Abstract
In situ chemical deposition sol-gel method has been used to prepare graphene oxide-BaCrO4 (GO-BaCrO4), which was then reduced to graphene-BaCrO4 (RGO-BaCrO4) under visible-light irradiation. X-ray diffraction, Fourier transform infrared spectroscopy, and transmission electron microscopy were used to study the morphological characteristics of the prepared composite material. This synthesis method offers effortless incorporation of a visible light active photocatalyst in a composite; the reduction of GO to RGO was carried out under visible light as well. Graphene sheets with high specific surface area and unique electronic properties can be used as a good support for BaCrO4 and enhance the photocatalytic activity for the degradation of methylene blue dye compared to pure BaCrO4. The poor photocatalytic activity of pure BaCrO4 is due to its fast charge recombination. Enhanced photocatalytic degradation activity is attributed predominantly to the presence of graphene, which serves as an electron collector and transporter to lengthen efficiently the lifetime of the photogenerated charge carriers from BaCrO4 nanoparticles. The modified surface of BaCrO4 acts as a good photocatalyst compared to unmodified BaCrO4. The optimum reaction time for synthesis and GO concentration in the composite to enhance photocatalytic activity are presented in this article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
During the last decades, photocatalysis has been extensively studied as a vital device for the degradation of organic dyes and pollutants in wastewater [1, 2]. Degradation of dyes by using semiconductor photocatalysts (such as TiO2, ZnO, CdS, BaCrO4, etc.) is a promising resolution for wastewater purification, but remains a great challenge [3–6]. Most of these photocatalysts work under UV light, which restricts their applications in sunlight since sunlight that reaches the Earth's surface contains only 4% UV light. Very few catalysts are active under visible light, but their efficiency is low due to fast charge recombination. Hence, attempts have been made to search newer materials or to reduce that recombination time. Among these materials, BaCrO4 (also called hashemite) with a bandgap of 2.63 eV is an attractive candidate for photocatalysis [6].
BaCrO4 is a chromate analog of barite, which is mostly used as an oxidizing agent in organic reactions. Recently, applications of BaCrO4 in photocatalysis have been proven in the degradation of methylene blue dye and mineralization of azure B [7]. Therefore, considering a safe and cost-effective photocatalyst in the visible region with enhanced activity, current obvious techniques such as doping and composite formation are used. One obvious challenge is to use graphene, a two-dimensional (2D) carbon nanostructure, as conductive carbon mats to anchor photocatalytic materials to form new composite hybrid materials with potential application in optoelectronics and energy conversion devices [8–11]. Such an attachment of photocatalyst particles on a graphene sheet may also prevent the restacking and agglomeration of graphene sheets during the reduction process due to Van der Waals interactions between them [12].
Graphene has an excellent electron accepting property; it can be expected that the introduction of graphene into the system will enhance the catalytic performance of the semiconductor [13]. Ng et al. reported the remarkable enhancement in the activity of TiO2 in the photocurrent generation and photocatalytic degradation of organic pollutants when combined with graphene [14]. The improved photocurrent generation is attributed to the ability of graphene sheets to capture and transport the charge as collecting electrodes. The faster rate of 2,4-d degradation observed with RGO-TiO2 points out the additional benefit of concentrating the organics near the photocatalyst surface. The incorporation of graphene in semiconductor photocatalyst shows remarkable enhancement in photocatalytic activity. In our previous work, graphene-loaded polymer composites of carbon nitride (g-C3N4) and poly(3-hexylthiophene) act as efficient photocatalysts for wastewater treatment; we found a threefold enhancement in the activity of the polymer composite when combined with graphene [15]. Graphene-wrapped bismuth vanadate (GW-BiVO4) photocatalyst with excellent performance has been successfully prepared via a one-step sol-gel method. The photocatalytic activity measurements demonstrate that the GW-BiVO4 photocatalyst show superior photoactivity in the degradation of methylene blue (MB) under visible-light irradiation. The significant enhancement in photoactivity can be ascribed to the efficient separation of photogenerated electrons in the BiVO4 and graphene coupling system and the concerted effects of individual components or their integrated properties [16].
