Abstract
The hydrothermal synthesis and optical properties of Nb5+-doped lithium metasilicate and lithium disilicate nanomaterials were investigated. The microstructures and morphologies of the synthesized Li2 -2x Nb2x SiO3 + δ and Li2 -2x Nb2x Si2O5 + δ nanomaterials were studied by powder X-ray diffraction and scanning electron microscopy techniques, respectively. The synthesized niobium-doped lithium metasilicate and lithium disilicate nanomaterials, respectively, are isostructural with the standard bulk Li2SiO3 (space group Cmc21) and Li2Si2O5 (space group Ccc2) materials. The photoluminescence spectra of the synthesized materials are studied. The measured optical properties show dependence of the dopant amounts in the structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Lithium ceramics are of research interest because of their technological applications. Among these ceramics, lithium silicates have been investigated as breeder materials for nuclear fusion reactors and as carbon dioxide absorbents in addition to other more well-known applications such as in thermal expansion glass-ceramics used in ceramic hobs [1–6]. The tetrahedral silicate ion (SiO4 2-), in the structure of silicates, provides good mechanical resistance and stability for the phosphor [7–11]. Lithium metasilicate and lithium disilicate, therefore, are suitable pyroelectric materials and used also in optical waveguide devices [12].
The synthesis of lithium silicate doped with La3+, Sm3+, Gd3+, Ho3+, Dy3[13–16], Nd3+[17], Na+[18], Eu3+, Ce3+, and Tb3+[19] ions has been reported previously. Also, Cu2+-doped [20], Cr4+-doped [21], Al3+-doped [22], Cr3+- and Tm3+-doped [23], V3+-, V4+-, and V5+-doped [24] lithium silicates have been synthesized.
Recently, we have reported the hydrothermal synthesis and optical properties of Sb3+-doped lithium metasilicate and lithium disilicate nanomaterials [25]. However, to the best of our knowledge, no work has been devoted to niobium-doped lithium silicates. Doping of Nb5+ causes conductivity [26] and generates metallic behavior in insulators [27], increases electrical resistivity and enhances hysteresis squareness and fatigue behavior [28, 29], decreases the dielectric constant maximum and Curie point [30], and so on. Also, Nb can be considered as a donor dopant for PZT materials [31].
In this research work, we report the synthesis and optical properties of Li2 - 2x Nb2x SiO3 + δ and Li2 - 2x Nb2x Si2O5 + δ nanomaterials under hydrothermal conditions. Also, we have studied the effect of dopant amount on the morphology of the synthesized nanomaterials, while keeping the other conditions unchanged. The effect of the dopant concentration on the morphology of the synthesized materials is investigated. Moreover, the optical properties of the synthesized Li2 - 2x Nb2x SiO3 and Li2 - 2x Nb2x Si2O5 nanomaterials are studied. The optical and catalytic properties of the synthesized materials were improved by doping Nb5+ in lithium silicates, so they are applicable in fabrication of optical devices and also as catalysts.
Methods
All the reagents used in the experiments were of analytical grade and used as received without further purification. Nb5+-doped lithium metasilicate and lithium disilicate nanomaterials are synthesized in a one-step hydrothermal process.
Synthesis of niobium-doped lithium metasilicate (Li2 - 2x Nb2x SiO3 + δ ) (x = 0.0025, 0.005)
Appropriate molar amounts of LiNO3 (MW = 68.95 g mol-1) (10 and 11.9 mmol, respectively), SiO2 · H2O (MW = 96.11 g mol-1) (20 and 23.92 mmol, respectively) and Nb2O5 (MW = 265.815 g mol-1) (0.0263 and 0.06 mmol, respectively) were dissolved in 60 mL of hot NaOH solution (0.67 and 0.80 M solution, respectively) under magnetic stirring at 80°C. The resultant solution was transferred and sealed in a Teflon-lined stainless steel autoclave of 100 mL capacity, under autogenous pressure and heated to 180°C for 96 h. The autoclave was then allowed to cool naturally to room temperature and the resulting white precipitate was recovered.
