Abstract
Purpose
Three VEGF SNPs (−2578) C/A, (+405) G/C and (+936) C/T were investigated in Tunisian exudative AMD patients in order to determine their association with the disease susceptibility and their influence to intravitreal bevacizumab therapy response.
Methods
145 AMD patients and 207 age-matched controls were included. 68 patients were treated with intravitreal bevacizumab. SNPs genotyping were performed using direct sequencing. The serum VEGF was assayed by ELISA (R&D).
Results
The (+405) CC and (+936) TT genotypes were higher in AMD patients than in controls (p = 5 × 10−6 and p = 0.021, respectively). The mean plasma levels of VEGF were statistically higher in AMD patients (84.22 pg/ml) than in controls (15 pg/ml). Three months after bevacizumab treatment, 52 patients (85.6%) were classified as good responders (GR) and 16 (14.4%) as poor responders (PR). The mean plasmatic-VEGF levels in GR patients was higher (86.61 ± 80.30 pg/ml) than in PR patients (47.12 ± 45.74 pg/ml) (p = 0.086). The patients with genotype homozygous TT (+936) would be PR compared to those carrying CT and CC genotypes. Whereas, those with AA (−2578) genotype would be GR compared with others genotypes (p = 0.014; p = 0.042 respectively).
Conclusions
Our results show that VEGF genetic variants may contribute to the susceptibility to neovascular AMD in Tunisian patients.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is a progressive chronic inflammation that affects the cells of the retinal pigment epithelium (RPE) and promotes the formation and deposition of phospholipid material retinal drusen. This maculopathy progresses to degeneration in two forms: “wet AMD” and “Dry AMD”. Indeed, most visual loss occurs in the late stages of the disease due to one of two processes: choroïdal neovascularization (CNV) and geographic atrophy [1, 2]. Etiological research suggests that AMD is a complex disease, caused by the interactions of several genetic and environmental factors. Different evidences have supported that in exudative AMD form, inflammatory lesions are associated with a high expression and synthesis of growth factors such as vascular endothelial growth factor (VEGF) which is a key regulatory factor in angiogenesis and vascular permeability in both physiological and pathological states [3]. VEGF expression has been shown in experimental choroidal neovascularization and shown to induce CNV growth in animal models [4]. Therefore, it would be involved in the development of CNV in this disease.
More recently, attention has been focused on the function of VEGF in light of its role as a therapeutic target and VEGF-inhibitors have been used in successful therapy of exudative AMD [5, 6]. The most important advance in the treatment of neovascular AMD is the development of anti-vascular endothelial growth factor (anti-VEGF) therapeutic agents that preserve and improve visual acuity by arresting choroidal neovascular growth and reducing vascular permeability. Thus, VEGF is a potential candidate for genetically influencing AMD susceptibility, based on its functional relevance to AMD pathophysiology.
VEGF gene exhibits many single nucleotide polymorphisms (SNPs) that might influence qualitatively and/or quantitatively its expression. Previous studies have shown that the genetic variations in the VEGF gene influence the rate of the VEGF protein synthesis [7–9]. The human VEGF-A gene is located on chromosome 6 (6p21.3) and is organized into eight exons [10]. Several different isoforms of VEGF are generated by alternate splicing of the VEGF-A gene [11]. Of these, the VEGF165 isoform (named according to the number of amino acids), is the most abundant and corresponds to a 23 kDa polypeptide, constituting a monomer of homodimeric human VEGF-A the role of vascular endothelial growth factor (VEGF) in inflammatory bowel disease [12].
In this context, three common VEGF SNPs (C-2578A, rs699947), (G + 405C, rs2010963) and (C + 936 T, rs3025039), in the promoter, the 5′Untranslate and the 3′Untranslated regions respectively, were investigated in Tunisian exudative AMD patients in order to determine their association with the disease susceptibility, their influence to the level of production of this glycoprotein and the response to intravitreal bevacizumab therapy.
Material and methods
Patients
One hundred and forty five unrelated Tunisian patients with exudative AMD were collected from the Department B of Ophthalmology, Hedi Rais Institute of Ophthalmology, Tunis, Tunisia.