Such beneficial aspects of 2D nanostructures need to be further exploited to design next-generation photocatalyst and solar cell devices. In this communication, we report the incorporation of graphene in BaCrO4 to form reduced graphene oxide-BaCrO4 composite systems (RGO-BaCrO4) via a chemical deposition sol-gel route, and its significant influence on photocatalytic degradation and removal of dye molecules from water had been studied. The RGO-BaCrO4 system displays excellent catalytic degradation of MB under visible-light irradiation. The photodegradation mechanism was proposed to be due to the promotion of charge separation efficiency caused by the synergy between graphene and BaCrO4.
Methods
Synthesis of RGO-BaCrO4 composites
Graphene oxide (GO) was prepared according to the modified Hummers method [17, 18]. Thirty milligrams of GO was dispersed in 50 ml of double-distilled water by sonication for half an hour to obtain a clear brown dispersion of graphene oxide. Potassium chromate (0.1 M) was added to the dispersion, and the mixture was stirred for about 30 min at room temperature. BaCO3 solution (0.1 M) was added to the above well-stirred reaction mixture, and stirring was continued for 12 h. It resulted into a pale green-colored product which was separated by centrifugation. The product was washed with acetone several times and dried at 45°C in a vacuum oven. Reduction of GO to RGO was carried out through a photoreduction method as reported by Ng et al. [14]. When composite particles are dispersed in ethanol and irradiated with visible light, on the surface of BaCrO4, electron-hole pairs are generated, and the positive holes are consumed by ethanol hole scavenger and photogenerated electrons to be injected into GO which carry out reduction. Continuous irradiation for about 30 min forms a dark-colored solution.
For comparison, pure BaCrO4 was prepared using the same sol-gel method without the addition of GO. The RGO-BaCrO4 composites with different amounts of GO (0, 10, 20, 30, and 40 mg) were prepared under the same conditions.
Characterization
Wide-angle (51 to 801, 40 kV/200 mA) powder X-ray diffraction (XRD) measurements were carried out by a polycrystalline X-ray diffractometer (X'Pert Pro, PANalytical, Almelo, The Netherlands). Fourier transform infrared (FT-IR) spectra were recorded with a FT-IR spectrophotometer (Model-Alpha, Bruker, Ettlingen, Germany). Transmission electron microscopy (TEM) image was obtained using a transmission electron microscope (CM200 TEM, Philips, Amsterdam, Netherlands). UV-visible absorption spectra were recorded with a UV-vis spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). Photoluminescence (PL) spectra were obtained using a spectroflurophotometer (RF-5301 PC, Shimadzu).
Evaluation of photocatalytic activity
The relative photocatalytic activities of the prepared materials (RGO-BaCrO4 composites and pure BaCrO4) were evaluated by photodegradation of MB. An amount of 100 mg of the composite photocatalyst was dispersed in 100 ml of MB solution (1 × 10−5 M) to achieve a concentration of 1 mg ml−1 (for every photodegradation experiment, the amount of the overall photocatalyst is the same). The mixed suspension was first stirred in dark for 30 min to reach the adsorption-desorption equilibrium of the MB dye. A Philips lamp (40 W/230 V) was placed 10 cm away from the reaction vessel, which was used to provide a full-spectrum emission without any filter to simulate the sunlight source. The photocatalytic reaction was started by turning on the Philips lamp. Three milliliter of the aliquot was extracted at various irradiation times and centrifuged to remove the photocatalyst. The concentration of residual MB in the upper clear layer was determined by recording the maximum absorbance of MB at 663 nm with the UV-vis spectrophotometer.
Results and discussion
Modified Hummers method was employed to synthesize GO by oxidizing graphite using concentrated sulfuric acid and potassium permanganate. XRD patterns of the prepared materials, i.e., RGO-BaCrO4, GO-BaCrO4, and GO, are shown in Figure 1 (patterns a, b, and c). The XRD pattern of the sol-gel product (RGO-BaCrO4) is shown in Figure 1 (pattern a). The XRD pattern of GO corresponding to the (002) inter-plane (Figure 1, pattern c) shows a dominant diffraction peak centered at 2θ = 10.17, indicating that a considerable oxidation of graphite has taken place.
