Retraction
This article was mistakenly published twice. For this reason this duplicate article has now been retracted. For citation purposes please cite the original:http://www.inljournal.com/?_action=articleInfo&article=22
Abstract
Antimony-doped tin oxide (ATO) thin films were prepared by dip coating method. The effect of antimony doping on the structural, electrical, and optical properties of tin oxide thin films were investigated. Tin(II) chloride dehydrate (SnCl2·4H2O) and antimony(III) chloride (SbCl3) were used as a host and a dopant precursor, respectively. X-ray diffraction analysis showed that the non-doped SnO2 thin film had a preferred (211) orientation, but as the Sb doping concentration increased, a preferred (200) orientation was observed. The lowest resistivity (about 5.4 × 10−3 ΩΩ cm) was obtained for Sb-doped films at 2 at.%. Antimony doping led to an increase in the carrier concentration and a decrease in Hall mobility. The transmittance of ATO films was observed to increase to 96% at 2 at.% Sb doping, and then, it was decreased for a higher level of antimony doping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Transparent conducting oxide (TCO) films are widely used in a variety of optoelectronic devices such as solar cells, displays, and electrochromic devices. In recent years, there has been a growing interest in the application of TCO films as electrodes in solar cell devices. Among the TCO films, the most appropriate material for the application seems to be tin oxide films, which are chemically inert, mechanically hard, and heat resistant. In addition, they exhibit low electrical resistivity and high optical transmittance. Either doped or non-doped, tin oxide thin films can be fabricated by a number of techniques: chemical vapor deposition[1], sputtering[2], sol-gel coating[3], and spray pyrolysis[4–6]. The sol-gel method has such advantages as cheap cost and flexible deposition technique. Such properties can be improved by doping tin oxide with, for example, antimony (Sb), indium (In), or fluorine (F). In fact, by increasing the doping concentration (>2%), a degenerate semiconductor is formed, displaying higher σ values (>103 Ω−1 cm−1). Since this doping level is not too high, doped SnO2 thin films are transparent for visible light, which makes them useful for a device application point of view. Also, the nature, quantity, and structural distribution of doping are important factors for the electrical properties of SnO2[7].
The current study investigates the characteristics of Sb-doped SnO2 thin films prepared by dip coating technique. The structural, electrical, and optical properties of the thin films are examined in relation to the increase in the antimony amount.
Methods
The antimony-doped tin dioxide sols were prepared using the same methodology utilized in our previous literature[8]. The SnO2 solution was obtained by dissolving 2.01 g of SnCl4·4H2O in 25 ml of absolute ethanol. To achieve Sb doping, antimony trichloride (SbCl3) was added to the precursor solution. The amount of SbCl3 to be added depends on the desired doping concentration. The doping concentration varied from 0 to 7 at.%. The solution was stirred at 70°C for 6 h in a closed container for the homogenous mixing of the solution and then was aged in the air for 24 h, i.e., until the solid materials dissolved. The thin films were deposited by dip coating technique on glass substrates, which had been cleaned ultrasonically in acetone, rinsed in DI water, and then dried by N2 blowing. The clean glass substrates were dipped vertically and carefully into the sol, left for a short time, and withdrawn from the bath at withdrawal speeds in the range of 1 to 10 mm/s. This was followed by drying and then sintering of the films between 400°C and 550°C for periods ranging from 6 to 24 h. To obtain higher thickness films, the sequence of dipping, drying, and then dipping again was performed a number of times. However, sintering was done only after the final dipping. The thickness of the films increased almost linearly with the number of times of dipping. The films were kept at 25°C and humidity of 40% RH. All measurements were performed in the same conditions and after the final annealing. The film thickness, t, was measured with a Tencor P10 profilometer, (KLA-Tencor Corporation, Milpitas, CA, USA). The measurement accuracy of this equipment for the thickness measurement is 0.1 nm. The sheet resistance, R, of the films was measured by the linear four-point method. The electrical resistivity, ρ, was determined by the relation ρ = Rt. The optical transmittance of the films was measured using a UV-visible spectrophotometer (Cary 500, Agilent Technologies, Inc., Santa Clara, CA, USA). Crystal structure identification and crystal size analysis were carried out by X-ray diffraction (XRD) (2000, SCintag Inc., Cupertino, CA, USA) with a Cu-Kα radiation source and a scan rate of 2°/min.