The diagnosis of AMD was established on the basis of clinical examination, fundus photographs and fluorescein angiography results. Fundus findings in each eye were classified based on a standardized set of diagnostic criteria established by the International Age-Related Maculopathy Epidemiologic Study [13]. Data obtained from each patient at the ophthalmology institute included age, gender, personal/familial history of AMD and risk factors (tobacco use, arterial hypertension, hypercholesterolemia and cardiovascular risk). Clinical and epidemiological characteristics of patients are summarized in Table 1.
Exudative AMD patients were classified in two groups. The first group (G1) composed by 117 patients with active neovascular form and divided into three subtypes including 40 (34,2%) predominantly classic cases without occult CNV, 53 (45,3%) occult CNV and 24 (20,5%) minimally classic CNV, according to the guidelines from the international classification. The second group (G2) included 28 patients with cicatritial lesion form.
According to the standard protocol for anti-VEGF therapy, 68 of G1 patients (58.11%) were treated with 3 initial monthly intravitreal bevacizumab injections (1.25 mg in 0.05 ml). After this therapy, improving visual acuity (VA), as a 2-lines gain (a margin of 10 ETDRS lettres) was used to compare response to treatment. Patients were classified into:
–Good responders (GR): defined as patients with a gain of ≥ 10 ETDRS letters and those who demonstrated stability in visual acuity (a gain or loss of < 5 lettres) after the 3 bevacizumab injections.
–Poor responders (PR): defined as patients with a loss of > 10 ETDRS letters in post-therapy.
Controls
Two hundred and seven subjects blood donors older than 50 years and having undergone a complete ophthalmological examination with a normal fundus test, served as control group.
All patients and controls were fully informed of the purpose and procedures of the study, and informed consent was obtained from all patients before they were enrolled in the study.
Methods
Venous blood of patients and controls was collected in EDTA tubes. Plasma was frozen at −20°C for determination of VEGF plasma levels and genomic DNA was extracted for molecular study using a salting-out procedure [14].
Determination of VEGF plasma levels
Plasmatic VEGF levels of 104 patients included in this study were measured using the Human VEGF Quantikine ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer recommended protocol. VEGF levels in patient samples were expressed in pg/ml. The normal mean value of VEGF is 15 ± 4.5 pg/ml. In 68 G1patients, this assay occurred before treatment.
Genotyping of VEGF gene polymorphisms
In the promoter region, the VEGF (−2578) C/A gene polymorphism was determined using the previously described PCR restriction fragment length polymorphism (RFLP) method. Primers used were as follows15:
Forward 2578 sens:
5′-ATAAGGGCCTTAGGACACCA-3′
Reverse 2578 Antis:
5′-GCTACTTCTCCAGGCTCACA-3′
PCR was carried out in a final volume of 20 μl containing 100 ng of genomic DNA, 1.5 mM MgCl2, 0.2 mM dNTP, 10 pmol of each primer and 0.5U of Taq DNA polymerase (Promega, USA). The PCR product were digested for 2 hours at 37°C with restriction endonuclease BglII (SIBENZYME) at a final concentration of 4 units, fragments were analyzed on 3% agarose gels stained with éthidium bromide. The A allele remained uncut, while the C allele was cut into two fragments of 212 and 264 bp.
The VEGF (+405) C/G in the 5′Untranslated region and (+936) C/T in 3′Untranslated region gene genotyping were performed by a polymerase chain reaction (PCR) in a final volume of 20 μl containing 5 pmol of each primer: 5′-ATTTATTTTTGCTTGCCATT-3′ (forward primer +405S) and 5′-GTCTGTCTGTCTGTCCGTCA-3′ (reverse primer +405AS); 5′-AAGGAAGAGGAGACTCTGCGC-3′ (forward primer +936S) and 5′-TATGTGGGTGGGTGTGTCTACAGG-3′ (reverse primer +936AS) respectively, 50 ng of genomic DNA, 1.5 mM of MgCl2, 0.2 mM dNTP, 10 pmol of each primer and 0.5U of Taq DNA polymerase (Promega, Madison, WI, USA).
The DNA was denatured at 94°C for 4 minutes, prior to 30 cycles of amplification. The conditions used for each cycle were denaturation for 30 seconds at 94°C, annealing for 30 seconds at 60°C, and extension for 1 minute at 72°C. The 30 amplification cycles were followed by a final extension step at 72°C for 5 minutes. The PCR products were resolved in 2% agarose gels stained with ethidium bromide. The amplified fragments were then sequenced in forward direction using the forwards primer in an ABI PRISM Dye Terminator Cycle Ready Reaction kit (Applied Biosystems) under recommended conditions. Sequenced samples were purified using Centri-Sep columns (Dye EXTm 2.0 Spin Kit, Qiagen) according to manufacturer’s instructions, loaded in a PE ABI Prisms 310 Genetic Analyzer (Perkin Elmer) and analyzed using ABI Prisms Navigator Software. The (+405) G/C and (+936) C/T alleles were observed as different fluorescence peaks in that position.