All the diffraction peaks of BaCrO4 that match the standard JCPDS data (JCPDS 35-0642) belonging to the single phase of barite BaCrO4 are present in both the bare BaCrO4 and GO-BaCrO4 compounds. The high-intensity characteristic peak of GO centered at 2θ = 10.17 corresponding to the (002) plane is completely reduced in the RGO-BaCrO4 composite after the photocatalytic reduction of GO-BaCrO4. Graphene layers have intrinsic nanocurvature distortions [19, 20] existing in a two-dimensional single crystalline structure; the interlayer distance of graphene, i.e., d, is slightly larger than that of bulk graphite. The reported d of graphene and graphite is approximately 3.4 [21] and 3.348 to 3.360 Å [20], respectively. Graphene oxide has the largest interlayer distance because of its intercalated H2O molecules and various oxide groups. The interlayer distance of GO is in the range of approximately 5 to 9 Å, depending on the number of intercalated water molecules. It is rational to assume that the interlayer distance order is GO > graphene > graphite. In the case of composite, the interlayer distance is found to be approximately 8.3 Å. This interlayer distance is greater than that of pure graphene but nearly equals that of GO; this might be due to the incorporation of BaCrO4 within the layers of graphene. The d value of BaCrO4 is found to 7.9 Å, calculated by considering the highest intensity index peak. When this BaCrO4 molecule incorporated between two layers of the graphene due to Van der Waals interaction, it separates the two layers by approximately 8.3 Å. These results explain why the interlayer spacing increased in the RGO-BaCrO4 composite. Some reports show that during the reduction of GO to RGO, a small hump appears between 2θ = 20 and 25°, but in this type of composite, the possibility of the small hump formation is ruled out since majority of high-intensity characteristic peaks of BaCrO4 are lying in the same region. This result shows that the layered GO has been exfoliated and that precursors adsorbed on the surface of graphene oxide and then the nucleation and growth of BaCrO4 crystallites took place in the lamellar structure of the graphene oxide, forming the GO-BaCrO4 composite which finally reduced to RGO-BaCrO4 composite. The absence of GO diffraction peak in the XRD pattern of RGO-BaCrO4 shows that the exfoliation of GO takes place during the synthesis.
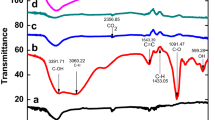
To study the nature of interaction between the graphene layer and BaCrO4, we performed FT-IR analysis. Figure 2 (spectra a, b, and c) shows the FT-IR spectra of BaCrO4, RGO-BaCrO4 composite, and GO. Comparing the FT-IR spectra of RGO-BaCrO4 composite and GO, a decrease in intensity of the representative absorption peaks of GO is observed. A small peak at 3,362 cm−1 (O-H stretching vibration), 1,713 cm−1 (C=O stretching of COOH groups), 1,632 cm−1 (C=C stretching vibration), and 1,143 cm−1 (tertiary C-OH groups stretching) dramatically decreased or even disappeared after photoreduction, indicating that the oxygen-containing functional groups in GO were reduced under visible-light environment. These functional groups hinder the charge transformation from BaCrO4 to GO layer. Hence, for better photocatalytic activity, we need to convert GO into RGO. The absorption band at 1,631 cm−1 observed in the FT-IR spectrum of the sol-gel product can be assigned to the skeletal vibration of graphene sheets [22]. The reduction in the intensity of BaCrO4 characteristic peak at 851 cm−1 in the RGO-BaCrO4 composite confirms that chemical interaction occurred during the composite formation reaction. The appearance of a new peak shown by a square at 2,312 cm−1 may be due to an effective charge transfer between BaCrO4 and RGO [23]. Gradual development of charge transfer peak at 2,312 cm−1 is presented (Additional file 1: Figure S1). Although reduction of graphene oxide can be achieved by using reductants like hydrazine, hydroquinone, NaBH4, and so on, it is claimed that the photoreduction is more effective in lowering the oxygen defect levels in graphene [24].
The chemical interaction between BaCrO4 and RGO influences the overall structure of the composites. The porosity of RGO-BaCrO4 composite (Table 1) is enhanced compared to the individual materials. This is related to the new pore space formed at the BaCrO4/RGO interface as a result of the chemical interactions. The improved porosity in the case of BaCrO4-RGO is due to reduction of oxygen-containing functional groups. The porous nature of the composites was seen clearly in the SEM images (Additional file 1: Figure S2). The FT-IR, XRD, and BET surface area analysis results reveal that the graphene oxide reacts with BaCrO4 and forms a composite by sol-gel reaction.