Synthesis of niobium-doped lithium disilicate (Li2 - 2x Nb2x Si2O5 + δ ) (x = 0.005, 0.0075, and 0.01)
Appropriate molar amounts of LiNO3 (MW = 68.95 g mol-1; 11.9, 10 or 9.9 mmol, respectively), SiO2 · H2O (MW = 96.11 g mol-1; 35.9, 30.22 or 30 mmol, respectively) and Nb2O5 (MW = 265.815 g mol-1; 0.06, 0.073, or 0.1 mmol, respectively) were dissolved in 60 mL of hot NaOH solution (1.20, 1.0, and 1.0 M solution, respectively) under magnetic stirring at 80°C. The resultant solution was transferred and sealed in a Teflon-lined stainless steel autoclave with 100 mL capacity, under autogenous pressure and heated to 180°C for 96 h. The solution was then allowed to cool naturally to room temperature and the resulting white precipitate was recovered.
Results and discussion
Powder X-ray diffraction analysis
Phase identifications were performed on a powder X-ray diffractometer Siemens D5000 (Siemens AG, Munich, Germany) using Cu-Kα radiation. The morphology of the obtained materials was examined with a Philips XL30 scanning electron microscope (Philips, Amsterdam, Netherlands) equipped with energy-dispersive X-ray (EDX) spectrometer. Absorption and photoluminescence spectra were recorded on a AnalytikJena Specord 40 (AnalytikJena UK, Wembley, UK) and a Perkin Elmer LF-5 spectrometer (PerkinElmer Inc., Waltham, MA, USA) respectively.
Figure 1a,b, respectively, shows the EDX spectra of the synthesized Nb5+-doped lithium metasilicate and lithium disilicate nanomaterials, which verify the doping and the compositional analysis of Nb5+ in the nanoparticles of lithium silicates.
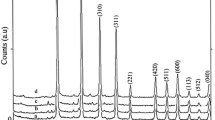
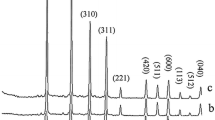
The crystal phases of the synthesized materials were examined by powder X-ray diffraction technique. Figures 2 and 3 show the powder X-ray diffraction (PXRD) patterns of the Nb5+-doped lithium metasilicate and lithium disilicate, respectively. The measured powder XRD data are in good agreement with those of corresponding undoped lithium metasilicate or lithium disilicate nanomaterials [25] and the obtained stable phases are, respectively, isostructural with Li2SiO3 (space group Cmc21) [25, 32–41] and Li2Si2O5 (space group Ccc2) [25, 42–44]. The measured data are in agreement with the respective Joint Committee on Powder Diffraction Standards (JCPDS) card for Li2SiO3 (JCPDS 29-0829) (a = 9.3808 Å, b = 5.3975 Å, and c = 4.6615 Å) and for Li2Si2O5 (JCPDS 15-0637) (a = 5.825 Å, b = 14.56 Å, and c = 4.796 Å). The standard crystallographic data for lithium metasilicate (JCPDS 29-0829) and lithium disilicate (JCPDS 15-0637) and the powder XRD data for respective hydrothermally synthesized undoped nanomaterials [25] are summarized in Tables 1 and 2 respectively. Also, the powder XRD data for respective hydrothermally synthesized Nb-doped lithium metasilicate and Nb-doped lithium disilicate are summarized in Tables 3 and 4 for comparisons. Moreover, the intense sharp diffraction patterns suggest that the as-synthesized products are well crystallized.
The doping limitations are 0 to 0.25 and 0 to 0.75 mol% of Nb5+ for lithium metasilicate and lithium disilicate, respectively. Excess mole percent concentration of the dopant agent in the reaction mixture, as shown in Figures 2 and 3, results in impurity peaks in the XRD patterns [45]. The diffraction line at 2θ ≈ 49° is assigned by its peak position to the excess Nb2O5[43]. Moreover, the formation of other phases of lithium silicates and raw materials was already detected for higher mole percent concentration of the dopant agent in the reaction mixture (Figures 2 and 3) [25, 41, 42, 46].