Statistical analysis
Snellen VA was converted to the logarithm of minimum angle of resolution (logMAR) VA for the purpose of statistical analysis. Change in VA was calculated as the difference between VA at baseline and VA at follow-up.
Statistical calculations were performed using SPSS for Windows 19.0 (SPSS, Chicago, IL, USA). As most data had a skewed distribution, numbers reported are median values unless indicated otherwise and non parametric test methods were used in statistical analyses. Continuous data were analyzed by Mann–Whitney U-test and categorical data by Fisher’s exact test. Factors that were significantly associated with s-VEGF after univariate analyses were then entered into multiple regression models to determine the independence of potential correlations and to estimate adjusted ORs (Exp(β)).
Genotype and haplotype analyses were performed with SNP Stats software. Comparisons between genotypes were adjusted using the Bonferroni multiple comparison correction and the reported p-values reflect this correction. The strength of the association between genotypes or alleles in each group was estimated by the calculation of the odds ratios (OR) and 95% confidence intervals (CI). Values of p < 0.05 were considered statistically significant.
Results
Genotype and allele frequencies
As shown in Table 2, the genotype and allele frequencies of the (+405) CC and (+936) TT were significantly higher in AMD patients than in controls [(OR: 3.86, 95% CI [2.03 - 7.42], p = 5 × 10−6 and OR: 8.89, 95% CI [1. 05–198.1], p = 0.021 respectively)] and [(OR: 1.79, 95%CI [1.3 - 2.48], p = 2 × 10−4 and OR: 1.95, 95%CI [1. 22–3.12], p = 0.003 respectively)]. While, the distribution of (−2578) C/A genotypes and alleles was similar among patients and controls.
Otherwise, the distribution of (−2578) C/A, (+405) C/G and (+936) C/T genotypes and alleles frequencies was similar in G1 and G2 patients (Table 1). Subtypes of neovascular AMD did not show statistically significant differences.
Haplotype analysis
Eight different combined haplotypes of these three commun VEGF SNPs were observed Table 3. The cumulative high risk ACT haplotype was more frequently found in patients with the active form of the disease (G1: 4.6%) compared to those with the scar form (G2: 2.8%), but the difference was not statistically significant (p = 0.49).
Response to anti-VEGF therapy
Among the 68 G1patients treated with anti-VEGF, at 3 months, 52 patients (85.6%) were classified as good responders and 16 (14.4%) as poor responders.
VEGF genotyping and response to intravitreal bevacizumab injections
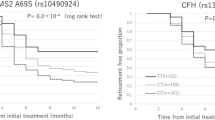
As summarized in Table 2, no difference was shown between the distribution of (+405) G/C genotypes and response to anti-VEGF therapy. However, the patients with genotype homozygous TT (+936) would be poor responders compared with those carrying CT and CC genotypes. Whereas, those with the AA (−2578) genotype would improve their visual outcome compared with AC and CC genotypes (p = 0.014; p = 0.042 respectively). Nevertheless, theses SNPs lose their significance in analysis of combined haplotypes.
Determination of VEGF plasma levels
Plasmatic VEGF levels in patients were ranged from 10 to 1050 pg/ml. The mean plasma levels of VEGF was statistically higher in AMD patients (84.22 pg/ml) than in controls (15 pg/ml), and in active form of AMD patients (83.39 pg/ml) compared to those with a scar form (30.60 pg/ml) (p = 0.04 and p = 0.035, respectively) (Figure 1). However, these results were not confirmed after multivariate analysis and adjustement for known covariates factors (age, gender and risk factors) (Exp (β): 0,95). We did not found any difference in plasmatic VEGF levels between the different types of CNV (p = 0.23). Additionally, the mean plasma levels of VEGF in GR patients was higher (86.61 ± 80.30 pg/ml) than in PR patients (47.12 ± 45.74 pg/ml) with a trend to significance (p = 0.086) (Figure 2).
