Abstract
Background
In the years 1970, 1980, 1985, 1995, 2005 and 2010, lichen mapping in the towns of Wetzlar and Giessen in Hesse was performed. The aim was to show the effects of immission load. Despite the application of modified test methods during the study period, the results can still be compared directly because they could be adapted to the requirements of the guidelines of the Association of German Engineers. Even the earlier study results could be interpreted partly within the scope of the guidelines. Parallel to the lichen mapping, comparative examinations of pH on tree bark were carried out.
Results
In both towns, the pH of the tree bark has been increasing continuously, presently almost reaching pre-industrial values. The increase was stronger in Wetzlar than in Giessen. In 1970, the lichen vegetation showed a complete depletion. Since then the number of species has risen significantly. This development happened faster and more intensely in Wetzlar. The comparison of the lichen vegetation between 1970 and 2010 also shows an increased existence of species that are favored by hypertrophic air contaminants. In the 2010 survey, some species considered as being promoted by global warming were found for the first time.
Conclusions
The increase of the bark pH can be explained by the decreasing acid pollution, primarily SO2. This is also assumed to be the main cause for the increasing number of lichen species. An explanation for Wetzlar's advantage is that the acid pollutants had been neutralized by a local industrial emittent of lime dust. With the reduction of dust emissions in Wetzlar due to the installation of filters, the bark pH in both towns steadily converged in the reference period. An examination of pre-industrial lichen studies shows that in both towns the original state of lichen vegetation has not been restored yet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
The use of lichens as bioindicators to assess air pollution has been a long tradition in Europe. Nevertheless, there are rare long-term mappings of lichens under standardized conditions. Comparative assessments of the air pollution situation are also hardly possible; as in previous studies, different methods have been observed, and the transfer to modern, regulated and standardized methods[1] fails as the original data are no longer available. The towns of Central Hesse, Giessen and Wetzlar, represent a positive exception from these points: they have been mapped repeatedly at irregular intervals since 1970, and the original survey data of the respective investigations are still available. In our study areas, the surveys were not all conducted using the same methodology. Thus, in the first mapping in 1970 for example, it was only distinguished whether crustose, foliose or fruticose lichens could be found on the studied trees[2, 3]. Therefore, we can only make a statement about those areas where only crustose lichens occurred, which at that time was almost exclusively the acid-enduring and toxitolerant species of Lecanora conizaeoides. The sole occurrence of this species indicates, by the standards of today's Association of German Engineers (VDI) guideline interpretation, an extremely high immission load with acidic pollutant gases - primarily SO2. Besides the recording of the lichen vegetation from 1970 in Giessen and 1985 in Wetzlar, measurements of the pH of the bark of the mapped trees were made, so that, in this case, a comparison with our current studies of 2010 was possible[4–7].
The present report describes the development of the lichen vegetation and bark pH in both towns on free-standing deciduous trees in the period between 1970 and 2010. The analysis of the lichen data were performed according to the currently valid guideline VDI 3957 Part 13[1]. Additionally, statements regarding the development of the climatic situation are made.
Methods
Sampling grid
A grid consisting of sampling units of 1 × 1 km was applied to the urban districts of Wetzlar and Giessen[1, 5–9]. The examination of lichens was carried out on 6 to 12 suitable trees/sampling unit. The number of tested units varied (increasing the tendency) from one survey to the next. For reasons of comparability, only those 34 units in Wetzlar and 29 areas in Giessen which have been mapped together in all survey years were evaluated.
Selection of trees
The majority of the trees met the requirements of the current VDI guideline[1]; they belong to the group of tree species which have subneutral bark under natural conditions (exceptions: some few trees, such as Quercus spp. and Robinia pseudoacacia). Trees that are oblique, injured, scrubbed or treated with herbicides or fungicides were usually excluded from the beginning - in accordance with the requirements of the guideline. In the first three study periods, the number of mapped trees varied; since 1995, the average number is 9.4 trees/sampling unit. Over the long study period, a decrease of Malus and Populus in favor of Acer Fraxinus and Tilia has to be stated. The percentage distribution of the trees examined in 2010 is reproduced in Figure1.
Recording of lichens
The lichen mapping procedure used in 1970 differed from the later ones as follows: half the circumference of free-standing deciduous trees that was most covered with lichens had been examined on their lichen growth from 50 to 200 cm from the ground[2, 3]. In addition, in these early days of bioindication with lichens, only crustose, foliose and fruticose lichens were distinguished from each other. Only since 1985, a quantitative recording of the existent species with the help of a lichen monitoring quadrat (Figure2; in VDI 3957 Part 13 Annex A[1]), was done.
A direct comparison of the first mapping with the following ones was not possible because of this strong deviation from subsequent methods. Therefore, only those areas from this early study were cartographically presented in which no lichens (lichens desert because of extremely high air pollution) or only the species of the toxitolerant L. conizaeoides, whose presence also indicates a very high air pollution load, were found. In 1970, its sole occurrence in some units signals a very unfavorable air quality situation, which probably no longer occurs in Central Europe today (and is therefore no longer considered in the current VDI guideline).
Since 1985, a monitoring quadrat has been fixed to the tree trunk, allowing a quantitative detection (frequency) of the occurring lichen species[5–9]. The monitoring quadrat has a size of 20 × 50 cm (Figure2) and is applied to the most lichen-covered side of the trunk. For comparability, this grid was used in all examinations. The VDI guideline supports this method for long-term studies (in VDI 3957 Part 13 Annex A[1]).
Calculation of the air quality index ‘LGI’
According to the guideline, the LGI is determined by the number (diversity) and the incidence (frequency) of lichen species within the monitoring quadrat[1]. In this method, a frequency-weighted lichen diversity value is taken as a criterion for evaluating the air quality situation within the sampling unit.