Compared to the nanomaterials of undoped lithium silicates, the diffraction lines in the powder XRD patterns of the Nb5+-doped lithium silicates nanomaterials shift to lower 2θ values and therefore to larger d values. For the most intensive diffraction line (200), a diffraction line shift of ∆2θ = 18.881° (pure)-18.80° (doped) = 0.081° (∆d = 4.7206 Å (doped)-4.7005 Å (pure) = 0.0201 Å) for Nb5+-doped lithium metasilicate; for the most intensive diffraction line (040), a diffraction line shift of ∆2θ = 24.78° (pure)-24.70° (doped) = 0.08° (∆d = 3.600 Å (doped)-3.589 Å (pure) = 0.011 Å) for Nb5+-doped lithium disilicate are calculated via Bragg’s law. Tables 5 and 6 show the crystal sizes of the Nb-doped materials in different dopant amounts via Debye-Scherrer equation.
Since the ionic radius of the Nb5+ (0.64 Å [46]) is closer to the ionic radius of Li+ (0.59 Å [46]) rather than the Si4+ (0.26 Å [46]), in the Nb5+-doped lithium metasilicate and lithium disilicate, it may be expected that the dopant ion will replace with Li+ ions in the structure. The larger radius of the dopant ion, compared to the Li+, may cause an expansion of the lattice parameter in the Nb5+-doped lithium silicate nanomaterials. Since both ionic radii and charges are not the same for the dopant and Li+ ions, it is also possible that the dopant ion takes an interstitial position in lattice rather than replacing any Li+ ions, where additional patterns will be observed in XRD pattern [47]. However, here, the powder XRD data measured for the doped samples are in accord with those of the undoped materials without any residual or impurity phase formation. The powder XRD patterns of the doped samples, therefore, suggest the fact that the dopant ions are indeed going to lattice positions rather than interstitial positions.
Moreover, on replacing Li+ ions, the dopant ions are bound to create some oxygen-related defect centers or Li+ vacancies for charge compensation. Therefore, it is believed that the dopant ions will be in a structurally disordered environment.
Cellref version 3 was used to refine the cell parameters from the measured powder XRD data of the synthesized doped nanomaterials. Compared to the standard crystallographic data for lithium metasilicate (JCPDS 29-0829) and lithium disilicate (JCPDS 15-0637), the refined unit cell parameters of the synthesized Nb-doped lithium metasilicate and lithium disilicate nanomaterials are a = 9.3702 Å, b = 5.3994 Å, c = 4.6643 Å and a = 5.826 Å, b = 14.6168 Å, c = 4.878 Å, respectively.
Microstructure analysis
Scanning electron microscopy (SEM) images of the pure lithium metasilicate and lithium disilicate are present in our previous work [25]. Figure 4 shows typical SEM images of the synthesized Li1.995Nb0.001SiO3 + δ nanoparticles. The synthesized sample is composed of multi-ply sheets (thickness and length of about 100 nm and 5 μm, respectively) joined together to form nanoflowers. The typical SEM images of the synthesized Li1.99Nb0.002Si2O5+δ and Li1.985Nb0.003Si2O5+δ are given in Figures 5 and 6, respectively. The synthesized Li1.99Nb0.002Si2O5 nanomaterial is composed of plate-like nanoparticles with homogenous dispersion (Figure 5b,c). The length of the nanoplates is approximately 0.7 to 0.8 μm. As shown in Figure 6, with increasing the dopant concentration in the structure to x Nb = 0.0075, the resultant nanoplates assemble to each other to form nanoflower-like structures. The length and thickness of the nanoplates are estimated to be 500 nm and 80 to 100 nm approximately.
SEM images of the hydrothermally synthesized Li 2 - 2 x Nb 2 x SiO 3 + δ ( x = 0.0025) nanoflowers. ( a , b ) The SEM image shows that the doped material is composed of plate like structure. (c , d) The image shows that the structure thickness size is about 80 nm and the length size is about 100 nm.