The morphology of RGO-BaCrO4 composites was characterized by TEM. It can be observed from the TEM images (Figure 3a,b,c,d) that the graphene sheets are covered densely with BaCrO4 crystals. However, a single BaCrO4 particle is clearly distinguished. The TEM images also present the structure of composites in which the graphene sheets are densely decorated with BaCrO4 particles. The graphene fringe can be explicitly identified (Figure 3b), convincing us of the presence of graphene under the BaCrO4 layer. The BaCrO4 particles exhibit barite structure and a narrow size distribution with an average crystallite size of 55 nm. Mixing the two starting materials by mechanical stirring for an appropriate time achieved the physical adsorption of BaCrO4 on the graphene oxide sheets. It is reasonable to consider that restacking of graphene sheets is well prevented due to the crystallization of BaCrO4 on the surface of graphene sheet during the sol-gel reaction. The good distribution of BaCrO4 particles and the single-layer structure of graphene will benefit the photocatalysis. The TEM image of the selected area (Figure 3c) indicates a well-defined crystallinity of BaCrO4.
Photoluminescence study was usually employed to study the excited-state behavior of semiconductor as well as its surface structure which gives information about electron-hole pair recombination after being irradiated with visible light. Figure 4 shows the PL spectra of pure BaCrO4 and RGO-BaCrO4 composite synthesized at different time intervals. An abrupt quenching in the PL intensity of BaCrO4 is observed in the spectra of RGO-BaCrO4 composites, showing that electron transfer from excited BaCrO4 to graphene took place effectively. Figure 4 shows the amplificatory image of the area in the range of 700 to 800 nm, showing that quenching extent is relative to the reaction time. Extending the time of the sol-gel reaction from 8 to 12 h decreases the emitting intensity gradually, while further prolonging the time to 14 h seems to exhibit no change. This shows that there is a contact time required to interact the BaCrO4 with graphene oxide, and that is 12 h. Under irradiation, electrons are photoexcited to the CB of BaCrO4, leaving positively charged holes in valence band (VB). In the absence of other materials, electrons will undergo a quick transition to VB owing to the instability of excited states, resulting in the emission of fluorescence and hence a low photocatalytic activity towards the degradation of pollutants. In the case of RGO-BaCrO4 composites, a heterojunction forms at the interface, where there is a space-charge separation region and electrons tend to flow from the higher to the lower Fermi level. As the calculated work function of graphene is 4.42 eV [25] and BaCrO4 has a bandgap of about 2.64 eV, graphene can accept the photoexcited electrons from BaCrO4, thus hindering the electron-hole pair recombination. As we mentioned above, moderately prolonging the reaction time may promote the bonding of BaCrO4 on graphene with Ba-C chemical interaction, leading to the formation of more homogeneous composites, and better linkage between the two materials must facilitate the electrons transfer, causing more decrease of emission [23].
Photocatalytic degradation of MB with pure BaCrO4, RGO-BaCrO4 composites, and without a photocatalyst was studied by a UV-visible spectrophotometer (Figure 5). RGO-BaCrO4 composites show enhanced degradation rate compared to pure BaCrO4. To compare the photocatalytic activities of the pure BaCrO4 and RGO-BaCrO4 composites with different reaction times, a series of photodegradation experiments were carried out using MB as a model pollutant under visible light. The comparative efficiency is presented in the Figure 6; the RGO-BaCrO4 composites with 12-h reaction time display a significantly improved MB photodegradation efficiency compared with pure BaCrO4, achieving a degradation percentage of more than 88% in 150 min. The MB photodegradation efficiency by RGO-BaCrO4 (8 h) was much lower than the 12-h composite sample which seems to be due to the instability of the sample, which may be attributed to the poor bonding between graphene and BaCrO4 (Figure 6).