For a couple of years, the lichen vegetation is dominated by hypertrophication-enduring species as a consequence of the effects of airborne nitrogen compounds. The overly rich nutrient supply has negative effects on the biodiversity of plants, animals and fungi in ecosystems, so that the occurrence of hypertrophication-indicating lichens is rated negatively. For this reason, the diversity values for hypertrophication-indicating lichens and for all other species (here called reference species) were calculated separately.
Data interpretation
The assessment of a sampling unit and thus its ‘air quality index’ results from the combination of the values of diversity for the indicators of hypertrophication and the reference species (Figure3). For example, within a fictitious unit area ‘X’ with a lichen diversity value of 16.5 for the reference species and 10.7 for the hypertrophication-indicating species, the intersection is represented by the magenta point. The sampling unit would therefore be given the color green.
Assessing air quality by combining diversity values of both reference species and indicators of hypertrophication. Modified from VDI 3957 Part 13[1].
In the guideline, the air quality is verbally classified as ‘very high’, ‘high’, ‘medium’, ‘low’ and ‘very low’; the LGI is numbered. The LGI is composed of two digits. The first digit of the index stands for the overall air quality and is coupled with the colors; the second digit (after the dot) is directly related to the diversity of the indicators of hypertrophication. The verbal assessment of the air quality index is compiled in Additional file1; some examples are given in Table1.
Using lichens as indicators of climate change
For the use of lichens as indicators of climate change, the records of the mapping of air quality were re-evaluated. The analysis was performed using lichen indicator values[10]. The indicator values provide information about the ecological requirements of a species with respect to certain environmental factors. They include a nine-step scale with 1 being the lowest and 9 the highest degree of the respective factor. Indicator values for lichens exist for the ecological factors light, temperature, continentality, moisture, reaction and nitrogen. For the evaluation of the climatic situation, the average indicator values of temperature and humidity were calculated for each measurement period since 1985.
In addition the climate indicator value of lichens was used for evaluation[11]. It is a combination of the indicator values for temperature and continentality and thus integrates both ecological indicator values. The climate indicator values of the lichens within a measuring surface are averaged and result in a climate index.
Measurement of bark pH
The bark pH was measured in the years 1970, 1985, 2005 and 2010 using the following method[4–7]:
-
The removal of the bark samples for the pH measurement (normally mixed samples from 10 trees of a species) was carried out on those trees of a study site on which lichens have been mapped.
-
If more than one tree species was mapped, the sampling was done separately for each tree species.
-
The samples were removed from the trunk 1 to 1.5 m in height, outside the monitoring quadrat, using a knife.
-
The thickness of the collected pieces of bark was <3 mm.
-
The bark was dried at room temperature and then stored at −20°C.
-
Dust, algae and lichens were removed from the bark with a brush.
-
The composite sample of a unit of investigation was crushed and homogenized in a mill.
-
Every 2 g of the powdered sample were shaken overnight with 15 ml of demineralized water.
-
The pH was measured using a pH meter.
Results and discussion
Temporal change in the bark pH
For reasons of better comparability, only those sampling units were included which had been sampled over the entire study period from 1970 to 2010. Often, even the examined trees were identical.
The results show a marked increase in the pH of all the species of trees that are given (Tilia spp., Populus spp., Malus domestica) and also in both investigated towns (Figure4). However, the initial pH of the various species of trees is different. While the limes - species with naturally acidic bark - started at a low pH, poplars and apple trees had higher pH even during times of high acid immission load. The reason therefore is the better buffering capacity of the poplars and apple trees in comparison with the lime.
In Giessen, where pH measurements on lime already exist from 1970, it is visible that the rise from 1970 to 1985 was insignificant. Since 1985, however, the pH has become markedly higher. This phenomenon can be easily explained by the evolution of the sulfur dioxide concentration (Figure5). Until 1987, the SO2 levels were high and fairly constant but then dropped drastically due to enhanced measures for the reduction of emissions and have stabilized at a low value since 2000.
Annual mean values of sulfur dioxide concentration in Wetzlar and Giessen. There are no data since 2004 for Giessen[12].
After the decline in the acid deposition, the pH of the bark has nearly achieved natural values. Since 2005, its further increase might also have another reason: the trend in the lichen abundance suggests that there is an increase of the effects of airborne nitrogen compounds (especially NH3). An increase in ammonia concentration leads to an increase of the pH of the tree bark.
Noteworthy is the differential development of bark pH in Wetzlar and Giessen. In Wetzlar in 1985, the acidification of all tree species was significantly lower than in Giessen, despite the comparable SO2 immissions in both towns. This phenomenon can be explained by a peculiarity pollution situation in Wetzlar. In addition to the acidic immissions, there were lime dust-emitting sources in the north of Wetzlar. In 1970, their basic dusts neutralized the acidic emissions partly leading to a reduced acidification of the local tree bark. Thus, it was not surprising that the first neutrophytic lichens (e.g., Physcia tenella[3]) were found in the north-east of Wetzlar, in the lee of the sources.
In Giessen, the dust deposition has only been measured since 1987. Based on the measurements of other comparable Hessian towns though, (e.g., Kassel[12, 13]) we can assume that the dust deposition in Giessen in 1970 was lower than that in Wetzlar. It was probably less than 200 mg/(m2 × day) and also contained no cement dust.
After the reduction of the acidic pollutant gases and the simultaneous reduction of lime dust emissions through the installation of filters, the relationships between the two towns have converged in 2010 (Figure6[12, 13]). Since many lichens have a close bond to the substrate and its pH, the development of the bark pH will play a role in the development of the lichen vegetation.