Results and discussion
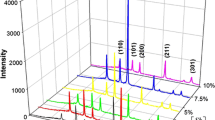
Figure1 shows the XRD patterns of the sol-gel ATO thin films with a 220-nm thickness deposited at 550°C as a function of Sb doping concentration. The preferred orientation changed with Sb doping. The non-doped SnO2 thin films had a preferred (211) orientation. However, as the Sb doping amount increased, the intensity of the (211) peak decreased and the (200) peak intensity increased.
Thus, the preferred (200) orientation was observed for Sb-doped films at 2 to 7 at.%. The preferred (200) orientation was also reported by Elangovan et al.[6]. This behavior with Sb doping implies that in the present case, antimony incorporation in SnO2 lattice has not affected the structural properties to a considerable extent. On the other hand, for higher dopant levels, the incorporation would take place at interstitial sites, and some precipitation like antimony oxides (Sb2O3, Sb2O4, and Sb2O5) could be induced[9]. As a result, with the increase of doping concentration, the deposited films lose the crystallinity, and the preferred orientation growth of SnO2 films may be suppressed by the precipitation. In the present case, antimony incorporation in SnO2 lattice has not affected the structural properties to a considerable extent. It is also clear that in Figure1, the crystallinity improves initially with antimony doping up to 7 at.% in the present case, but it decreases progressively beyond 7 at.% doping concentration, as observed by Shanthi et al.[10].
The effect of doping on the electrical properties of ATO thin films has been investigated. Figure2 shows the variation of the resistivity (ρ) with different Sb doping concentration (atomic percentage). The resistivity of ATO thin films decreases initially with an increase in the Sb doping concentration to about 4 × 10−4 Ω cm for 2 at.% of Sb but increases again for further doping. The observed minimum is significantly less than that for pure SnO2 thin films (5.4 × 10−3 Ω cm). The decrease in resistivity which may be attributed to the substitution of Sn4+ by Sb5+[10], as their ionic radii match (Sn4+ 0.071 nm and Sb5+ 0.065 nm). The variation in the resistivity of tin oxide thin films with antimony doping is explained on the basis of the presence of Sb in two oxidations states, namely Sb5+ and Sb3+. The possible mechanism may be as follows. When SnO2 is doped with Sb, a part of the lattice Sn4+ atoms are replaced by Sb5+, resulting in the generation of conduction electrons and thus the decrease of resistivity[11, 12]. Hence, a continuous decrease of resistivity is observed until Sb doping concentration ≤ 2 at.%. Beyond 2 at.% of Sb, the resistivity increases again (Figure2). This is because beyond 2 at.% of Sb doping, a part of Sb5+ ions reduces to the Sb3+ state, resulting in the formation of acceptor sites and concomitant loss carriers[13–16]. The reduction of Sb5+ to Sb3+ has been verified by Terrier et al.[12] by estimation of the lattice parameter of the doped SnO2 films. Since the ionic radius of Sn4+ is less than that of Sn3+ but higher than that of Sn5+, an increase in the lattice parameter of SnO2 phase is observed beyond 2 at.% of Sb doping. This reduction of Sb5+ to Sb3+ can be attributed to the increase in the resistivity above an optimum level of Sb doping.
It is observed that this substitution increases the carrier concentration and thereby decreases resistivity. Thus, we could obtain the thin films which have the lowest resistivity at 2 at.% Sb doping level. The resistivity ρ is proportional to the reciprocal of the product of carrier concentration n and Hall mobility μ, as in the following equation:
As shown in Figure3, Hall mobility decreased from 0.9024 to 0.2901 cm2/Vs with Sb doping. The increase in Hall mobility may be attributed to the (1) increase in the addition of antimony at the tin site and (2) a decrease in grain boundary scattering. It is known that grain boundary scattering and ionized impurity scattering are two major scattering mechanisms determining the mobility variation of such extrinsic doped semiconductors. The resultant mobility is given as follows:
where μ is the resultant mobility; μgb is the mobility due to grain boundary scattering; and μis is the mobility due to ionized impurity scattering. These observations are in close coincidence with those of Shanthi[10], Agashe[17], and Advani[18]. Figure4 shows the carrier concentration of thin films with an increase in Sb doping concentration. The substitution of Sn4+ by Sn5+ led to an increase in the carrier concentration because the radii of the two ions matched. The carrier concentration of SnO2 thin films was 2.004 × 1019 cm−3 and the value increased continuously with Sb doping to 6 × 1019 cm−3 at 8 at.% Sb doping concentration. The effect of doping on the optical properties of ATO thin films has been investigated. Figure5 shows the transmittance spectra of ATO thin films with a thickness of 220 nm in the range of 300 to 2,000 nm. Maximum transmittance is found to be 96% (at 502 nm) for the ATO film doped with 2 at.% of Sb, which is attributed to the low scattering effect and thickness uniformity of the film due to surface smoothness of the film. However, the transmittance is found to decrease gradually if the antimony concentration is increased above 2 at.%. The decrease in transmittance with the increase in dopant concentration may be attributed to the increase in cluster size and surface roughness of the film, which promotes the diffuse and multiple reflections at the surface and increases the absorption. These observations are well in agreement with the results illustrated by Advani et al. and Manifacier[18, 19], Jarzebski[20], Ambrazeviciene[21], and Shanthi[22]. In the case of heavily doped semiconductors with carrier concentration being approximately 1019 to 1021 cm−3, the Drude model can be generally used to represent the decrease in the transmittance[23–25]. Briefly, the model indicates that the transmittance drop in the near infrared region is associated with the plasma frequency (ωp) that can be expressed as follows:
where n is the carrier concentration; e, the electronic charge; ε0, the permittivity of free space; ε∞, the high-frequency permittivity; m*, the conductivity effective mass. Below the plasma frequency, the films are characterized by a high reflectance, which functions as a screen of the incident electromagnetic wave[25]. As ωp is proportional to the square root of the carrier concentration, the increase in the carrier concentration led to the lowering of the transmittance level near the infrared region.