SEM images of the hydrothermally synthesized Li 2 - 2 x Nb 2 x Si 2 O 5 + δ ( x = 0.005) nanoparticles. (a, b, c and d) The SEM image shows that the doped material is composed of plate like structure. (e, f) The high resolution images show that the thickness size is about 70 nm and the length size of the plate is about 500 nm.
Optical properties
The emission spectra of pure Li2SiO3 and Li2Si2O5 are shown in Figures 7 and 8. In the excitation spectrum of the synthesized Li2SiO3 and Li2Si2O5 nanomaterials, a band is observed with maxima at 360 and 250 nm, respectively. Accordingly, in the emission spectrum of the synthesized Li2SiO3 nanomaterials, an intense peak appears at 410.03 nm. In comparison, an intense peak at 291.45 nm is observed in the emission spectrum of the synthesized Li2Si2O5 nanomaterials. With increasing reaction time, no shift is observed in the emission spectrum of the obtained Li2SiO3 and Li2Si2O5 nanomaterials. However, increasing band intensities in the emission spectra of both compounds are observed with increasing reaction time. In the emission spectrum of Nb5+-doped lithium metasilicate nanoflowers (Figure 9), under excitation with light at 234 nm, the main emission band is located at 360 nm with shoulders at 310, 340, and 425 nm. The shoulder appeared at 310 nm is assigned to the band edge emission. Also, the broad band with maxima at 360 nm and the shoulder at 340 nm are assigned to the trap state emission of the nanoparticles. Considering that the energy gap of bulk lithium silicates is above 3.3 eV, the purple-blue photoluminescence which appeared as a shoulder at 425 nm (approximately 2.92 eV) is probably due to a triplet to ground state transition of a neutral oxygen vacancy defect, as suggested by ab initio molecular orbital calculations for many other well-studied metal oxides. Also, the emission band related to the Nb (V) centers in the structure is expected to be superimposed on the shoulder at 425 nm [44]. In comparison, the synthesized Nb5+-doped lithium disilicate nanoparticles exhibit an intense broad emission band (λ ex = 229 nm) at 420 nm (approximately 2.95 eV) (Figure 10) assigned to the oxygen-related defects and Nb5+ centers in the structure, which shows an increasing intensity with increasing the dopant concentration in the structure.
Conclusion
In summary, nanoplates and nanoflowers of Nb5+-doped lithium metasilicate and lithium disilicate were synthesized successfully by employing a simple hydrothermal method. The molar ratio of Li/Si and the dopant concentration in the reaction mixture affect the crystal phase and morphology of the final product, respectively. The synthesized Nb-doped stable phases are isostructural with the corresponding undoped Li2SiO3 or Li2Si2O5 materials. The synthesized nanomaterials exhibited emerging PL optical properties in the UV-Visible region which shows dependence on the dopant amounts in the structure. These materials are expected to have potential application in light emitting devices and as catalysts.
Authors’ information
SK got his B.S. degree in Applied Chemistry from the University of Birjand in 2007. He got his M.Sc. degree in Inorganic Chemistry from the University of Tabriz in August 2010. He is now finishing his Ph.D. studies in Inorganic Chemistry in the Faculty of Chemistry of the University of Semnan, Iran. AA got his B.S. and M.Sc. degrees in Chemistry from the University of Tabriz, Iran in 1972 and 1974, respectively. He got his Ph.D. degree in Inorganic Chemistry from the University of Paris, France in 1978. He is now a professor in Inorganic Chemistry at the University of Tabriz, Iran. MD got his B.S. and M.Sc. degrees in Chemistry and in Inorganic Chemistry from the University of Tabriz, Iran in 2004 and 2006, respectively. He got his Ph.D. degree in Inorganic-Solid State Chemistry from the University of Tabriz, Iran in 2010. She is now a postdoctorate student and associate professor in the research group of Prof. Rostami at the School of Engineering Emerging Technologies, University of Tabriz, Iran and in the Department of Inorganic Chemistry in the same university. AB got his B.S. and M.Sc. degrees in Chemistry and in Inorganic Chemistry from the University of Tabriz, Iran and from the University of Urmia in 2004 and 2006, respectively. He got his Ph.D. degree in Inorganic Chemistry from University of Tabriz, Iran in 2010. He is now an associate professor in the University of Payamenoor, Tehran. HM is now a M. Sc. student in Inorganic Chemistry in Azad University (Ardabil branch). SS is now a Ph.D. student in Faculty of Natural Science at University of Tabriz. AE got his M. Sc degree from university of Azad, Branch Of tehran in 2011.