Trend in lichen-indicated air quality situation in Wetzlar and Giessen
As the basic data from previous mapping examinations are still available, it was possible to compare the current results with those of earlier studies[2, 3, 5–9, 14, 15]. Over the period from 1970 to 2010, the number of lichen species shows a steady increase (Figure7). The approximate 10-fold increase in the number of species is a first indication of an improving air quality throughout the study period. For methodological reasons, it is not possible to differentiate between the species numbers of Wetzlar and Giessen in the first two studies. Until 1985, a difference between the species numbers of Wetzlar and Giessen is assigned. This imbalance to the disadvantage of Giessen will be retained until 2010 but with a much weaker intensity recently. Giessen gradually catches up with Wetzlar concerning the lichen diversity. Additionally, there has been a conspicuous increase in the number of species in the short period between 2005 and 2010 in both towns.
Figure8 shows the development in Wetzlar. In the period around 1970, the immission load with the components sulfur dioxide and lime dust was extremely high (Figures5 and6) so that in the industrial district in the north of Wetzlar, no lichens were found at all - there existed a ‘lichen desert’. Adjacent to the lichen-free zone, only the crustose lichen L. conizaeoides was recorded in almost the entire town of Wetzlar. The sole occurrence of this species shows an extremely high air pollution. For 1970, a further lichen-based differentiation of the former air quality outside the ‘Lecanora zone’ is not possible due to methodological reasons.
Such unfavorable conditions, however, could also be identified in Frankfurt/Main, 70 km away from Wetzlar. In the period around 1970, the ‘lichen desert’ in Frankfurt was even larger than that in Wetzlar and affected the town center[16].
In 1985, the colors red and orange still dominated. At the outer edge of the study area, there were, however, already sampling units with medium and, in the north-west, even an area with high air quality. The significant improvement in the ambient air quality is obvious, and the average air quality index (LGI) value is 2.1 (Table2). At this point, the studied areas have an average hypertrophication index of 1.2, which shows a very small effect of nutrient pollution.
In 1995, there was only one red-colored unit in the far north of Wetzlar. Otherwise, low to moderate air quality conditions dominated; in the south, there are already two areas with high air quality indices. The sampling unit most severely affected is still located in the town's center and in the north industrial area of Wetzlar. The average hypertrophication index value increases from 1.2 to 1.5, which could be an evidence for an increase in the load caused by airborne fertilizer materials.
By 2005, the situation had again improved as 10 sampling units in the control area were given the color green, indicating a high level of air quality in these areas. However, there is also a distinctive increase in indicator species for hypertrophication (Table2), especially in the north of the investigated area.
In the survey year 2010, no area is associated with the color red anymore, but on the other hand, the number of units with the LGI 4 (green) has increased, compared with that in 2005, from 10 to 17. This means that half of the measuring units have a high air quality now. From 2005 to 2010, the hypertrophication index increased only moderately because the effect of eutrophicating air pollutants in Wetzlar is still low.
A basically similar pattern to Wetzlar can also be seen in Giessen, albeit at a lower level (Figure9). Although there were no units without lichens in 1970, large parts of the study area were inhabited only by the toxitolerant and acidophytic L. conizaeoides (color magenta).
In 1985, the situation improved significantly, although there were still two units regarded as highly charged. The average air quality index of 1.1 is a whole level lower than the corresponding initial value in Wetzlar. Hypertrophication indicating lichen species were extremely rare; according to this, the average of the hypertrophic index is 1.0.
The further improvement of the air quality until the year 1995 was not as fast and was not to such a great extent, as observed in Wetzlar. On the other hand, the average hypertrophication index remained at a lower value than in Wetzlar (Table2).
By 2005, Giessen had caught up on Wetzlar: nearly two thirds of all the investigated areas measured only medium to low loads (in Wetzlar, these are at the same time about 80 % of all comparison areas). The mean hypertrophication index had increased slightly but still remained well below that of Wetzlar.
By 2010, Giessen was on the same level as Wetzlar in terms of its air quality: there was neither a red nor an orange sampling unit. The improvement is much more obvious than in Wetzlar. Although the number of areas with green color was still lower than that of Wetzlar, there already was a blue-colored area in Giessen, which shows a very high air quality.
Table2 summarizes the trends of air quality indices and the effects of the hypertrophication of Wetzlar and Giessen from 1985 to 2010. Again, the initial advantage in Wetzlar's lichen-indicated air quality in comparison to Giessen is shown. It is clear, however, that Giessen continuously caught up after the sulfur dioxide played no more essential role in air pollution in both towns (Figure9). Thus, the original advantage of Wetzlar due to its lime dust neutralizing the SO2 immissions ended. Both towns have improved their air quality by 2010. With the increase of the air quality index values, the hypertrophic index values in both towns rose. Considering, however, the maximum values of the year 2010, one might conclude that the conditions in the two Central Hessian towns in terms of hypertrophication risk from airborne nitrogen compounds are still considered to be low to medium.
A comparison of the percentage distribution of the five air quality categories in Giessen over the survey period (Figure10) shows that sampling units with very low air quality (color red) continuously decline and no longer appear in the current study. Conversely, the number of units with high air quality increases. In 2010, for the first time, even a blue-colored measuring unit appears. The results for Wetzlar show a similar pattern.
Evolution trends of different lichen species between 1985 and 2010
Between 1985 and 2010, there were some characteristic differences in the development of the species, which shall be illustrated by typical examples (Figures11 and12).