Association of VEGF polymorphisms with serum VEGF levels
If the polymorphism (−2578) C/A showed no relationship with s-VEGF levels of the mutant homozygous genotype (+405) GG and the wild homozygous genotype (+936) CC showed a higher s-VEGF levels than the other genotypes but the differences failed to reach significance.
Additionally, haplotype combinations analysis did not provide any significant association with s-VEGF levels variations.
Discussion
The current study showed that (+405) G/C and (+936) C/T VEGF polymorphisms were statically associated with exudative AMD in the Tunisian patients corroborating the large study of Haines et al., who attributed to the some SNPs of VEGF, VLDLR and LRP6 genes, a role in the risk of AMD development [15]. These results were in close agreement with those previously published by Janik-Papis et al., in a Polish population [16] and by Lin et al., in Taiwan Chinese population [17]. Inversely, it has been reported in other studies that the positive association between VEGF polymorphisms of the gene and susceptibility to the occurrence of AMD could not be confirmed in the Rotterdam study and in Anglo-Celtic subpopulation [18–20]. These controversial results are due to differences in the size of the study, patients heterogeneity, the choice of analyzed SNPs located in the promoter region and/or in the coding regions and the methods used for their genotyping. It is now well established that genetic susceptibility to complex diseases such as AMD, is rather due to the cumulative effect of several predictive alleles identified in the full haplotype information [17, 18]. Among combined haplotypes observed in this cohort, the risk ACT variant was more frequently found in patients with the active form of the disease (G1patients) compared to those with the scar form (G2 patients), but the difference was not statistically significant. The small numbers of subjects in G2 could induce a loss of statistical power to detect this difference.
The angiogenesis process is highly controlled through the balance of pro- and anti-angiogenic factors. VEGF is the key pro-factor in this process. Specific polymorphisms in the VEGF gene have been associated with a variation of protein levels [7, 21, 22]. The data from this study support this hypothesis by showing that both in controls and G2 patients compared to the G1 subjects, a significant decrease in s-VEGF was observed, confirming once again that VEGF is the major stimulus for the development and growth of choroidal neovascularization in the exudative AMD because wet AMD involves neovascularization, the best approach for the treatment of CNV appears to be the use of anti-VEGF drugs. At present, several molecules have been used in intravitreal therapies approved by Food and Drug Administration (FDA) and the European Medecines Agency (EMEA).
Among these anti-VEGF agents, several studies report that the bevacizumab (Avastin*; Genentech Inc) gives the best results for the treatment of neovascular AMD [23–26].
The effectiveness of such treatments depends on their molecular weight, the binding affinity of the various isoforms of VEGF and their pharmacokinetics lesional prior retinal barrier. In this study, taking into account of the availability of bevacizumab exposure in plasma, this molecule would be more effective in patients with a high concentration of plasmatic VEGF levels compared to those with the rate of protein relatively low. Indeed, a difference in the distribution of plasma levels of VEGF was found among good responders compared with poor responders. These results corroborate those of Carneiro et al., who show that bevacizumab significantly reduced the plasma levels of VEGF after 28 days after intravitreal injections in patients with exudative AMD compared to injections of ranibizumab, with two main potential determinants of their systemic adverse effects including the blood levels of plasmatic VEGF and the degree of systemic anti-VEGF inhibition.
About the relationship between SNPs of the VEGF gene studied and response to intravitreal injection of bevacizumab, this study revealed, when each SNP was analyzed individually, that the patients mutated homozygous AA (−2578) genotype were rather classified as good responders whereas those with risk homozygous genotype TT(+936) belonged to the group of poor responders. Nevertheless, theses SNPs lose their significance in analysis of combined haplotypes. In the literature, unanimity is not yet fully established for the use of these genetic biomarkers to predict the visual evolution in response to anti-VEGF therapy in the exudative AMD. Indeed, if in the promoter region of VEGF gene, the SNP (C-2578A, rs699947) seems to be also, a predictor of success to bevacizumab treatment in Japan population [27], our results, concerning the SNP (C936T, rs3024039) in the 3′Untranslated region, are in discordance with a Qu Y. in Chinese population and Boltz A. studies that provide no evidence for an association between this SNP and the response to bevacizumab treatment [28, 29]. In Korean neovascular AMD cohort, Un Chul Park et al., do not find statistically significant effect of VEGF-SNPs (C936T, C-2578A and C-460 T) on visual outcome after ranibizumab treatment [30]. In 5′Untranslate region, the SNP (G405C, rs2010963), despite the fact that it is associated with susceptibility to AMD, the response to bevacizumab was independent of this polymorphisme. This is in agreement with study of Imai et al. found that VEGF (−2578; rs699947) and PDEF (+rs113628) were associated with vision changes at 1 month and 3 months post-bevacizumab therapy respectively but VEGF (+405; rs2010963) may not be genetic biomarker to estimate visual outcomes in response to intravitreal bevacizumab treatment for neovascular AMD [27].
