Abstract
Nanocrystalline titania-based sulfonic acid (TiO2-Pr-SO3H) was used as an efficient and reusable catalyst for the synthesis of quinoxalines. The clean and mild acidity condition, quantitative yields of products, short reaction time, and low reaction temperature are attractive features of this method, making it suitable for heat- or acid-sensitive substrates, particularly in drug synthesis. In practice, this method affords an advantageous combination of satisfactory yields, easy product isolation, and purification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Among various classes of nitrogen-containing heterocyclic compounds, quinoxaline derivatives are important components of several pharmacologically active compounds[1, 2]. These compounds are widely used as anticancer and anthelmintic agents[3], antiviral, and antibacterial[4]. Also, quinoxalines have been reported for their applications as dyes[5] and building blocks in the synthesis of organic semiconductors[6], and they also serve as useful rigid subunits in macrocyclic receptors or molecular recognition[7] and chemically controllable switches[8]. Therefore, the preparation of these compounds has gained considerable attention in recent years.
Improved methods have been reported for the synthesis of quinoxalines; some of the newly used reagents include alumina[9], montmorillonite K-10[10], sulfated TiO2[11–13], clayzic[14], zirconium (IV)-modified silica gel[15], PEG-400[16], heteropoly acid[17], ZrO2/M x O y /MCM-41[18], cellulose sulfuric acid[19], and Ga(OTf)3[20]. These procedures, although effective, have various drawbacks such as requirement of long reaction times (24 h[14]), potential hazards, difficulty in catalyst preparation (e.g., preparation of ZrO2/M x O y /MCM-41 involves a complex procedure and calcination at 500°C) and low yields. Hence, introduction of new methods to circumvent these problems is still in demand.
In recent years, there has been considerable growth of interest in the catalysis of organic reactions by solid acid catalysts. Solid acid catalysts provide numerous opportunities for recovering and recycling the catalysts from reaction mixtures. These features can lead to improved processing steps, better process economics, and environmentally friendly industrial manufacturing.
In the present study, the synthesis of chemically bound adsorbed sulfonic acid on TiO2 (TiO2-Pr-SO3H) by the reaction of (3-mercaptopropyl) trimethoxysilane and TiO2 and oxidation of the thiol groups with H2O2 is reported. The characterization of TiO2-Pr-SO3H was performed by means of various techniques such as Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and thermogravimetric analysis (TGA). The acidic property of the synthesized catalyst was evaluated by the calculation of Hammett acidity function. The assessment of the catalyst was carried out in the preparation of quinoxalines under mild conditions at room temperature (Scheme 1).
Results and discussion
In an initial study, in order to examine the catalytic activity of catalyst, we examined the reaction of 1,2-phenylenediamine (1 mmol) with benzil (1 mmol) in various solvents (EtOH, THF, MeCN, EtOAc, and toluene) and also under solvent-free classical heating conditions in the presence of different amounts of the catalyst. The best result was achieved by carrying out the reaction in the presence of 10 mg of TiO2-Pr-SO3H in EtOH (Table 1, entry 9). The feasibility of the reaction was successfully tested by producing good yields of the products using various 1,2-dicarbonyl compounds and O-phenylenediamines (Table 2). The present protocol also works well for condensation of aliphatic 1,2-diamines and 1,2-dicarbonyl compounds to give the corresponding quinoxaline derivatives in moderate yields.
Another advantage of the present methodology is the reusability of the catalyst. After completion of the reaction, the catalyst was removed by simple filtration, then washed with acetone, dried at 100°C for 2 h, and reused for three consecutive reactions without any appreciable change in its catalytic activity (Table 2, entry 1). The infrared (IR) spectra and TGA of the recovered catalyst after the third run showed the same spectra of the unused catalyst, which show that the catalyst remained unchanged after three usages.
The possible mechanism for the preparation of quinoxalines in the presence of TiO2-Pr-SO3H as a promoter is shown in Scheme 2. On the basis of this mechanism, TiO2-Pr-SO3H catalyzes the reaction by the electrophilic activation of 1,2-dicarbonyls,making these compounds susceptible to nucleophilic attack by the diamine. Successive elimination of H2O results in the formation of quinoxaline derivatives and regenerates TiO2-Pr-SO3H in the reaction mixture. A similar mechanism has been proposed for this reaction with the sulfated TiO2catalyst[10].