Conclusions
Transparent conducting ATO thin films were prepared by dip coating method via sol-gel route. A preferred (211) orientation was observed for the non-doped SnO2 thin films, but as the Sb doping concentration increased, a preferred (200) orientation appeared for Sb-doped films at 3 to 7 at.%. With the increase in the Sb doping concentration, the polyhedron-like grains became rounder and smaller. The carrier concentration of SnO2 thin films was 2.004 × 1019 cm−3, and the value increased continuously with Sb doping to 6 × 1019 cm−3 at 8 at.% Sb doping concentration, while the Hall mobility decreased from 0.9024 to 0.2901 cm2/Vs. The resistivity decreased to 2 at.% Sb doping concentration; thereafter, it increased. Thus, the lowest resistivity (about 5.4 × 10−3 Ω cm) was obtained for the Sb-doped films at 2 at.%. The most effective transparent conducting ATO thin film was the Sb-doped films at 2 at.%.
Authors' information
TMH is a professor of Material Science in the Physics Department, Faculty of Science in Al-Azhar University, Gaza, Palestine. He got his Ph.D. (May 1998) in Solid State Physics from Moscow State University. His research interests include solid state physics, thin film coating, material science, and nanotechnology (nanoparticles, nanowires, nanorods). NKH is an assistant professor of Physical Chemistry in the Chemistry Department in Al-Quds Open University, Gaza, Palestine. He got his Ph.D. (February 2009) in Physical Chemistry from Ain Shames University, Cairo, Egypt. His main research areas are thin film coating and nanostructure and their characterizations.
References
Fang TH, Chang WJ: Effect of freon flow rate on tin oxide thin films deposited by chemical vapor deposition. Appl Surf Sci 2003, 220: 175. 10.1016/S0169-4332(03)00817-1
Ma J, Hao X, Huang S, Huang J, Yang Y, Ma H: Comparison of the electrical and optical properties for SnO2:Sb films deposited on polyimide and glass substrates. Appl Surf Sci 2003, 214: 208–213. 10.1016/S0169-4332(03)00344-1
Lee SC, Lee JH, Oh TS, Kim YH: Fabrication of tin oxide film by sol-gel method for photovoltaic solar cell system. Sol Energy Mater Sol Cells 2003, 75: 481–487. 10.1016/S0927-0248(02)00201-5
Fukano T, Motohiro T: Low-temperature growth of highly crystallized transparent conductive fluorine-doped tin oxide films by intermittent spray pyrolysis deposition. Sol Energy Mater Sol Cells 2004, 82: 567–575. 10.1016/j.solmat.2003.12.009
Thangaraju B: Structural and electrical studies on highly conducting spray deposited fluorine and antimony doped SnO2 thin films from SnCl2 precursor. Thin Solid Films 2002, 402: 71–78. 10.1016/S0040-6090(01)01667-4
Elangovan E, Ramesh K, Ramamurthi K: Studies on the structural and electrical properties of spray deposited SnO2:Sb thin films as a function of substrate temperature. Solid State Commun 2004, 130: 523–527. 10.1016/j.ssc.2004.03.015
Mishra KC, Johnson KH, Schmidt PC: Electronic structure of antimony-doped tin oxide. Phys Rev B: Condens Matter 1995, 51: 13972–13976. 10.1103/PhysRevB.51.13972
Hammad TM, Tamous HM, Hejazy NK: Effect of argon plasma treatment on the electrical and optical properties of sol gel antimony-doped tin dioxide thin films fabricated by dip coating. Int J Mod Phys B 2007, 21: 4399. 10.1142/S0217979207037776
Kim KH, Lee SW, Shin DW, Park CG: Effect of antimony addition on electrical and optical properties of tin oxide film. J Am Ceram Soc 1994, 77: 915–921. 10.1111/j.1151-2916.1994.tb07247.x