The effect of graphene concentration in the composites on the photodegradation of MB has been studied. The 1-g composite sample prepared with 30 mg of GO (approximately 3.0 wt.%) presents a better photocatalytic activity than the sample with 10 mg (approximately 1.0 wt.%) and 20 mg (approximately 2.0 wt.%) of GO. However, increasing the concentration of GO to 40 mg (approximately 4.0 wt.%) does not further improve the photocatalytic activity as expected. We presume that a very high concentration of graphene exhibits a strong absorption to light, thus reducing light absorption on BaCrO4 surface, resulting in the decrease of photoexcited electrons (Figure 7). The morphology of the RGO-BaCrO4 composites after the photodegradation was characterized with TEM. It can be observed that graphene sheets are still covered densely by BaCrO4 nanoparticles, and few BaCrO4 particles exfoliate from the graphene supports. From the above discussion, we plot the detailed mechanism for the formation of RGO-BaCrO4 composite as shown in Scheme 1. No significant differences were found when comparing the FT-IR spectra of the used and unused RGO-BaCrO4 composites. This shows that the RGO-BaCrO4 composite has good recyclability (Additional file 1: Figure S3).
Conclusions
We present a simple sol-gel method to prepare RGO-BaCrO4 composites using GO, potassium chromate, and barium carbonate as starting materials. The resulting products exhibit uniform distribution of BaCrO4 nanoparticles on the graphene sheets and show enhanced photocatalytic activity towards MB degradation. The efficiency of the RGO-BaCrO4 composites depends on the duration of the sol-gel reaction. Prolonging the reaction time moderately can obtain more homogeneous products and cause more quenching of photoluminescence. The photodegradation of MB was carried out using different photocatalysts under irradiation of simulated sunlight. The RGO-BaCrO4 composites prepared with 30 mg of GO (approximately 3.0 wt.%) and 12 h of reaction time show excellent photocatalytic activity. Such interesting composites may find significant applications in both environmental protection and dye-sensitized solar cell.
Author’s information
SBG is a research student working on a major research project sanctioned by UGC New Delhi (India). He has a 3-year research experience and published three research papers at the international level. He is pursuing his Ph.D. degree at RTM Nagpur University, India. He is presently working on the development of nanoscale materials and their applications as an environmental photocatalyst. SRT is an associate professor of chemistry at the Government Institute of Science, Nagpur (India). He is also a visiting professor at the Nanotechnology Department, Science College, Nagpur (India). He obtained his Ph.D. from the Department of Chemistry, RTM Nagpur University, Nagpur, India. His main research activity includes the synthesis of advanced materials in different morphologies at the nanoscale and studying their impact on physical properties. He has published more than 25 research papers at the international level.
References
Hernandez-Alonso MD, Fresno F, Suarez S, Coronado JM: Development of alternative photocatalysts to TiO 2 : challenges and opportunities. Energy Environ. Sci. 2009, 2: 1231–1257. 10.1039/b907933e
Chun OW, Mingliang C, Kwangyoun C, Cheolkyu K, Zeda M, Lei Z: Synthesis of graphene-CdSe composite by a simple hydrothermal method and its photocatalytic degradation of organic dyes. Chin. J. Catal. 2011, 32: 1577–1583. 10.1016/S1872-2067(10)60264-1
Rehman S, Ullah R, Butt AM, Gohar ND: Strategies of making TiO 2 and ZnO visible light active. J. Hazard Mater. 2009, 170: 560–569. 10.1016/j.jhazmat.2009.05.064
Yang JL, An SJ, Park WI, Yi GC, Choi W: Photocatalysis using ZnO thin films and nano needles grown by metal–organic chemical vapor deposition. Adv. Mater. 2004, 16: 1661–1664. 10.1002/adma.200306673
Liu X, Pan L, Lv T, Zhu G, Sun Z, Sun C: Microwave-assisted synthesis of CdS-reduced graphene oxide composites for photocatalytic reduction of Cr(VI). Chem. Commun. 2011, 47: 11984–11986. 10.1039/c1cc14875c
Thakare SR, Patil SR, Choudhary MD: Undoped, single phase barite BaCrO 4 photocatalyst for the degradation of methylene blue under visible light. Indian J. Chem. Sect. A. 2010, 49: 54–58.
Pare B, Singh V, Jonnalagadda SB: BaCrO 4 assisted visible light induced advanced oxidation process for the mineralization of azur B. Indian J. Chem. Sect. A. 2011, 50: 1061–1065.