In order to show the efficiency of this method, Table 3 compares the results from the preparation of 2,3-diphenylquinoxaline in the presence of TiO2-Pr-SO3H and some of the other catalysts. In comparison with previously reported methods, our catalyst promotes the reaction very effectively and gives the desired product in a very short time at high yield, and very low amount of the catalyst is needed. Also, our synthesized catalyst can be recovered simply by filtration and can be reused in the next runs without a significant yield decrease of the products. Moreover, our procedure is environmentally friendly as it does not use any toxic auxiliary or solvent.
Conclusions
In conclusion, we have described a novel and efficient method for the preparation of quinoxalines catalyzed by TiO2-Pr-SO3H, as a new and efficient reagent, under mild conditions. High yields of the products, short reaction times, ease of the preparation and recyclability of the reagent, solvent-free nature of the reaction, and easy work-up are among the other advantages of this method which make this procedure an alternative to the useful available methods.
Methods
General
Chemicals were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (St. Louis, MO, USA) chemical companies. All of the yields refer to the isolated products. Identification of the products was confirmed by comparison of their physical constants with those of authentic samples. The purity determination of the substrate and reaction monitoring were accompanied by thin-layer chromatography (TLC) on silica gel POLYGRAM SILG-UV254 (Carl Roth GmbH, Karlsruhe, Germany) plates.
Instrumentation
Thermogravimetric analyses were conducted using a TGA PYRIS 1 (PerkinElmer Instruments, Waltham, MA, USA) thermoanalyzer instrument. Samples were heated from 25°C to 600°C at ramp 10°C/min under N2 atmosphere. Wide-angle XRD measurements were performed at room temperature on a Siemens D-500 X-ray diffractometer (Munich, Germany), using Ni-filtered Cu-Kα radiation (λ = 0.15418 nm). IR and FT-IR spectra were obtained in KBr wafers on Shimadzu IR-470 (Kyoto, Japan) and PerkinElmer RX1 (Waltham, MA, USA) spectrophotometers respectively. TEM analysis was performed on a Philips model CM 10 (FEI Co., Hillsboro, OR, USA) instrument. Sample was prepared by sonicating a small amount of powder in methanol and then placing a drop of the mixture on a 3-mm TEM Cu grid having a lacey carbon support film. Scanning election microphotographs were obtained on a LEO 1430VP instrument. The absorption spectra in the UV-visible regions were recorded by a PerkinElmer LAMBDA 25 recording spectrophotometer. 1H and 13C NMR spectra were measured in CDCl3 with a Brucker DRX-400AVANCE (Karlsruhe, Germany) spectrometer at 400 and 100 MHz, respectively.
Catalyst preparation
Synthesis of 3-mercaptopropyltitania
To 20 g of TiO2 (anatase, from Sigma-Aldrich) in dry toluene, 25 mL of (3-mercaptopropyl) trimethoxysilane was added, and the reaction mixture was refluxed for 24 h. After this period, the mixture was filtered to obtain 3-mercaptopropyltitania (MPT), which was washed with acetone and dried.
Oxidation of 3-mercaptopropyltitania
MPT was oxidized with 10 wt.% H2O2 in methanol (20 mL) for 24 h at room temperature. The prepared sample was then treated with 1 N H2SO4 at ambient temperature for complete protonation, and then the mixture was filtered and washed with H2O and acetone to obtain TiO2-Pr-SO3H catalyst (Scheme 3).
Catalyst characterization
FT-IR analysis
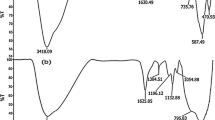
FT-IR spectra for the pure TiO2 and TiO2-Pr-SO3H samples are shown in Figure 1. In the case of TiO2, the peaks at 3,446 and 1,773 cm−1 correspond to the -OH stretching and bending vibrations of the adsorbed water, respectively. The spectrum of functionalized TiO2 by sulfonic acid displays almost the same pattern as that of pristine TiO2, but the band at 3,000 to 3,600 cm−1 that centered at 3,444 cm−1 is flattened in sulfonated TiO2 which can be attributed to the modification of TiO2. Also, CH stretching vibrations of silylating agent was observed at 2,963 and 2,908 cm−1, and the bands at 1,040 and 914 cm−1 can be collectively attributed to Si-O stretching vibrations. Furthermore, the band at 1,375 cm−1corresponds to the stretching frequency of S = O in SO3H.
Wide-angle X-ray diffraction
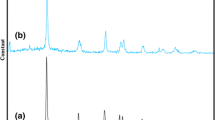
XRD patterns of TiO2 and TiO2-Pr-SO3H are given in Figure 2. XRD patterns clearly show anatase lines. It seems that the peak intensities of TiO2-Pr-SO3H are almost the same as those of TiO2, and the sulfate modification does not change the phase of TiO2. The average crystallite sizes of TiO2 and TiO2-Pr-SO3H, determined using Debye-Scherrer equation, are 15.3 and 20 nm, respectively, which demonstrated that sulfate modification increases the size of TiO2.