The development of the frequency of L. conizaeoides is shown in Figure11. This toxitolerant and strongly acidophytic species shows the decrease of acid emissions at best. In Wetzlar as well as in Giessen, it was the most common - and sometimes only - lichen at times of high SO2 pollution. Until 1995, its frequency declined due to the reduction of sulfur dioxide. However, this happened in Wetzlar much more strongly than in Giessen. In 2005, L. conizaeoides could hardly be found in Wetzlar, and the further decline is also visible in Giessen; in 2010, it had virtually disappeared. The behavior of this lichen correlates very well with the reduction of SO2 concentration in the period shown here (Figure5). The stronger decline of the species in Wetzlar can be interpreted as follows: in 1985, the concentration of SO2 was still so high that the basic lime dust could hardly reduce the effect of bark acidification. With the following decline of acidic pollutants, the pH of the tree bark increased more quickly due to the more effective neutralizing of the limestone dust. In the period from 1985 to 2010, the acidophytic lichen Hypogymnia physodes showed a similar distribution pattern.
X. parietina shows a different behavior (Figure11). In 1985, this neutrophytic and nutrient-loving lichen species was very poorly developed in both towns due to the acidic pollutant gases. The following increase was hesitant in 1995. Measuring units that were occupied in 1985 remained without evidence of the species in 1995, even though it was found in other units. In 2005, it had established itself in many areas, and the frequency at the examined trees was increased significantly. The well-known preference of X. parietina for higher pH manifests itself clearly in Wetzlar. From the beginning of the study, it preferred the lime dust-affected north of the town, and its distribution pattern was complementary to that of the previously described acidophytes H. physodes and L. conizaeoides. In 2010, both in Wetzlar and Giessen, the number of populated areas increased in comparison to 2005 again, and their frequency on the trees rose. Other indicators of hypertrophication like X. candelaria, X. polycarpa, Phaeophyscia orbicularis, Physcia adscendens and P. tenella increased considerably in this period. Typical of these species is that they emerged first in the north of Wetzlar near the lime dust emitters due to their high bark pH claims.
Although the pattern of the development of Parmelia sulcata (Figure12, moderately acido- to subneutrophytic, moderately sensitive) at first glance seems to be the same as the one of the hypertrophication indicators, upon closer examination, differences can be detected. P. sulcata avoids explicitly - and including in 2010 - the towns' centers. In Wetzlar, the living conditions for the species are obviously better in the south, from which one could derive acidophytic features. However, while the acidophytes declined during the study period (see L. conizaeoides, Figure11), P. sulcata became more common in both towns. This species belongs to a large lichen group that prefers average bark pH conditions. It tolerates only moderate hypertrophication and has a modest sensitivity to air pollution. Thus, P. sulcata is a typical representative of the ‘reference species’ whose occurrence indicates a favorable air quality. Other representatives of this group (with a similar distribution pattern) are Melanohalea exasperatula, Melanelixia glabratula, and Melanelixia subaurifera. These species are slightly more sensitive than P. sulcata; they were first found with some single individuals in a few areas measured in Wetzlar in 1995. In Giessen, they were not found before 2005, but by now, they populate many sampling units there.
Species such as Ramalina farinacea (Figure12) indicate an even higher degree of air quality. Until 1995, they had no possibilities of existence because of the unfavorable immission situation in Wetzlar and Giessen. Throughout the following years, they started settling in both towns, though hitherto only with a few individuals. R. farinacea is a typical representative of a rather demanding species in terms of air quality. It is one of the few fruticose lichens which have returned so far. Occasionally, one now finds representatives of the genus Usnea in both towns. They are so sensitive to air pollution that they were initially only found in a few and very small individuals (and therefore hard to determine). Based on the fact that these species appear every once in a while in a measuring area but disappear in the following mapping and emerge in another one, it can be concluded that the air quality in the study areas of Wetzlar and Giessen is still slightly below the possibilities of existence for R. farinacea and species of the genus Usnea. A few years ago, similar observations were made, for example, for the fruticose lichen Evernia prunastri; today, this species has firmly anchored in Central Hesse even within the towns. The further observation of ‘pioneer species’ is of particular interest as they will clearly show an improvement of the air quality.
Use of lichen indicator values
Surveys in the field have shown that each lichen species has certain demands to abiotic environmental factors (such as acidity or nutrients of their substrates). Consequently, species prefer environmental conditions that meet these requirements and avoid those that do not fit them. Conversely, the presence and quantity of these factors can be logically deduced from the occurrence of a species with specific environmental requirements. With the use of lichens as indicators of environmental characteristics, one should, however, be aware that their predictive value relates to the average and long-term quality and quantity of environmental parameters in the investigated sites. Additionally, interactions of several environmental factors may affect that result.
The ecological behavior is valuated according to a nine-point scale, with 1 being the lowest and 9 the largest scale means of the respective factor[10]. Average indicator values for a sampling unit can be calculated both unweightedly (only assessing, if the species occur or not) and weightedly (including their frequency).
The temporal development of the reaction value, which allows a statement on the acidity of the substrates colonized by lichens, is shown in Figure13. The increase of the lichen reaction value follows the bark pH (Figure4). Since 1985 the pH has gradually increased almost back to pre-industrial values as a result of the decreasing acid immissions, thereby allowing for the reintroduction of subneutrophytic and neutrophytic lichen species. As demonstrated by the lichen reaction values the largest pH-increase occurred between 1985 and 1995, related to the recently drastically reduced SO2 concentrations (Figure5).
As can be seen in Figure14, changes in the composition of lichens could be caused by a higher load of hypertrophicating air pollutants. This is supported by the increase in nutrient indicator values. In Figures8 and9 (also in Table2), we have already pointed out the increase of hypertrophication indicating lichens in almost all measuring units. However, the value in 1985 has to be queried. It is conceivable that at that time, due to the high SO2 levels, hypertrophication-indicating species could not yet exist despite already existing exposure to nitrogen compounds.
