Abstract
The deposition of semen, bacteria and debris in the uterus of the mare after breeding normally induces a self-limiting endometritis. The resultant fluid and inflammatory products are cleared by 48 hours post cover. Mares that are susceptible to persistent post-breeding endometritis (PPBEM) have impaired uterine defence and clearance mechanisms, making them unable to resolve this inflammation within the normal time. This persists beyond 48 hours post-breeding and causes persistent fluid accumulation within the uterus. Mares with PPBEM have an increased rate of embryonic loss and a lower overall pregnancy rate than those without the condition. To enhance conception rates, mares at high risk need optimal breeding management as well as early diagnosis, followed by the most appropriate treatment. This article reviews the pathogenesis, diagnosis and treatment of PPBEM and the management of affected mares.
Similar content being viewed by others
Introduction
Persistent post-breeding endometritis (PPBEM) is the third most common medical condition of adult female horses [37] and the major reason for failure to conceive [14]. It affects approximately 15% of Thoroughbred mares after natural cover [47]. Due to its association with decreased fertility, it is of major concern to breeders and veterinary practitioners [44].
Early embryonic death rates are three times higher in mares with this condition than in normal mares [24], making it an important cause of loss to the bloodstock industry.
The aim of this article is to review the pathogenesis, diagnosis and treatment of PPBEM and the management of affected mares. It aims to provide breeders with a better understanding of the disease and enable them to optimise their breeding management in order to reduce its incidence. It also aims to update practitioners, allowing them to detect such mares early and administer the most appropriate and successful treatment.
Pathophysiology of PPBEM
Inflammation of the endometrium is caused by a response to exogenous materials introduced directly into the uterus at breeding [40]. These include components of the semen, extender (in the case of AI), bacteria and other debris [39, 40, 11].
The normal endometrial inflammatory response, which is triggered by these antigens, is a predictable, physiological event [40]. It is most commonly seen within a half to one hour of breeding [19] and is necessary to clear dead spermatozoa and bacteria from the uterine lumen [39].
The influx of polymorphonuclear neutrophils (PMNs) into the uterine lumen and their phagocytic activity after opsonisation of the target [19, 39, 40] is followed by myometrial contractions regulated by prostaglandin F2α and oxytocin [39]. This uterine defence mechanism peaks at around six to 12 hours post insemination [19]. In the normal mare, most of the inflammatory products are cleared by physical uterine clearance mechanisms within 48 hours of cover [19]. Because the embryo leaves the uterine tube and enters the uterus on about days five to six post-ovulation [5], the uterine inflammation has to be under control by 96 hours postovulation to maximise survival of the embryo [39]. A mare that is susceptible to PPBEM is unable to clear such fluid by 96 hours and the resulting prolonged inflammation generates an embryo-toxic environment [39, 40].
In addition to this, premature lysis of the corpus luteum is caused by complement products. Leukotriene B4, prostaglandin E and prostaglandin F2α [32], and subsequent progesterone deficiency, all contribute to embryo mortality [32]. Prolonged inflammation may be caused by several factors. Post-ovulatory artificial insemination (AI) more than 12 hours post-ovulation may influence the predisposition towards PPBEM, as rising progesterone levels may decrease uterine defence and uterine clearance [19, 45]. In contrast, AI undertaken between 12 hours pre-ovulation and four to eight hours post-ovulation has no influence on fluid accumulation or pregnancy rate [45]. Two artificial inseminations within 24 hours during oestrus (in the absence of seminal plasma) profoundly reduce fertility, since the second insemination takes place when the uterine inflammation is at its peak [3]. Although spermatozoa can survive in a uterine environment already inflamed by an initial insemination, their motility is progressively reduced due to their aggregation with PMNs [2]. This results in a reduction in the number of viable spermatozoa reaching the uterine tube for fertilisation [3, 41].
The use of spermatozoa with reduced seminal plasma (as in frozen/thawed semen or sperm 'packed' from fresh semen by centrifugation) results in a more marked and prolonged inflammatory response [40], because seminal plasma is a modulator of sperm-induced inflammation [39, 40] and protects viable spermatozoa from opsonisation and phagocytosis [40].