In view of these results, the question is what about the genetic profile associated with long-term visual outcome? The follow-up of further study with larger sample sizes and standard intervals to record the results of visual acuity should be performed to confirm these results and to answer this question.
In conclusion, this study found that VEGF genetic variants may contribute to the susceptibility to neovascular AMD in Tunisian patients and were also associated with vision changes at 3 months of anti-VEGF therapy. However, our findings need to be replicated in additional studies. Further expression studies are needed to investigate the potential pharmacologic role of these variants in antiangiogenesis AMD therapy at long-term.
References
Klein R, Peto T, Bird A, Vannewkirk MR: The epidemiology of age-related macular degeneration. Am J Ophthalmol 2004, 137: 486–495.
VanNewkirk MR, Nanjan MB, Wang JJ, Mitchell P, Taylor HR, McCarty CA: The prevalence of age-related maculopathy: the visual impairment project11received December 30, 1999. Accepted march 27, 2000. Ophthalmology 2000, 107: 1593–1600.
Bhattacharya R, Sinha S, Yang S-P, Patra C, Dutta S, Wang E, Mukhopadhyay D: The neurotransmitter dopamine modulates vascular permeability in the endothelium. J Mol Signal 2008, 3: 14.
Kondo S, Asano M, Suzuki H: Significance of vascular endothelial growth factor/vascular permeability factor for solid tumor growth, and its inhibition by the antibody. Biochem Biophys Res Commun 1993, 194: 1234–1241.
Martin DF, Maguire MG, Ying G, Grunwald JE, Fine SL, Jaffe GJ: Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011, 364: 1897–1908.
Heier J, Shapiro H, Singh A Sr, Group MS: Randomized, controlled phase iii study of ranibizumab (lucentistm) for minimally classic or occult neovascular age-related macular degeneration: Two-year efficacy results of the marina study. Invest Ophtalmol Vis Sci 2006, 47: 2959.
Renner W, Kotschan S, Hoffmann C, Obermayer-Pietsch B, Pilger E: A common 936 c/t mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res 2001, 37: 443–448.
Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE: Identification of polymorphisms within the vascular endothelial growth factor (vegf) gene: correlation with variation in vegf protein production. Cytokine 2000, 12: 1232–1235.
Ruggiero D, Dalmasso C, Nutile T, Sorice R, Dionisi L, Aversano M, Bröet P, Leutenegger A-L, Bourgain C, Ciullo M: Genetics of vegf serum variation in human isolated populations of cilento: importance of vegf polymorphisms. PLoS One 2011, 6: e16982.
Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW: The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of rna. Mol Endocrinol 1991, 5: 1806–1814.
Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes J, Abraham J: The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991, 266: 11947–11954.
Ferrara N: Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004, 25: 581–611.
Bird A, Bressler N, Bressler S, Chisholm I, Coscas G, Davis M, De Jong P, Klaver C, Klein B, Klein R: An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol 1995, 39: 367–374.
Miller S, Dykes D, Polesky H: A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988, 16: 1215.
Haines JL, Schnetz-Boutaud N, Schmidt S, Scott WK, Agarwal A, Postel EA, Olson L, Kenealy SJ, Hauser M, Gilbert JR: Functional candidate genes in age-related macular degeneration: significant association with vegf, vldlr, and lrp6. Invest Ophthalmol Vis Sci 2006, 47: 329–335.
Janik-Papis K, Zaras M, Krzyzanowska A, Wozniak K, Blasiak J, Szaflik J, Szaflik JP: Association between vascular endothelial growth factor gene polymorphisms and age-related macular degeneration in a polish population. Exp Mol Pathol 2009, 87: 234–238.