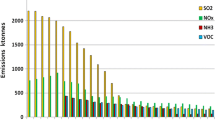
As a main cause for the hypertrophication, airborne nitrogen compounds come into question. Their concentrations, however, do not show any significant changes in the technical measurements in Hesse for the period in question[12].
One explanation for this apparent contradiction lies in the fact that technically measured nitrogen oxides have no direct relevance for the nutrition of lichens. Plants and lichens are known to metabolize especially NO3 and NH3/NH4[17–20]. These nitrogen compounds are often not detected in environmental monitoring due to technical problems in measuring. The amount of nitrogen emitted in the form of ammonia in Central Europe has approximately the same magnitude as the total emission of nitrogen in form of both, NO and NO2. The ammonia mainly comes from agricultural sources (livestock, manure spreading). It is degraded within a few hours to ammonium or ammonium salts. Therefore, elevated ammonia concentrations can only be measured in the immediate vicinity of sources. The secondary products (ammonium or ammonium salts), however, can be transported as aerosols over long distances. Due to the transport, it appears that these compounds exist over a wide area[21]. While it has been assumed that the NH3 emissions from car exhausts are rather negligible, recent studies published significant concentrations of ammonia from the exhaust gases of occupied, cold, or aged catalysts[22–25]. According to Umweltbundesamt[26], the petrol-fueled vehicles emit between 20 and 50 mg ammonia/km, depending on the catalyst type and the traffic flow. These findings would also explain why the nitrogen enrichment in lichens is particularly high in the towns, and therein mainly in the vicinity of heavily frequented roads, despite the lack of agricultural emissions[27, 28]. It is further stated that the diversity of nitrophytic lichens, starting from the edges of the roads, gradually decreases[29]. All these facts suggest a significant role of ammonia (or its reaction product ammonium) in the effects of nitrogen compounds on lichens[30]. Only until recently, a metrological reference exists to the increase of ammonia in Hesse (Figure15[31]). As demonstrated, the NH3 levels have increased in different sampling sites in Germany since 2000, amongst those for instance the site in Linden near Giessen[31]. These results provide a plausible explanation for the increase of the nutrient indicator values (Figure14).
Figure16 indeed shows an increase in the lichen diversity of reference species for the two towns, indicating an overall improvement in the air quality conditions. This increase becomes even more evident when one omits the calculation of Lecanora conizaeoides, a crustose lichen tolerant to SO2 and therefore the dominant species on tree bark until 1985. However, the simultaneous - and even higher - increase in hypertrophication indicators also points to a significant exposure to substances with nutrient effects.
Lichens as indicators of climate change
If we follow the change in the epiphytic lichen vegetation over long periods of time, it can be seen that apart from the decrease of acidophytes (prefer acidic substrates) and the simultaneous increase in nitrophytes (prefer nutrient-rich substrates), further changes in species composition take place. While lichens that are adapted to cool environmental conditions decrease (or at least do not increase) recently, such species preferring warm-temperate and more humid conditions have spread. In the present study, we have regarded species as indicators of climate change that were entitled in the corresponding VDI guideline (guideline VDI 3957 Part 20, personal communication). Moreover, we added lichens that have a temperature indicator value of 7 to 9[10]. Their presence shows a generally more balanced climate with mild winters, increased annual average temperatures and increased humidity.
In a previous investigation in Hesse, there appeared to be an immigration trend in the lichens on rocks and walls that demonstrated a significant relationship between the measured temperatures and the occurrence of heat-loving lichens[32, 33]. In the Netherlands and in north-western and western Germany, such a change had also been identified for epiphytic lichens a few years ago[28, 34, 35]; now, it is occurring in Central Hesse as well, whereas in 1970 and 1980, not a single heat indicator grew on the examined trees. In 1985, the first of such lichen species could be detected.
In 1995, two species appeared, while there were already five species in 2005 which are assigned to the group of heat indicators. By the year 2010, within 5 years, the number had increased to 14. Our calculations of the average indicator values for temperature show that lichens respond to the changing climatic conditions. In 25 years, the average indicator value for temperature has risen by half a unit (Figure17).
This observation is consistent with the increase in temperature (Figure18; Prof. Schönwiese, personal communication). Between 1820 and 2010, a significant increase in the mean annual values of almost 2°C could be recorded; during the study period from 1970 to 2010, it amounted to almost half a degree.
As can be seen in Figures19 and20[36], the climate change is particularly noticeable through milder winters. This fact supports the Atlantic lichen species: according to the ‘Law of the Minimum’, formulated by Justus Liebig, the minimum factor limits the growth. As is happening recently, the frost periods are getting shorter and less severe, wherefore also species from the southwest of Europe or the sub-Mediterranean climate area have a chance to survive in Hesse.
Development of the temperature (summer half year) in Frankfurt/Main City[36].
Development of the temperature (winter half year) in Frankfurt/Main City[36].
Furthermore, it should be noted that the rainfall in Hesse has increased in recent decades and predominates in the winter[36]. This trend is reflected in the average indicator values of lichens. In Figure21, an (albeit slight) increase in the indicator values for humidity can be seen.
In the publication by Kirschbaum and Wirth, an index of climate change, the climate index ‘KI’, was introduced[11]. It is a combination of both the indicator values for temperature and oceanity (the latter is complementary to the indicator value for continentality) and thus integrates the statements of both values. The climatic values are averaged and a climate index (KI) for every measuring unit is calculated. Since the heat indicators are still relatively rare and occur with less frequency on the trees, a weighting of the results in the calculation of the KI - according to the frequency of their occurrence - was omitted. (Because of their low frequency, their values would not influence the results in comparison to the other mostly common species.) Moreover, in this way it was possible to calculate an unweighted index for 1970; a calculation of a weighted index was not possible due to methodological reasons.