It is commonly assumed that mares that are susceptible to PPBEM are older maiden [21, 31] or multi-parous mares [21, 8, 9, 18] with a history of repeated fluid accumulation [31] and low fertility rates [47]. However, new studies by Veronesi et al. [42] show that the average age of normal mares (14 years) and susceptible mares (16 years) is not significantly different. Rigby et al. [34] also suggest that myometrial dysfunction in mares with PPBEM is not dependent on age or pregnancy number.
Delayed uterine clearance of bacteria, fluid and debris following mating [27] may be caused by many different factors. These include: decreases in the frequency, intensity and duration of the myometrial activity [27, 39, 34]; vascular changes in the endometrium [39, 34]; altered hormonal responses [39, 34]; and, altered mucus production [10, 11]. Other factors in multiparous mares include altered neuromuscular interactions [34], or impaired lymphatic drainage [11] due to partial dilation of the uterus [39] or caudo-ventral displacement of the uterus [21]. All of these conditions enhance intrauterine inflammation and fluid accumulation [39, 14].
The vulva, vestibule, vagina and cervix normally act as physical barriers protecting the uterus from external contamination [19, 39, 32] (Figure 1). There is a higher predisposition towards PPBEM in mares with previous foaling injuries [18], poor perineal conformation [18], altered conformation of the vulva [16] (Figure 2) and incomplete closure or persistent relaxation of the vulvar lips [32, 16]. Therefore, the above abnormalities can all affect barrier function, causing air, faeces and urine to enter the reproductive tract [18]. In addition, cervical incompetence has consequences for both barrier function and uterine clearance. It may include either insufficient relaxation during oestrus with impaired cervical drainage [18, 31] or improper closure during dioestrus [32], predisposing to bacterial colonisation before breeding.
Physical clearance mechanisms in PPBEM
The equine uterus is strongly dependent on a physical clearance mechanism, which is based on ciliary beating and uterine contraction [12]. An imbalance or breakdown of this sensitive system increases the risk of PPBEM in mares [39].
The equine endometrium consists of mucus secreting and ciliated cells [12]. The cilia beat 13 times per second and help to transport mucus, fluid, bacteria and other debris along the longitudinal folds of the endometrium and the cervix [12]. However, dilated capillary spaces between the folds and damaged, scarred or atrophic folds disturb this precise clearance mechanism [12]. Ulceration, degeneration and lack of cilia are suggested to have the same effect [12].
Mucus overlies, hydrates and lubricates the endometrium [20]. It also prevents bacteria from binding to cell receptors [20, 11]. Water, ions, specific antibacterial proteins (including lactoferrin, lysozyme and immunoglobulins) and mucins are the main components of the mucus-gel [17, 46, 28]. Mucins are highly hydrated glycoproteins [20, 46]. A change in the hydration of their polysaccharide chains is believed to disturb the viscosity and elasticity of the mucus [12]. As a consequence, pathogens trapped in the mucus and fluid are not cleared [46, 28, 12].
Smooth muscle cells form the inner circular and the outer longitudinal layer of the myometrium [30]. It is hypothesised that contractile defects of the myometrium increase the susceptibility of mares to PPBEM [27, 34]. Troedsson et al. [38] showed that the myometrial electrical activity, which was measured 20 hours after bacterial challenge, was increased in both resistant and susceptible mares. However, resistant mares showed a greater increase in frequency, intensity and duration of uterine electrical activity compared to susceptible mares [38].
