Abstract
Background
In this study, poly(vinyl chloride) (PVC) is considered one of the most versatile plastics. It is the second largest manufactured resin by volume worldwide.
Results
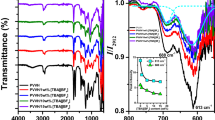
To study the optical properties of modified PVC containing 1,3,4-thiadiazole and phthalyl groups in the repeating units, thin films of the prepared polymers (P I, P II, P III, P IV, P V, and PVC) were casted from tetrahydrofuran solvent with a thickness of 30 μm. The optical properties were studied in the range of 200 to 600 nm, and the optical data were analyzed and interpreted in terms of the theory of phonon-assisted direct electronic transitions; the measured Eg of pure and modified PVC increases in the following order:
Also, the refractive index was measured.
Conclusions
The results showed that for all compositions, the refractive index decreases with increasing wavelength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Modification of polymers has received greater attention as it brings specific properties to the modified material, such as enhanced thermal stability, multiphase physical responses, compatibility, impact response, flexibility, and rigidity [1]. Poly(vinyl chloride) (PVC) is one of the most versatile plastics. It is the second largest manufactured resin by volume worldwide [2].
The modification of PVC lies basically in the dechlorination process, including both substitution and elimination. Because of the peculiarities of the material relevant to its applications, the chemical functionalization of PVC seems permanent to improve and modify the polymer properties [3].
Synthesis of polymer-bound chelating ligands has been reported. A number of ligands including polydentate amines, crown ethers, phosphines, and bipyridines have been bound with mainly polystyrene divinylbenzene copolymers [4, 5].
These studies are mostly concerned with ion-binding and catalytic aspects. Very little information appears to exist as to whether, and to what extent, such organic compounds can influence the properties of the macromolecule. Recently, scientists were able to modify PVC by introducing aromatic and heterocyclic moieties through halogen displacement reaction. PVC, thus modified, showed improved overall photochemical stability and optical properties [6, 7]. In this paper, we present results of such a study on the optical properties of pure and modified PVC films.
Methods
Optical measurements
The best definition of the energy gap is the minimum energy difference between the lowest minimum of the conduction band and the highest maximum of the valance band [8]. The value and shape of the mobility gap in PVC depend on the proportion conditions such as substrate temperature, degree of impurity, and defect of the material. Any variation in such parameters leads to a shift in the absorption edge towards higher or lower energy. The absorption coefficient can be determined from the normal incidence transmission through plane-parallel plate of thickness (d), and interference and multiple reflections are neglected. The reflectance (R) and transmittance (T) are related by Equation 1:
where B is a constant. This equation can be rewritten as follows:
where A is the optical absorbance (A = −log T).
So, the absorption coefficient α(ω) in terms of absorbance becomes
For band-to-band transitions, the energy dependence of the absorption coefficient is of the form
where hν is the photon energy, Eg is the energy gap, r is a constant depending on the type of electronic transitions which takes the value 1/2 for allowed direct transition and 3/2 for forbidden direct transition, and the absorption coefficient α ≥ 104 cm−1. The energy dependence absorption coefficient is in the form
where Eg is the minimum energy gap, Ep is the phonon energy, (+) represents absorbance, (−) represents emission, r takes the values 2 for allowed indirect transition and 3 for forbidden indirect transition, and the absorption coefficient α < 104 cm−1. By plotting (αhν)1/r vs. hν for a fixed r value, the extrapolation of the linear part could be used to define Eg[7].
Results and discussion
The relation of (αhν)2 and (αhν)1/2 vs. photon energy for the PVC sample is shown in Figure 1 for allowed transition. For modified samples shown in Figures 2, 3, 4, 5, and 6, the shift in the energy gap could be attributed to the formation of polarons in the modified films. The evidence of polaron formation is that the reaction in the band-to-band transition is due to the shifting of the band density of state toward the energy gap. The effect of the organic addition on the values of phonon energies is also investigated, and the results are shown in Table 1.
The energy gaps were measured by adopting the data of energy gap (see Table 1 and Figures 1, 2, 3, 4, 5, and 6). The Eg for PVC in the presence of additives increases in the following order:
The presence of an ion pair of electrons at different atoms, such as sulfur and nitrogen, for the stereochemistry of the polymer leads to the change of the value of energy gap rather than PVC.