As can be seen in Figure22 the climate index for the forty-year study period increased, as well. From this, a tendency to a more Atlantic climate with more balanced seasonal temperatures and precipitation can be concluded. It is remarkable that the increase is not substantially caused by those species that are both heat and hypertrophication indicators; hereby, it is obviously not a synergetic effect between increased airborne nitrogen compounds and the climate change, as is being considered by some authors[37, 38].
Conclusions
Conclusions regarding air pollution
Both the results of the bark pH measurements and the results of the lichen surveys show the improvement of the air quality in the Hessian towns of Wetzlar and Giessen over a period of 40 years; they are estimated as being representative for large parts of Hesse (and beyond). The reduction in immissions, particularly in sulfur dioxide, has had a positive impact on the livelihoods of lichens. The same is expected for the hazard risks in plants, animals and humans; lichens here act as early warning systems[39].
The reduction in the acid forming gases is, however, opposed to an increase in hypertrophicating air pollutants. In comparison with strong agriculturally oriented areas (‘manure belt’ in the north-west of Germany and the Netherlands), the conditions in Hesse are not yet worrisome. The concentrations of ammonia in parts of the Netherlands are twice as high[28] in comparison to the ones in Hesse (about 6 μg NH3/m3)[31], and also in England, much higher ammonia concentrations have been measured often[40]. Actually, the spread of nitrophytic species there is much more serious[19].
The same was observed in an investigation in the area south of Stuttgart and a survey in Rastatt, southwest of Karlsruhe[41]. While the hypertrophication index was denoted with 5 in all the measuring areas there, the average in Wetzlar and Giessen was 2.5 over the same period and reached a maximum value of 4 in only two of the 64 sampling units. Nevertheless, particular attention should be given to the problem in order to avoid negative impacts to sensitive ecosystems.
A further improvement of the air quality in Central Hesse is possible, whereof many sensitive lichen species would benefit. Almost 300 years ago, Dillenius described lichen species from the vicinity of Giessen that have not returned until today (including representatives of the extremely sensitive genres Anaptychia Lobaria and Sticta[42]). In the middle of the nineteenth century, the authors Rochleder and Heldt reported having collected species in the forests around Giessen, such as Evernia prunastri Usnea barbata Ramalina fraxinea Ramalina fastigiata Pseudevernia furfuracea and Xanthoria parietina growing there in great quantities on trunks of oaks, birches, spruces and pines[43]. Of these partly very sensitive species, only E. prunastri P. furfuracea and X. parietina have come back, while the rest currently seems to avoid the environmental conditions in Central Hesse. Such descriptions from the period about 150 years ago virtually exist to an unlimited extension (such as[44–48]).
Observations of the temporal development of lichens over the entire study period of 40 years show an increase in the numbers of species and their frequency; a stabilization of the species seems to have not yet occurred. Similar results on the base of repeated lichen mappings were obtained in other parts of Germany (such as[34, 49]).
Further documentation of this, for the most part, positive development of the air quality seems to be reasonable. It is to be hoped that the lichen biocenoses will regain their pre-industrial diversity. Particular attention should be given to the further development of airborne nitrogen compounds. Due to the growing world population (and the associated increase in food production), a global reduction of nitrogen emissions is not expected as it cannot be counteracted technically (with filters, flue gas scrubbing) currently[30].
While low nitrogen doses initially have a positive impact on the ecosystems, the diversity of species decreases with excessive supply. This phenomenon is also observed in lichen associations[40].
While higher plants possess protection mechanisms (such as the cuticle) and obtain their nutrients over the (buffered) soil, which is a delayed and more indirect response to changes in the composition of air, the response of the poikilohydric, all-year-round metabolically active lichens is more direct and clear and also because these organisms absorb their nutrients exclusively via the air.
Conclusions regarding climate change
Our studies were originally designed for the detection of ambient air pollution[1]. It appears, however, that the method is also suitable for indicating climatic changes. Recently, lichen mappings confirming to the ‘immission guideline’ and - simultaneously – to the ‘climate directive’ were carried out in Bavaria[50]. It arose that the method following the climate directive provided better correlations of the epiphytic lichen composition and climate parameters than the data confirming to the immission guideline. This is explained by the greater variability and the number of examined trees and by a larger examination area on the tree in the climate directive. As our study was conducted with the small monitoring quadrat (Figure2), it can be assumed that the results would have been even clearer if it had been worked according to the specifications of the climate directive.
With the lichens, both climate trends (higher temperatures and rainfall) are recorded. In contrast to many higher plants, their metabolism is not limited to the growing season. These poikilohydric organisms are metabolically active in the wet winter half year with higher humidity due to their ability to perform photosynthesis even at very low temperatures. Not only do they integrate the influences of the growing season into their response to climate change, but also the particularly important winter half year. In this respect, lichens are well suited to act as early warning systems for the climate change.
References
VDI 3957 Part 13: Biological measurement procedures for determining and evaluation the effects of ambient air pollutions by means of lichens (bioindication): Mapping the diversity of epiphytic lichens as an indicator of air quality. Berlin: Beuth; 2005.
Naumann C Thesis. In Die Rindenepiphyten im Stadtgebiet von Giessen in ihrer Abhängigkeit von stadtbedingten Faktoren. Giessen University, Department of Biology; 1969.
Naumann C Thesis. In Die Verbreitung von rindenbewohnenden Flechten und Algen im Raum Wetzlar und ihre Expositionsabhängigkeit. Giessen University, Department of Geography; 1970.