Diagnosis of susceptible mares
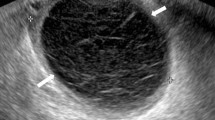
While PPBEM is easily diagnosed, its root causes are not always thoroughly investigated. A comprehensive assessment requires a detailed breeding history [44, 22]. Any changes in perineal, vulvar or cervical conformation that predispose the mare to PPBEM should also be evaluated before breeding [44, 22, 16]. It is very important to check that the cervix opens properly in oestrus and closes in dioestrus. Routine pre-breeding uterine swabs should be obtained from 'at-risk' mares, using double-guarded swabs. These minimise contamination with cervical, vaginal or perineal bacterial flora [11], offering the possibility of isolating organisms that are characteristic of susceptible mares, such as non-haemolytic Escherichia coli and β-haemolytic Streptococcus [1, 10, 26]. Bacteriology and cytology should always be conducted together, as the detection of PMNs together with potential pathogens is a much better indicator of the condition than bacteriology alone [26]. Following this initial examination, confirmation of a diagnosis of PPBEM is achieved using transrectal ultrasound examination 24 to 48 hours after breeding. The presence of free fluid in the uterine lumen (more than 15 to 20 mm in diameter) [45, 4, 7] is diagnostic for the condition [27, 7, 22, 15] (Figure 3). The earlier the diagnosis is made, the earlier the practitioner is able to start an effective treatment. This greatly enhances the chance of the mare carrying a foal to term. It is not uncommon that mares with a long history of normal fertility can spontaneously acquire PPBEM, giving the clinician no opportunity for prophylactic intervention [44].
Treatment options
The aim of the treatment is to completely clear fluid accumulation, contaminants and inflammatory products within 96 hours [19]. This ensures that the embryo encounters a healthy endometrium at day five [5], thus facilitating implantation [10]. A single dose of oxytocin (10-20 IU, IM) is usually enough to clear uterine fluid less than 20 mm in diameter [31]. If not, it should be repeated every four to six hours until the fluid is completely cleared [31].
In mares with intra-uterine fluid accumulation of more than 20 mm in diameter, treatment should consist of uterine lavage [31]. One to two litres of warm, sterile saline or a 0.05% povidone-iodine solution (5 ml of a 10% povidone-iodine solution to each litre of balanced salt solution) [6] are infused via a large-bore equine embryo-flushing catheter until the recovered fluid is clear. This should be followed by administration of uterotonic substances such as oxytocin [31]; 10 IU preovulatory and 25 IU post-ovulatory, IV or IM [14].
Another option is the administration of cloprostenol (a prostaglandin analogue; 250 μg IM daily, starting four hours after breeding) [22] that clears the fluid more rapidly than PGF 2α and has a longer duration of activity compared to oxytocin [13]. It is known that this drug has a negative effect on progesterone levels during early pregnancy (day two until day seven postovulation) when given throughout the periovulatory period until two days post-ovulation, but it does not decrease pregnancy rates, as the levels rise again at day seven and seem to be normal at day nine [25].
The mare is re-examined 24 hours after initial treatment. If fluid is still present, treatment is repeated [31]. For susceptible mares, treatment should be timed with breeding and it should not be delayed until ovulation occurs [22, 31]. As long as the mare is in oestrus, the cervix remains open, allowing the practitioner to perform several flushes before ovulation, if necessary [31]. Treatment should be delayed for four hours after breeding [33]. This allows enough time for the motile sperm to enter the uterine tube [33].
The day upon which oxytocin is administered also plays an important role in the contractility of the endometrium [14]. It is suggested that mares that are susceptible to post-breeding endometritis should have more time between breeding (24-48 hours before ovulation) and ovulation [14, 29]. Oxytocin treatment should be started in the pre-ovulatory period, as the response to oxytocin by the endometrium is higher when progesterone levels are low and oestrogen levels are high [14].
If oxytocin treatment is used after ovulation, the dose should be increased to achieve an effect [14, 29]. However, care has to be taken, as tetanic contractions can occur if more than 25 IU of oxytocin are administered, leading to retention of uterine fuid [8]. It is also suggested that an inflamed endometrium is more sensitive to oxytocin than a non-inflamed endometrium [42]. It is very important that the treatment is based on the individual mare, as standard treatment may not be effective in all cases [31].
Optimal breeding management
Optimising the breeding management of the mare is the most important aspect in reducing the susceptibility of the mare for post-breeding endometritis, but it is also an aspect that is often underestimated. Prevention is always better than cure.
Choosing the right time for breeding or inducing ovulation (2,500 IU of hCG when the follicle has a diameter more than 35 mm) [35, 31] reduces the need for more than one insemination during oestrus [32, 31]. Thus the spermatozoa are not exposed to an inflammatory uterine environment [2, 40].