Huang X, Qi X, Boeyab F, Zhang H: Graphene-based composites. Chem Soc. Rev. 2012, 41: 666–686. 10.1039/c1cs15078b
Rao CNR, Sood AK, Subrahmanyam KS, Govindaraj A: Graphene: the new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48: 7752–7777. 10.1002/anie.200901678
Manga KK, Zhou Y, Yan YL, Loh KP: Multilayer hybrid films consisting of alternating graphene and titania nanosheets with ultrafast electron transfer and photoconversion properties. Adv. Funct. Mater. 2009, 19: 3638–3643. 10.1002/adfm.200900891
Guo CX, Yang HB, Sheng ZM, Lu SZ, Song QL, Li CM: Layered graphene/quantum dots for photovoltaic devices. Angew. Chem. Int. Ed. 2010, 49: 3014–3017. 10.1002/anie.200906291
Nethravathi C, Rajamathi M: Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 2008, 46: 1994–1998. 10.1016/j.carbon.2008.08.013
Xiang Q, Yu J, Jaroniec M: Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41: 782–796. 10.1039/c1cs15172j
Ng YH, Iwase A, Kudo A, Amal R: Reducing graphene oxide on a visible-light BiVO 4 photocatalyst for an enhanced photoelectrochemical water splitting. J. Phys. Chem. Lett. 2010, 1: 2607–2612. 10.1021/jz100978u
Gawande SB, Thakare SR: Ternary polymer composite of graphene, carbon nitride, and poly(3-hexylthiophene): an efficient photocatalyst. ChemCatChem. 2012, 4: 1759–1763. 10.1002/cctc.201200277
Gawande SB, Thakare SR: Graphene wrapped BiVO 4 photocatalyst and its enhanced performance under visible light irradiation. International Nano Letters 2012, 2: 11–18. 10.1186/2228-5326-2-11
Hummers WS, Offeman RE: Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80: 1339–1339. 10.1021/ja01539a017
Kovtyukhova NI, Ollivier PJ, Martin BR, Mallouk TE, Chizhik SA, Buzaneva EV, Gorchinskiy AD: Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11: 771–778. 10.1021/cm981085u
Fujimoto H: Theoretical X-ray scattering intensity of carbon with turbostratic stacking and AB stacking structure. Carbon 2003, 41: 1585–1592. 10.1016/S0008-6223(03)00116-7
Li ZQ, Lu CJ, Xia ZP, Zhou Y, Luo Z: X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45: 1686–1695. 10.1016/j.carbon.2007.03.038
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS: Synthesis of graphene based nanosheets via chemical reduction of exfoliated graphite. Carbon 2007, 45: 1558–1565. 10.1016/j.carbon.2007.02.034
Seger B, Kamat PV: Electrocatalytically active graphene-platinum nanocomposites. Role of 2-D carbon support in PEM fuel cells. J. Phys. Chem. C. 2009, 113: 7990–7995. 10.1021/jp900360k
Yang Y, Liu T: Fabrication and characterization of graphene oxide/zinc oxide nanorods hybrid. Appl. Surf. Sci. 2011, 257: 8950–8954. 10.1016/j.apsusc.2011.05.070
Akhavan O: Photocatalytic reduction of graphene oxides hybridized by ZnO nanoparticles in ethanol. Carbon 2011, 49: 11–18. 10.1016/j.carbon.2010.08.030
Xiong ZG, Zhang LL, Ma JZ, Zhao XS: Photocatalytic degradation of dyes over graphene-gold nanocomposites under visible light irradiation. Chem. Commun. 2010, 46: 6099–6101. 10.1039/c0cc01259a
Acknowledgments
The authors gratefully acknowledge UGC, New Delhi, (no. F-39-696/2010(SR) and F-14-11/2008(Inno./ASIST) for the financial assistance to carry out this work through the major research project and innovative programme. The authors are also thankful to SAIF, IIT Bombay, and STIC Cochin (India) for characterizing the materials and their support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SBG carried out the complete experimental part of this article. SRT drafted the experimental finding into a well-documented manuscript. Both authors read and approved the final manuscript.
Electronic supplementary material
40089_2012_68_MOESM1_ESM.doc
Additional file 1: Supporting information. FT-IR spectra of the selected area and SEM images of the composites. (DOC 230 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gawande, S.B., Thakare, S.R. Synthesis of visible light active graphene-modified BaCrO4 nanocomposite photocatalyst. Int Nano Lett 3, 37 (2013). https://doi.org/10.1186/2228-5326-3-37
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-37