Shanthi S, Subramanian C, Ramasamy P: Growth and characterization of antimony doped tin oxide thin films. J Cryst Growth 1999, 197: 858. 10.1016/S0022-0248(98)01066-5
Grosse P, Schmitte FJ: Preparation and growth of SnO2 thin films and their optical and electrical properties. Thin Solid Films 1982, 90: 309–315. 10.1016/0040-6090(82)90382-0
Terrier C, Chatelon JP, Berjoan R, Roge JA: Sb-doped SnO2 transparent conducting oxide from the sol-gel dip-coating technique. Thin Solid Films 1995, 263: 37–41. 10.1016/0040-6090(95)06543-1
Kaneko H, Miyake K: Physical properties of antimony-doped tin oxide thick films. J Appl Phys 1982, 53: 3629–3634. 10.1063/1.331144
Mulla IS, Soni HS, Rao VJ, Sinha APB: Deposition of improved optically selective conductive tin oxide films by spray pyrolysis. J Mater Sci 1986, 21: 1280–1288. 10.1007/BF00553263
Nakanishi Y, Suzuki Y, Nakamura T, Hatanaka T, Fukuda Y, Fujisawa A, Shimoka G: Coloration of Sn-Sb-O thin films. Appl Surf Sci 1991, 48–49: 55. 10.1016/0169-4332(91)90307-6
Kojima M, Kato H, Gatto M: Optical and electrical properties of amorphous Sb‐Sn‐O thin films. Philos Mag B 1996, 73: 289. 10.1080/01418639609365824
Agashe C, Takwale MG, Marathe BR, Bhide VG: Structural properties of SnO2: F films deposited by spray pyrolysis technique. Thin Solid Films 1988, 17: 99–117. 10.1016/0165-1633(88)90010-X
Advani GN, Jordan AG, Lupis CHP, Longini RL: A thermodynamic analysis of the deposition of SnO2 thin films from the vapor phase. Thin Solid Films 1979, 62: 361–368. 10.1016/0040-6090(79)90012-9
Manifacier JC: Thin metallic oxides as transparent conductors. Thin Solid Films 1982, 90: 297–308. 10.1016/0040-6090(82)90381-9
Jarzebski ZM, Marton JP: Physical properties of SnO2 materials. J Electrochem Soc 1976,123(333C):199C-205C. 10.1149/1.2133010
Ambrazeviciene V, Galdikas A, Grebinskij S, Mironas A, Tvardauskas H: Gas-sensing properties of chemically deposited SnOx films doped with Pt and Sb. Sens. Actuators 1993, B 17: 27–33.
Shanthi S, Subramanian C, Ramasamy P: Investigations on the optical properties of undoped, fluorine doped and antimony doped tin oxide films. Cryst Res Technol 1999, 34: 1037. 10.1002/(SICI)1521-4079(199909)34:8
Hamberg I, Granqvist CG: Evaporated Sn‐doped In2O3 films: basic optical properties and applications to energy‐efficient windows. J Appl Phys 1986, 60: R123. 10.1063/1.337534
Marcel C, Naghavi N, Couturier G, Salardenne J, Tarascon JM: Scattering mechanisms and electronic behavior in transparent conducting ZnxIn2Ox+3 indium–zinc oxide thin films. J Appl Phys 2002, 91: 4291. 10.1063/1.1445496
Coutts TJ, Young DL, Li X: Characterization of transparent conducting oxides. MRS Bull 2000, 25: 58. 10.1557/mrs2000.152
Acknowledgments
The authors would like to gratefully appreciate the financial support from the DAAD and Saarland University, Germany. We also acknowledge the editor who made the significant revision and contribution towards our article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
NKH made the experiment on the thin films and performed tests on the samples. TMH carried out the characterization and wrote the manuscript. TMH gave the final approval of the version to be published. All the authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hammad, T.M., Hejazy, N.K. Retracted: Structural, electrical, and optical properties of ATO thin films fabricated by dip coating method. Int Nano Lett 2, 7 (2012). https://doi.org/10.1186/2228-5326-2-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-2-7