene promoter region-1131T/C polymorphism is associated with risk of ischemic stroke and elevated triglyceride levels: a meta-analysis. Cerebrovasc Dis 2012, 33: 558–565. 10.1159/000338781
Zhai G, Li M, Zhu C: APOA5 -1131T/C polymorphism is associated with coronary artery disease in a Chinese population: a meta-analysis. Clin Chem Lab Med 2011, 49: 535–539.
AshokKumar M, Subhashini NG, SaiBabu R, Ramesh A, Cherian KM, Emmanuel C: Genetic variants on apolipoprotein gene cluster influence triglycerides with a risk of coronary artery disease among Indians. Mol Biol Rep 2010, 37: 521–527. 10.1007/s11033-009-9728-7
Jang Y, Paik JK, Hyun YJ, Chae JS, Kim JY, Choi JR, Lee SH, Shin DJ, Ordovas JM, Lee JH: The apolipoprotein A5 -1131T>C promoter polymorphism in Koreans: association with plasma APOA5 and serum triglyceride concentrations, LDL particle size and coronary artery disease. Clin Chim Acta 2009, 402: 83–87. 10.1016/j.cca.2008.12.024
Henneman P, Schaap FG, Havekes LM, Rensen PC, Frants RR, van Tol A, Hattori H, Smelt AH, van Dijk KW: Plasma apoAV levels are markedly elevated in severe hypertriglyceridemia and positively correlated with the APOA5 S19W polymorphism. Atherosclerosis 2007, 193: 129–134. 10.1016/j.atherosclerosis.2006.05.030
Olano-Martin E, Abraham EC, Gill-Garrison R, Valdes AM, Grimaldi K, Tang F, Jackson KG, Williams CM, Minihane AM: Influence of apoA-V gene variants on postprandial triglyceride metabolism: impact of gender. J Lipid Res 2008, 49: 945–953. 10.1194/jlr.M700112-JLR200
Can Demirdogen B, Sahin E, Turkanoglu Ozcelik A, Bek S, Demirkaya S, Adali O: Apolipoprotein A5 polymorphisms in Turkish population: association with serum lipid profile and risk of ischemic stroke. Mol Biol Rep 2012, 39: 10459–10468. 10.1007/s11033-012-1926-z
Chandak GR, Ward KJ, Yajnik CS, Pandit AN, Bavdekar A, Joglekar CV, Fall CH, Mohankrishna P, Wilkin TJ, Metcalf BS, Weedon MN, Frayling TM, Hattersley AT: Triglyceride associated polymorphisms of the APOA5 gene have very different allele frequencies in Pune, India compared to Europeans. BMC Med Genet 2006, 7: 76. 10.1186/1471-2350-7-76
Ruiz-Narvaez EA, Yang Y, Nakanishi Y, Kirchdorfer J, Campos H: APOC3/A5 haplotypes, lipid levels, and risk of myocardial infarction in the Central Valley of Costa Rica. J Lipid Res 2005, 46: 2605–2613. 10.1194/jlr.M500040-JLR200
Smith CE, Tucker KL, Lai CQ, Parnell LD, Lee YC, Ordovas JM: Apolipoprotein A5 and lipoprotein lipase interact to modulate anthropometric measures in Hispanics of Caribbean origin. Obesity (Silver Spring) 2010, 18: 327–332. 10.1038/oby.2009.216
De Nicola L, Conte G: Waist: hip ratio is a better predictor of cardiovascular risk than BMI in patients with moderate CKD. Nat Clin Pract Nephrol 2008, 4: 592–593.
Qiao Q, Nyamdorj R: Is the association of type II diabetes with waist circumference or waist-to-hip ratio stronger than that with body mass index? Eur J Clin Nutr 2010, 64: 30–34. 10.1038/ejcn.2009.93
Chen CC, Wang WS, Chang HY, Liu JS, Chen YJ: Heterogeneity of body mass index, waist circumference, and waist-to-hip ratio in predicting obesity-related metabolic disorders for Taiwanese aged 35–64 y. Clin Nutr 2009, 28: 543–548. 10.1016/j.clnu.2009.04.017
Martinelli N, Trabetti E, Bassi A, Girelli D, Friso S, Pizzolo F, Sandri M, Malerba G, Pignatti PF, Corrocher R, Olivieri O: The -1131T>C and S19W APOA5 gene polymorphisms are associated with high levels of triglycerides and apolipoprotein C-III, but not with coronary artery disease: an angiographic study. Atherosclerosis 2007, 191: 409–417. 10.1016/j.atherosclerosis.2006.04.009
Soter MO, Gomes KB, Fernandes AP, Carvalho M, Pinheiro PS, Bosco AA, Silva DD, Sousa MO: -1131T>C and SW19 polymorphisms in APOA5 gene and lipid levels in type 2 diabetic patients. Mol Biol Rep 2012, 39: 7541–7548. 10.1007/s11033-012-1588-x
Evans D, Bode A, von der Lippe G, Beil FU, Mann WA: Cerebrovascular atherosclerosis in type III hyperlipidemia is modulated by variation in the apolipoprotein A5 gene. Eur J Med Res 2011, 16: 79–84. 10.1186/2047-783X-16-2-79
Mattei J, Demissie S, Tucker KL, Ordovas JM: Apolipoprotein A5 polymorphisms interact with total dietary fat intake in association with markers of metabolic syndrome in Puerto Rican older adults. J Nutr 2009, 139: 2301–2308. 10.3945/jn.109.109900
Acknowledgement
This work was supported by a grant from Deputy of research of Babol University of Medical Science. We would like to thank the staff of Cellular and Molecular Research Center of Babol University of Medical Sciences especially Dr. Akhavan Niaki. We also thank Pars laboratory for supplying subjects. We have especial thanks to Prof. A.A. Owji and Dr. Shokrpour for their proficient help in editing the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Prepared figure panels explaining the methodology: SS. Conceived and designed the study: SH SS. Performed the experiments: SS. Analyzed the data: SH SS KH. Contributed samples/reagents/materials/analysis tools: SH FJ SS. Wrote the paper: SH SS. Read and approved the final manuscript SH FJ KH SS.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Halalkhor, S., Jalali, F., Tilaki, K.H. et al. Association of two common polymorphisms of apolipoprotein A5 gene with metabolic syndrome indicators in a North Iranian population, a cross-sectional study. J Diabetes Metab Disord 13, 48 (2014). https://doi.org/10.1186/2251-6581-13-48
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6581-13-48