SEM analysis
The representative SEM images of TiO2 and TiO2-Pr-SO3H are shown in Figure 3a,b,c,d with ×30,000 and ×50,000 magnifications. All of the SEM images exhibit a cloud-like structure and small spherical-shaped particles. In TiO2-Pr-SO3H, aggregation of TiO2 nanoparticles was clearly seen. However, the SEM micrographs of the catalyst show some modifications with respect to TiO2such that the primary surface structure of TiO2 has changed.
TEM analysis
TEM images of TiO2-Pr-SO3H are shown in Figure 4. TiO2-Pr-SO3H particles are seen in different sizes ranging from 20 to 100 nm, and the average crystallite size of TiO2-Pr-SO3H, determined using XRD, was 20 nm. As can be seen in Figure 4, the particles are slightly corroded in the modification path. This is indicated in the figure by the arrow.
Thermal analysis
TGA was performed for the characterization of TiO2-Pr-SO3H in comparison with TiO2 (Figure 5). The TGA curve of TiO2 displays a weight loss (4 wt.%) below 100°C which corresponds to the loss of the physically adsorbed water. Also, there is a slight weight loss (3 wt.%) in the range of 100°C to 600°C, which possibly corresponds to the dehydroxylation of TiO2.
TGA of the catalyst shows an initial weight loss (4 wt.%) due to the desorption of water below 100°C. This is followed by a second weight loss that started at 245°C, corresponding to the loss of the covalently bound organic group. From this weight loss, it is calculated that the loading of the organic group bound to the titania surface was 2.52 mmol g−1. Also, from the TGA, we understood that TiO2-Pr-SO3H has a greater thermal stability (until 245°C) relative to TiO2.
Surface acidity studies
The Hammett acidity function (H0) can effectively express the acidity strength of an acid in organic solvents. It can be calculated using the following equation:
Here, ‘I’ represents the indicator base (mainly substituted dinitroanilines), and [IH+]s and [I]s are respectively the molar concentrations of the protonated and unprotonated forms of the indicator. The pK(I)aq values are already known (for example the pK(I)aq value of 4-nitroaniline is 0.99) and can be obtained from many references. According to the Lambert-Beerlaw, the value of [I]s/[IH+]s can be determined and calculated using the UV-visible spectrum.
In our experiment, 4-nitroaniline was chosen as the basic indicator, and CCl4was chosen as the solvent because it is aprotic. The maximal absorbance of the unprotonated form of 4-nitroaniline was observed at 329 nm in CCl4. As Figure 6 shows, the absorbance of the unprotonated form of the indicator in TiO2-Pr-SO3H was weak as compared to the sample of the indicator in CCl4, which indicated that the indicator was partially in the form of [IH+]. The results obtained are listed in Table 4, which shows the acidity strength of TiO2-Pr-SO3H.
General procedure
To a mixture of a substituted 1,2-diketone (1 mmol) and 1,2-diamine (1 mmol) in ethanol (5 mL), TiO2-Pr-SO3H (10 mg) was added. The mixture was stirred at room temperature and the reaction was monitored by TLC. After completion, the reaction mixture was heated for a few minutes. The catalyst was removed by filtration, the filtrate was concentrated, and the solid residue was recrystallized from ethanol to give the pure product.
References
Sarges R, Howard HR, Browne RC, Label LA, Seymour PA: 4-Amino[1,2,4]triazolo[4,3-a]quinoxalines. A novel class of potent adenosine receptor antagonists and potential rapid-onset antidepressants. J Med Chem 1990, 33: 2240–2254. 10.1021/jm00170a031
Brown KL: Chemistry and enzymology of vitamin B 12 . Chem Rev 2005, 105: 2075–2150. 10.1021/cr030720z
Sakata G, Makino K, Kuraswa Y: Recent progress in the quinoline chemistry: synthesis and biological activity. Heterocycles 1998, 27: 2481–2515.