Sänger K Thesis. In Flechtenwachstum und pH-Wert von Baumrinden. Giessen University, Department of Biology; 1972.
Biecker S Thesis. In Flechtenkartierung im Raum Wetzlar. Giessen University, Department of Biology; 1986.
Hansen H Thesis. In Flechtenkartierung im Raum Giessen. Giessen University, Department of Biology; 1986.
Kirschbaum U, Windisch U, Vorbeck A, Hanewald K: Mapping lichen diversity in Wetzlar and Giessen as indicator of air quality. (Comparison between the surveys of 1970, 1985, 1995 and 2005). Gefahrstoffe - Reinhaltung der Luft 2006, 66: 272–280.
Marx A Thesis. In Immissionsbezogene Flechtenkartierung im Raum Wetzlar. Giessen University, Department of Biology; 1996.
Schiek JE Thesis. In Immissionsbezogene Flechtenkartierung von Giessen, 1995. University of Applied Sciences Giessen, Department of KMUB; 1996.
Wirth V: Ökologische Zeigerwerte von Flechten – erweiterte und aktualisierte Fassung. Herzogia 2010, 23: 229–248.
Kirschbaum U, Wirth V: Flechten erkennen – Umweltgüte bewerten. Wiesbaden: Hessisches Landesamt für Umwelt und Geologie; 2010.
HLUG: Immissions- und Klimadaten. Wiesbaden: Hessisches Landesamt für Geologie; 2010.
HLUG: Bericht über Staubniederschlagsmessungen im Land Hessen von 1985–1992. Schriftenreihe der Hessischen Landesanstalt für Umwelt: Umweltplanung, Arbeits- und Umweltschutz, Wiesbaden 1994, 175: 1–249.
Weber W Thesis. In Flechten als Bioindikatoren zur Bewertung der lufthygienischen Situation der Stadt Lahn. Giessen University, Department of Biology; 1980.
Kirschbaum U, Marx A, Schieck JE: Beurteilung der lufthygienischen Situation Giessens und Wetzlars mittels epiphytischer Flechten. Angew Bot 1996, 70: 78–96.
Kirschbaum U Thesis. In Auswirkung stadteigener Faktoren auf die Verbreitung von Flechten und Algen im Raum Frankfurt. Giessen University, Department of Biology; 1969.
Van Dobben HF: Decline and recovery of epiphytic lichens in an agricultural area in The Netherlands (1900–1988). Nova Hedwigia 1996, 62: 477–485.
Van Dobben HF, De Bakker AJ: Re-mapping epiphytic lichen biodiversity in the Netherlands: effects of decreasing SO2 and increasing NH3. Acta Botanica Nederlandica 1996, 45: 55–71.
Van Herk CM: Mapping of ammonia pollution with epiphytic lichens in the Netherlands. Lichenologist 1999, 31: 9–20.
Van Herk CM: Epiphytes on wayside trees as an indicator of eutrophication in the Netherlands. In Monitoring with Lichens - Monitoring Lichens. NATO Science Series, IV, vol. 7. Edited by: Nimis PL, Scheidegger C, Wolseley PA. Kluwer: Dordrecht; 2002:285–289.
Bayerisches Landesamt für Umweltschutz (Ed): Ammoniak und Ammonium. Augsburg: Reihe Umweltwissen; 2004.
Umweltbundesamt (Ed): Daten zur Umwelt. Dessau-Roßlau; 2009.
Frahm JP: Der Einfluss von Ammoniak auf Stickstoff liebende Flechten in verkehrsbelasteten Gebieten. Immissionsschutz 2006, 11: 64–167.
Landesanstalt für Umwelt, Messungen und Naturschutz Baden-Württemberg (Eds): Ammoniak in der Umwelt – Messprogramme und Messergebnisse 2003–2007. Karlsruhe; 2008.
Bartholmeß H, Schlottman K, Nobel W: Immissionsmessungen mit Passivsammlern und Flechtenkartierungen im Zusammenhang mit dem Eintrag von Stickstoff in die Umwelt. Gefahrstoffe - Reinhaltung der Luft 2011, 71: 165–172.
Umweltbundesamt: Handbuch Emissionsfaktoren des Straßenverkehrs 2.1. Wien; 2004. Bd 107, 02/2004 Bd 107, 02/2004
Stapper NJ, Franzen-Reuter I, Frahm JP: Stickstoffgehalte in der Blattflechte Parmelia sulcata als Indikator atmosphärischer Stickstoffeinträge in einer Großstadt am Beispiel Düsseldorf. Immissionsschutz 2005, 3: 84–89.
Van Herk CM: Climate change and ammonia from cars as notable recent factors influencing epiphytic lichens in Zeeland, Netherlands. Bibliotheca Lichenologica 2009, 99: 205–244.
Vorbeck A, Windisch U: Flechtenkartierung München. Eignung von Flechten als Bioindikatoren für verkehrsbedingte Immissionen. In Materialien für Umwelt und Entwicklung 173. Edited by: Bayerisches Staatsministerium für Landesentwicklung und Umweltfragen. München; 2001:1–174.
Hauck M: Ammonium and nitrate tolerance in lichens. Environ Pollut 2010, 158: 1127–1133.
Dämmgen U, Matschullat J, Zimmermann F, Strogies M, Grünhage L, Scheler B, Conrad J: Emission reduction effects on bulk and wet-only deposition in Germany – evidence from long-term observations. Part 3. Sulphur and nitrogen compounds. Outlook and future requirements. Gefahrstoffe - Reinhaltung der Luft in press in press
Cezanne R, Eichler M, Kirschbaum U, Windisch U: Flechten als Anzeiger des Klimawandels. Sauteria. 2008, 15: 159–174.