The tail of the absorption edge is exponential, indicating the presence of localized states in the energy band gap. The amount of tailing can be predicted to a first approximation by plotting the absorption edge data in terms of an equation originally given by Urbach. The absorption edge of nonmetallic materials gives a measure of the energy band gap, and the exponential dependence of the absorption coefficient, α, on photon energy, hν, is found to hold over several decades for a polymeric material and takes the following form:
where α0 is a constant and Et is interpreted as the width of the tails of localized states in the gap region. To evaluate the values of α0 and Et, α should be in the logarithmic scale as a function of photon energy hν as shown in Figure 2. The reciprocal of the slope of each line yields the magnitude of Et and its values for different polymeric films. It is clear that modification increases the width of the tail of localized states and decreases the energy gap of PVC thin films. Using Equation 6 at a constant temperature, a graph representing α on the y-axis and energy E (eV) on the x-axis (Figure 7) in the range of the Urbach tail would yield a straight line.
The refractive index is a suitable state parameter directly correlated to the material density. The refractive index, n(λ), was determined from the absolute values of the transmittance and reflectance of the investigated films using the following formula:
where k is the extinction coefficient and R is the optical reflectance. The extinction coefficient can be obtained from the relation where k = αλ/ 4π. Plots in Figure 8 represent the dispersion in the refractive index for pure and modified PVC thin films in the investigated range of wavelengths. Inspection of Figure 8 indicates that for all compositions, the refractive index decreases with increasing wavelength.
Table 2 illustrates the refractive index values for all polymers at a wavelength of 600 nm (for the values of other polymer wavelength, see Additional file 1).
Experimental
All the polymers were prepared as described by [9] (Scheme 1). A solution of PVC or modified PVC (5%) in tetrahydrofuran (THF) was used to prepare the films. The polymeric films were prepared by casting the polymers (P I, P II, P III, P IV, P V, and PVC) onto leveled glass plates. The films were prepared by evaporation technique at room temperature for 24 h. To remove the possible residual THF solvent, the film samples were further dried at room temperature for 3 h under reduced pressure. The samples have the same thickness of 30 μm (measured using a micrometer type 2610 A, Mainz, Germany). The optical absorbance (A) of the samples was measured as a function of the wavelength (λ) which ranged from 200 to 600 nm using a computerized Shimadzu UV-160A UV–vis spectrophotometer (Kyoto, Japan) with a full-scale absorbance up to 2.5. The light sources are halogen lamp and socket deuterium lamp, and the detector is a Si photodiode. All measurements were performed at room temperature.
Conclusions
The study on the effect of PVC modification with 1,3,4-thiadiazole and phthalyl groups in the repeating units on its optical properties has shown that the energy gap of modified polymers decreases in comparison with that of pure PVC, and for all compositions, the refractive index decreases with increasing wavelength.
References
Amit B, Rawlins JW, Ray P (Eds): Polymer crafting and crosslinking. Hoboken: Wiley; 2009.
Claudiu L, Edina R, Bogdan M, Teodora Z, Gheorghe H: UPB Sci Bull. Series B 2010, 72: 127–140.
Moulay S: Prog Polym Sci. 2010, 35: 303–331. 10.1016/j.progpolymsci.2009.12.001
Gustafsson B, Hakansson M, Jagner S: Inorg Chim Acta. 2003, 350: 209–214.
Genhua Z, Pan C: Polymer. 2005, 46: 2802–2810. 10.1016/j.polymer.2005.01.071
Yousif E, Hameed A, Bakir E: Journal of Al-Nahrain University (Science). 2007, 10: 7–12.
Mohammed A, Emad Y, Atheel A, Yang F: Iraqi J Polymers. 2009,13(2):27–36.
Tauc J: Amorphous and liquid semiconductor. New York: Plenum; 1974.
Yousif E, Ayad H, Adel K, Yang F, Noora A, Abdualbasit G: Aust J Basic Appl Sci. 2009, 3: 1786–1794.
Acknowledgments
The authors would like to thank Al-Nahrain University, Universiti Tunku Abdul Rahman, and Universiti Kebangsaan Malaysia for the research grants UKM-GUP-NBT-08-27-113 and UKM-OUP-2012-139 and for the financial support and technical assistance on this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EY developed the concept, analyzed the data, and drafted the manuscript. MA, JS, and NS performed the PVC modification, optimization study, and statistical analysis. BMA, HH, and YW advised on the methods of tests. All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yousif, E., Abdallh, M., Hashim, H. et al. Optical properties of pure and modified poly(vinyl chloride). Int J Ind Chem 4, 4 (2013). https://doi.org/10.1186/2228-5547-4-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-4-4