Ali MM, Ismail MMF, El-Gabby MSA, Zahran MA, Ammar TA: Synthesis and antimicrobial activities of some novel quinoxalinone derivatives. Molecules 2000, 5: 864–873. 10.3390/50600864
Sonawane ND, Rangnekar DW: Synthesis and application of 2-styryl-6,7-dichlorothiazolo[4,5- b ]-quinoxaline based fluorescent dyes: part 3. J Heterocycl Chem 2002, 39: 303–308. 10.1002/jhet.5570390210
Dailey S, Feast JW, Peace RJ, Sage IC, Till S, Wood EL: Synthesis and device characterisation of side-chain polymer electron transport materials for organic semiconductor applications. J Mater Chem 2001, 11: 2238–2243. 10.1039/b104674h
Elwahy AHM: Synthesis of new benzo-substituted macrocyclic ligands containing quinoxaline subunits. Tetrahedron 2000, 56: 897–907. 10.1016/S0040-4020(99)01072-8
Crossley JC, Johnston LA: Laterally-extended porphyrin systems incorporating a switchable unit. Chem Commun 2002. 10.1039/B111655J
Jafarpour M, Rezaeifard A, Danehchin M: Easy access to quinoxaline derivatives using alumina as an effective and reusable catalyst under solvent-free conditions. Appl Catal A: Gen 2011, 394: 48–51. 10.1016/j.apcata.2010.12.022
Huang TK, Wang R, Shi L, Lu X: Montmorillonite K-10: an efficient and reusable catalyst for the synthesis of quinoxaline derivatives in water. Catal Commun 2008, 9: 1143–1147. 10.1016/j.catcom.2007.10.024
Krishnakumar B, Swaminathan M: Solvent free synthesis of quinoxalines, dipyridophenazines and chalcones under microwave irradiation with sulfated Degussa titania as a novel solid acid catalyst. J Mol Catal A: Chem 2011, 350: 16–25. 10.1016/j.molcata.2011.08.026
Krishnakumar B, Swaminathan M: A recyclable and highly effective sulfated TiO 2 -P25 for the synthesis of quinoxaline and dipyridophenazine derivatives at room temperature. J Organomet Chem 2010, 695: 2572–2577. 10.1016/j.jorganchem.2010.08.055
Krishnakumar B, Velmurugan R, Jothivel S, Swaminathan M: An efficient protocol for the green synthesis of quinoxaline and dipyridophenazine derivatives at room temperature using sulfated titania. Catal Commun 2010, 11: 997–1002. 10.1016/j.catcom.2010.04.021
Dhakshinamoorthy A, Kanagaraj K, Pitchumani K: Zn2+-K10-clay (clayzic) as an efficient water-tolerant, solid acid catalyst for the synthesis of benzimidazoles and quinoxalines at room temperature. Tetrahedron Lett 2011, 52: 69–73. 10.1016/j.tetlet.2010.10.146
Sharma RK, Sharma C: Zirconium(IV)-modified silica gel: preparation, characterization and catalytic activity in the synthesis of some biologically important molecules. Catal Commun 2011, 12: 327–331. 10.1016/j.catcom.2010.10.011
Zhang XZ, Wang JX, Sun YJ, Zhan HW: Synthesis of quinoxaline derivatives catalyzed by PEG-400. Chin Chem Lett 2010, 21: 395–398. 10.1016/j.cclet.2009.12.015
Heravi MM, Bakhtiari K, Bamoharram FF, Tehrani MH: Wells-Dawson type heteropolyacid catalyzed synthesis of quinoxaline derivatives at room temperature. Monatsh Chem 2007, 138: 465–467. 10.1007/s00706-007-0594-5
Ajaikumar S, Pandurangan A: Efficient synthesis of quinoxaline derivatives over ZrO 2 /M x O y (M = Al, Ga, In and La) mixed metal oxides supported on MCM-41 mesoporous molecular sieves. Appl Catal A Gen 2009, 357: 184–192. 10.1016/j.apcata.2009.01.021
Shaabani A, Rezayan AH, Behnam M, Heidary M: Green chemistry approaches for the synthesis of quinoxaline derivatives: comparison of ethanol and water in the presence of the reusable catalyst cellulose sulfuric acid. C R Chimie 2009, 12: 1249–1252. 10.1016/j.crci.2009.01.006
Cai JJ, Zou JP, Pan XQ, Zhang W: Gallium(III) triflate-catalyzed synthesis of quinoxaline derivatives. Tetrahedron Lett 2008, 49: 7386–7390. 10.1016/j.tetlet.2008.10.058
Acknowledgments
The authors acknowledge the editor who made the significant revision and contribution towards improving the standard of our article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Both authors - SVA and SSB - contributed equally in this article. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Atghia, S.V., Beigbaghlou, S.S. Nanocrystalline titania-based sulfonic acid (TiO2-Pr-SO3H) as a new, highly efficient, and recyclable solid acid catalyst for preparation of quinoxaline derivatives. J Nanostruct Chem 3, 38 (2013). https://doi.org/10.1186/2193-8865-3-38
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-38