Kirschbaum U, Hanewald K: Flechten als Anzeiger der Luftgüte und des Klimawandels. Wiesbaden: Hessisches Landesamt für Umwelt und Geologie; 2009.
De Bruyn UH, Linders W, Mohr K: Epiphytische Flechten im Wandel von Immissionen und Klima - Ergebnisse einer Vergleichskartierung 1989/2007 in Nordwestdeutschland. Umweltwiss Schadst Forsch 2009, 21: 63–75.
Stapper NJ, Franzen-Reuter I, Frahm JP: Epiphytische Flechten als Wirkungsindikatoren für Klimaveränderungen im Raum Düsseldorf. Gefahrstoffe - Reinhaltung der Luft 2011, 71: 173–178.
Schönwiese CD, Staeger T, Steiner H, Brinckmann S: Analyse der Klimaveränderungen in Hessen für den Zeitraum 1901 bis 2003. Abschlussbericht zum INKLIM-Forschungsvorhaben Nr. 2004.090353876–3 im Auftrag des Hessischen Landesamtes für Umwelt und Geologie (HLUG). Inst. für Atmosphäre und Umwelt der Univ. Frankfurt/Main; 2006.
Van Herk CM, Aptroot A, van Dobben HF: Long-time monitoring in the Netherlands suggests that lichens respond to global warming. Lichenologist 2002, 34: 141–154.
John V: Stickstoff und Wärme–Synergieeffekte oder gegenseitigeVerschleierung der Wirkung auf Flechten? VDI-KRdL Schriftenreihe 2007, 37: 109–118.
Cislaghi C, Nimis PL: Lichens, air pollution and lung cancer. Nature 1997, 387: 463–464.
Wolseley PA, James PW, Theobald MR, Sutton MA: Detecting changes in atmospheric lichen communities at sites affected by atmospheric ammonia from agricultural sources. Lichenologist 2006, 38: 161–176.
Bartholmeß H, Müller K, Nobel W, Winkelbauer W: Biomonitoring im Rahmen der Ökosystembewertung eines Automobil-Produktionsstandorts. Gefahrstoffe - Reinhaltung der Luft 2011, 71: 339–346.
Dillenius JJ: Catalogus plantarum sponte circa Gissam nascentium cum appendice. Joh. Maximilian á Sande, Frankfurt/M; 1719.
Rochleder F, Heldt W: Untersuchungen einiger Flechtenarten. Annalen der Chemie und Pharmacie 1843, XLVIII: 1–18.
Theobald GL: Die Flechten der Wetterau. Naturhistorische Abhandlungen aus dem Gebiete der Wetterau 1858, 313–390.
Bauer PM: Übersicht der in dem Großherzogthum Hessen beobachteten Flechten. Berichte der oberhessischen Gesellschaft für Natur- und Heilkunde 1859, 7: 13–26.
Zu Solms-Laubach R: Verzeichniß der von mir in der Gegend von Braunfels und Laubach gesammelten und bestimmten Lichenen. Berichte der oberhessischen Gesellschaft für Natur- und Heilkunde 1863, 10: 66–71.
Uloth W: Beiträge zur Kryptogamenflora der Wetterau. Berichte der oberhessischen Gesellschaft für Natur- und Heilkunde 1865, 11: 93–97.
Lorch W: Übersicht der bisher in der Umgebung von Marburg (Hessen) beobachteten Flechten. Jb Naturw Ver Elberfeld 1896, 8: 1–24.
Heemann S, Stetzka KM: Die sauren Zeiten sind vorbei – Ergebnisse der immissionsbezogenen Flechtenkartierung Dresdens und Veränderungen der Flechtendiversität. Gefahrstoffe - Reinhaltung der Luft 2010, 70: 141–146.
Windisch U, Vorbeck A, Eichler M, Cezanne R: Untersuchung der Wirkung des Klimawandels auf biotische Systeme in Bayern mittels Flechtenkartierung. In Edited by: Bayerisches Landesamt für Umwelt. 2011.
Acknowledgements
This study was financially supported by the Hessisches Landesamt für Umwelt und Geologie (HLUG). The views expressed herein by the authors are their own and do not necessarily reflect the views or policies of governmental agencies. We thank Anna Wirtz for performing the pH analysis and Alexander Vorbeck for preparing the final version of figures and maps. We would also like to thank all those who have carried out earlier mappings, kept their basic data over decades, retrieved them from dusty corners, and made them available to us. Special acknowledgement is extended to Prof. Dr. L. Steubing (University of Giessen). Without her commitment and stimulation, the early mappings would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
UK provided substantial contributions to the concept and design, acquisition of funding, project management, and data acquisition in the field (lichen mapping). He drafted the manuscript and created the original version of the figures. RC and ME did the acquisition of the field data (lichen mapping) and the critical revision of the manuscript. KH provided substantial contributions to the concept and acquisition of funding. UW did the acquisition of data (lichen mapping), management, analysis and interpretation of data, and revision and translation of the manuscript. All authors read and approved the manuscript.
Electronic supplementary material
12302_2011_67_MOESM1_ESM.tiff
Additional file 1: Valuation of the components of the air quality index. The verbal assessment of the air quality index compilation. (TIFF 69 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kirschbaum, U., Cezanne, R., Eichler, M. et al. Long-term monitoring of environmental change in German towns through the use of lichens as biological indicators: comparison between the surveys of 1970, 1980, 1985, 1995, 2005 and 2010 in Wetzlar and Giessen. Environ Sci Eur 24, 19 (2012). https://doi.org/10.1186/2190-4715-24-19
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2190-4715-24-19