References
Kudo H, Okuno K, Ohira S: Tritium release behavior of ceramic breeder candidates for fusion reactors. J. Nucl. Mater. 1988, 155: 524.
Wen G, Zheng X, Song L: Effects of P 2 O 5 and sintering temperature on microstructure and mechanical properties of lithium disilicate glass-ceramics. J. Acta. Mater. 2007, 55: 3583. 10.1016/j.actamat.2007.02.009
Yamaguchi T, Nair BN, Nakagawa K: Membranes for high temperature CO 2 separation: part II-lithium silicate based membranes. J. Membr. Sci. 2007, 294: 16. 10.1016/j.memsci.2007.01.028
Essaki K, Kato M, Nakagawa K: CO 2 removal at high temperature using packed bed of lithium silicate pellets. J. Ceram. Soc. Japan 2006, 114: 739. 10.2109/jcersj.114.739
Pfeiffer H, Bosch P, Bulbulian S: Synthesis of lithium silicates. J. Nucl. Mater. 1998, 257: 309. 10.1016/S0022-3115(98)00449-8
Mosqueda HA, Vazquez C, Bosch P, Pfeiffer H: Chemical sorption of carbon dioxide (CO 2 ) on lithium oxide (Li 2 O). J. Chem. Mater. 2006, 18: 2307. 10.1021/cm060122b
Ignatovych M, Holovey V, Vidczy T, Baranyai P: Spectral study on manganese-and silver-doped lithium tetraborate phosphors. J. Radiat. Phys. Chem. 2007, 76: 1527. 10.1016/j.radphyschem.2007.02.066
Kumar GB, Buddhudu S: Synthesis and emission analysis of RE 3+ (Eu 3+ or Dy 3+ ):Li 2 TiO 3 ceramics. J. Ceram. Int. 2009, 35: 521. 10.1016/j.ceramint.2007.09.107
Romanowski WR, Sokolska I, Dsik GD, Golab S: Investigation of LiXO3 (X = Nb, Ta) crystals doped with luminescent ions: recent results. J. Alloys Compd. 2000, 300301: 152.
Hreniak D, Speghini A, Bettinelli M, Strek W: Spectroscopic investigations of nanostructured LiNbO 3 doped with Eu 3+ . J. Lumin. 2006, 119–120: 219.
Yang X, Ning G, Li X, Lin Y: Synthesis and luminescence properties of a novel Eu3 +-doped γ-LiAlO2 phosphor. J. Mater. Lett. 2007, 61: 4694. 10.1016/j.matlet.2007.03.011
Ilyushin GD: Phase relations in the LiOH-TiO 2 -SiO 2 -H 2 O system at 500°C and 0.1 GPa. J. Inorg. Mater. 2002, 9: 927.