Lin J-M, Wan L, Tsai Y-Y, Lin H-J, Tsai Y, Lee C-C, Tsai C-H, Tseng S-H, Tsai F-J: Vascular endothelial growth factor gene polymorphisms in age-related macular degeneration. Am J Ophthalmol 2008, 145: 1045–1051. e1042
Boekhoorn SS, Isaacs A, Uitterlinden AG, van Duijn CM, Hofman A, de Jong PT, Vingerling JR: Polymorphisms in the vascular endothelial growth factor gene and risk of age-related macular degeneration: the rotterdam study. Ophthalmology 2008, 115: 1899–1903.
Richardson AJ, Islam F, Guymer RH, Cain M, Baird PN: A tag-single nucleotide polymorphisms approach to the vascular endothelial growth factor-a gene in age-related macular degeneration. Mol Vis 2007, 13: 2148–2152.
Haas P, Steindl K, Aggermann T, Schmid-Kubista K, Krugluger W, Hageman GS, Binder S: Serum vegf and cfh in exudative age-related macular degeneration. Curr Eye Res 2011, 36: 143–148.
Hansen TF, Spindler K-LG, Lorentzen KA, Olsen DA, Andersen RF, Lindebjerg J, Brandslund I, Jakobsen A: The importance of − 460 c/t and + 405 g/c single nucleotide polymorphisms to the function of vascular endothelial growth factor a in colorectal cancer. J Cancer Res Clin Oncol 2010, 136: 751–758.
Awata T, Inoue K, Kurihara S, Ohkubo T, Watanabe M, Inukai K, Inoue I, Katayama S: A common polymorphism in the 5′-untranslated region of the vegf gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes 2002, 51: 1635–1639.
Carneiro ÂM, Costa R, Falcão MS, Barthelmes D, Mendonça LS, Fonseca SL, Gonçalves R, Gonçalves C, Falcão‒Reis FM, Soares R: Vascular endothelial growth factor plasma levels before and after treatment of neovascular age‒related macular degeneration with bevacizumab or ranibizumab. Acta Ophthalmol (Copenh) 2012, 90: e25-e30.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T: Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the anchor study. Ophthalmology 2009, 116: 57–65. e55
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S: Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006, 355: 1432–1444.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY: Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006, 355: 1419–1431.
Imai D, Mori K, Horie-Inoue K, Gehlbach PL, Awata T, Inoue S, Yoneya S: Cfh, vegf, and pedf genotypes and the response to intravitreous injection of bevacizumab for the treatment of age-related macular degeneration. J Ocul Biol Dis inform 2010, 3: 53–59.
Qu Y, Dai H, Zhou F, Zhang X, Xu X, Bi H, Pan X, Wang H, Jiang H, Yin N: Vascular endothelial growth factor gene polymorphisms and risk of neovascular age-related macular degeneration in a chinese cohort. Ophthalmic Res 2010, 45: 142–148.
Boltz A, Ruiß M, Jonas JB, Tao Y, Rensch F, Weger M, Garhöfer G, Frantal S, El-Shabrawi Y, Schmetterer L: Role of vascular endothelial growth factor polymorphisms in the treatment success in patients with wet age-related macular degeneration. Ophthalmology 2012, 119: 1615–1620.
Park UC, Shin JY, Kim SJ, Shin ES, Lee JE, McCarthy LC, Newcombe PJ, Xu C-F, Chung H, Yu HG: Genetic factors associated with response to intravitreal ranibizumab in korean patients with neovascular age-related macular degeneration. Retina 2014, 34: 288–297.
Acknowledgment
This work was granted and supported by the Tunisian Immunology Research Laboratory (LR03SP01) and Oculogenetic Research Unity. Tunis El Manar University. Tunisia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
No benefits in any form have been received or will be received from commercial party related directly or indirectly to the subject of this manuscript.
Authors’ contributions
IH: carried out the molecular genetic studies and drafted the manuscript. IS: follow up of molecular studies, sequence alignment and statistical analysis. AC: clinical monitoring and identification of patients. FK: clinical monitoring and identification of patients. RB: clinical monitoring and identification of patients. SJ: technical part of the study. MM: technical part of the study. TBA: Director of the research laboratory. LM: clinical department head. YG: follow up of this study, correction of manuscript and corresponding author. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Habibi, I., Sfar, I., Chebil, A. et al. Vascular endothelial growth factor genetic polymorphisms and susceptibility to age-related macular degeneration in Tunisian population. Biomark Res 2, 15 (2014). https://doi.org/10.1186/2050-7771-2-15
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2050-7771-2-15