Ganesan M: Li 1- x Sm 1+ x SiO 4 as solid electrolyte for high temperature solid-state lithium batteries. J. Ionics. 2007, 13: 379. 10.1007/s11581-007-0110-2
Ganesan M, Dhananjeyan MVT, Sarangapani KB, Renganathan NG: Lithium ion conduction in sol-gel derived lithium samarium silicate solid electrolyte. J. Alloy Comp. 2008, 450: 452. 10.1016/j.jallcom.2006.10.126
Ganesan M: Synthesis and characterization of lithium holmium silicate solid electrolyte for high temperature lithium batteries. J App Electrochem. 2009, 39: 947. 10.1007/s10800-008-9754-5
Ganesan M: A new promising high temperature lithium battery solid electrolyte. J. Electrochem. Comm. 2007, 9: 1980. 10.1016/j.elecom.2007.05.012
Takeda N, Itagaki Y, Sadaoka Y: Ionic conductivity of Li x La 10- x (SiO 4 ) 6 O 3- x sinters. J. Cer. Soc. Japan. 2008, 116: 803. 10.2109/jcersj2.116.803
Victoria L, Trejo M, Fregoso-Israel E, Pfeiffer H: Textural, structural, and CO 2 chemisorption effects produced on the lithium orthosilicate by its doping with sodium (Li 4- x Na x SiO 4 ). J. Chem. Mater. 2008, 20: 7171. 10.1021/cm802132t
Naik YP, Mohapatra M, Dahale ND, Seshagiri TK, Natarajan V, Godbole SV: Synthesis and luminescence investigation of RE 3+ (Eu 3+ , Tb 3+ and Ce 3+ )-doped lithium silicate (Li 2 SiO 3 ). J. Lumin. 2009, 129: 1225. 10.1016/j.jlumin.2009.06.001
Elbatal HA, Mandouh Z, Zayed H, Marzouk SY, Elkomy G, Hosny A: Gamma ray interactions with undoped and CuO-doped lithium disilicate glasses. J. Physica B: Cond. Mat. 2010, 405: 4755. 10.1016/j.physb.2010.08.071
Deng D, Xu S, Ju H, Zhao S, Wang H, Li C: Broadband near-infrared emission from Cr 4 + -doped transparent glass-ceramics based on lithium silicate. J Chem Phys Lett 2010, 486: 126. 10.1016/j.cplett.2009.12.063
Nakazawa T, Yokoyama K, Noda K: Ab initio MO study on hydrogen release from surface of lithium silicate. J. Nucl. Mater. 1998, 258–263: 571.
Rodriguez VD, Rodriguez-Mendoza UR, Martin IR, Lavin V, Nunez P: Site distribution in Cr 3+ and Cr 3+ -Tm 3+ -doped alkaline silicate glasses. J. Lumin. 1997, 72–74: 446.
Abd E, All S, Ezz-Eldin FM: Beam interactions with materials and atoms. Nucl. Inst. Met. Phys. Res. B 2010, 268: 49. 10.1016/j.nimb.2009.09.038
Alemi A, Khademinia S, Dolatyari M, Bakhtiari A: Hydrothermal synthesis, characterization, and investigation of optical properties of Sb 3+ -doped lithium silicates nanostructures. Int. Nano Lett. 2012, 2: 20. doi:10.1186/2228-5326-2-20 10.1186/2228-5326-2-20
Fu LF, Browning ND: Defects in Co-doped and (Co, Nb)-doped TiO 2 ferromagnetic thin films. J. App. Phys. 2006, 100: 123910. 10.1063/1.2402979
XU JW, Wang H, Jiang MH, Liu XY: Properties of Nb-doped ZnO transparent conductive thin films deposited by rf magnetron sputtering using a high quality ceramic target. J. Bull. Mat. Sci. 2010, 33: 119. 10.1007/s12034-010-0016-x
Klissurska RD, Brooks KG, Reaney IM, Pawlaczyk C, Kosec M, Setter N: Effect of Nb doping on the microstructure of sol-gel-derived PZT thin films. J. Am. Ceram. Soc. 1995, 78: 1513. 10.1111/j.1151-2916.1995.tb08846.x
Griswold EM, Sawyer M, Amm DT, Calder ID: The influence of niobium-doping on lead zirconate titanate ferroelectric thin films. Can. J. Phys. 1991, 69: 260. 10.1139/p91-043
Pereira M, Peixoto AG, Gomes MJM: Effect of Nb doping on the microstructural and electrical properties of the PZT ceramics. J. European Ceram. Soc. 2001, 21: 1353. 10.1016/S0955-2219(01)00017-6
Hardtl KH, Hennings D: Distribution of A-site and B-site vacancies in (Pb, La)(Ti, Zr)O3 ceramics. J. Am. Ceram. Soc. 1972, 55: 230–231. 10.1111/j.1151-2916.1972.tb11267.x
Gutiérrez GM, Cruz D, Pfeiffer H, Bulbulian S: Low temperature synthesis of Li 2 SiO 3 : effect on its morphological and textural properties. J. Res. Lett. Mater. Sci. 2008.
Zhang B, Easteal AJ: Effect of HNO 3 on crystalline phase evolution in lithium silicate powders prepared by sol-gel processes. J. Mater. Sci. 2008, 43: 5139. 10.1007/s10853-008-2736-5
Fuss T, Moguš-Milanković A, Ray CS, Lesher CE, Youngman R, Day DE: In situ crystallization of lithium disilicate glass: effect of pressure on crystal growth rate. J. Non-Cryst. Sol. 2006, 352: 4101. 10.1016/j.jnoncrysol.2006.06.038
Soares PC, Zanotto ED, Fokin VM, Jain H: TEM and XRD study of early crystallization of lithium disilicate glasses. J. Non-Cryst. Sol. 2003, 331: 217. 10.1016/j.jnoncrysol.2003.08.075
Zheng X, Wen G, Song L, Huang X: Effects of P 2 O 5 and heat treatment on crystallization and microstructure in lithium disilicate glass ceramics. J. Acta Mater. 2008, 56: 549. 10.1016/j.actamat.2007.10.024
Mahmoud MM: Crystallization of lithium disilicate glass using variable frequency microwave processing. Virginia: Blacksburg; 2007.
Ge S, Wang Q, Li J, Shao Q, Wang X: Controllable synthesis and formation mechanism of bow-tie-like Sb 2 O 3 nanostructures via a surfactant-free solvothermal route. J. All. Comp. 2010, 494: 169. 10.1016/j.jallcom.2010.01.064
Deng Z, Chen D, Tang F, Ren J, Muscat AJ: Synthesis and purple-blue emission of antimony trioxide single-crystalline nanobelts with elliptical cross section. J. Nano. Res. 2009, 2: 151.
Grund CS, Hanusch K, Breunig JH, Wolf HU: Antimony and antimony compounds. In Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH; 2006.
De Jong BHW, Beerkins RGC, Van Nijnatten PA, Bourhis EL: Glass. In Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH; 2005.
Peiniger M, Piel H: A superconducting Nb 3 Sn coated multicell accelerating cavity. J. Nucl. Sci. 1985, 32: 3610.
Moura S, Hernane R: Melting and purification of niobium. In Single Crystal–Large Grain Niobium Technology: AIP Conference Proceedings, 927th. Melville: American Institution of Physics; 2007:165.
Zhou Y, Qiu Z, Lu M, Zhang A, Ma Q: Preparation and spectroscopic properties of Nb 2 O 5 nanorods. J. Lumin. 2008, 128: 1369. 10.1016/j.jlumin.2008.01.001
Marrero-Lopez D, Pena-Martinez J, Ruiz-Morales JC, Perez-Coll D, Martin-Sedeno MC, Nunez PJ: Boletin De La Sociedad Espanola De Structural and electrical characterisation of Nb 5+ and Cr 6+ substituted La 2 Mo 2 O 9 Cerámica y Vidrio. 2008, 47: 213.
Moritani K, Tanaka S, Moriyama H: Production behavior of irradiation defects in lithium silicates and silica under ion beam irradiation. J. Nucl. Mat. 2000, 281: 106. 10.1016/S0022-3115(00)00364-0
Lide DR: CRC Handbook of Chemistry and Physics. Boca Raton, FL: CRC Press. Taylor and Francis; 2006.
Acknowledgements
The authors express their sincere thanks to the authorities of Tabriz University for financing the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors (AA, SK, SWJ, MD, AB, HM, SS and AE) participated in the experiments and read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Alemi, A., Khademinia, S., Joo, S.W. et al. Hydrothermal synthesis and investigation of optical properties of Nb5+-doped lithium silicate nanostructures. Int Nano Lett 4, 4 (2014). https://doi.org/10.1186/2228-5326-4-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-4-4