Early breeding enhances the chance of accumulated fluid being expelled before the cervix starts to close rapidly after ovulation. In addition, the natural immune defence mechanisms of the endometrium are more pronounced during oestrus than in dioestrus [19, 31]. With regard to breeding hygiene, bandaging the mare's tail and cleaning the vulva and perineal area with clean water help to minimise contamination during cover [31].
AI with fresh or cooled semen is another means by which uterine inflammation can be reduced (but not eliminated), as exogenous bacterial contamination is limited [31]. It is very important to know that old mares bred with frozen semen are at a higher risk of developing PPBEM, as seminal plasma is reduced by the process of cryopreservation [43, 40]. Therefore, its suppressive effect on post-breeding endometritis is lost [40].
New trends in insemination, for example the use of sexsorted (frozen-thawed) semen, require low sperm dose insemination techniques to achieve pregnancy [36, 15, 23]. The uterotubal junction (UTJ) is the sperm reservoir in the mare [23]. Therefore, transrectally-, transendoscopically- or ultrasonographically-guided deep intrauterine horn insemination with a flexible catheter, onto or close to the UTJ papilla, is becoming more and more popular [36, 15, 23]. Approximately one to 25 × 106 progressively motile spermatozoa in volumes between 20 and 1,000 μl are needed [23]. This low number of spermatozoa is preferred when inseminating into the uterine horn ipsilateral to the dominant pre-ovulatory follicle to avoid increased inflammation in normal mares [15].
However, it should be borne in mind that these methods require a very skilled and experienced inseminator and that they seem to result in lower pregnancy rates in problem mares [36].
Correction of anatomical defects, for example by Caslick's vulvoplasty operation, can protect and prevent the reproductive tract from constant external contamination, air aspiration [11] and ascending inflammation [31].
Conclusion
Much research has been performed over the last few decades to determine the reasons for this multi-factorial condition in mares. There are also successful and accepted treatment programmes, which help improve pregnancy rates by completely clearing fluid early after breeding. However, more effective and rapid prognostic and diagnostic tests are required for improved management of PPBEM.
References
Albihn A, Baverud V, Magnusson U: Uterine microbiology and antimicrobial susceptibility in isolated bacteria from mares with fertility problems. Acta Veterinaria Scandinavia. 2003, 44: 121-129. 10.1186/1751-0147-44-121.
Alghamdi A, Troedsson MH, Laschkewitsch T, Xue JL: Uterine secretion from mares with post-breeding endometritis alters sperm motion characteristics in vitro. Theriogenology. 2001, 55: 1019-1028. 10.1016/S0093-691X(01)00462-9.
Alghamdi AS, Foster DN, Troedsson MH: Equine seminal plasma reduces sperm binding to polymorphonuclear neutrophils (PMNs) and improves the fertility of fresh semen inseminated into inflamed uteri. Reproduction. 2004, 127: 593-600. 10.1530/rep.1.00096.
Barbacini S, Necchi D, Zavaglia G, Squires EL: Retrospective study on the incidence of postinsemination uterine fluid in mares inseminated with frozen/thawed semen. Journal of Equine Veterinary Science. 2003, 23: 493-496. 10.1016/j.jevs.2003.10.003.
Betteridge KJ, Eaglesome MD, Mitchell D, Flood PF, Beriault R: Development of horse embryos up to twenty two days after ovulation: observations on fresh specimens. Journal of Anatomy. 1982, 135: 191-209.
Brinsko SP: How to perform uterine lavage: indications and practical techniques. Proceedings American Association of Equine Practitioners. 2001, 47: 407-411.
Brinsko SP, Rigby SL, Varner DD, Blanchard TL: A practical method for recognizing mares susceptible to post-breeding endometritis. Proceedings American Association of Equine Practitioners. 2003, 49: 363-365.
Cadario ME, Merritt AM, Archbald LF, Thatcher WW, LeBlanc MM: Changes in intrauterine pressure after oxytocin administration in reproductively normal mares and in those with a delay in uterine clearance. Theriogenology. 1999, 51: 1017-1025. 10.1016/S0093-691X(99)00047-3.
Cadario ME, Thatcher WW, Klapstein E, Merrit AM, Archbald LF, Thatcher MJ, LeBlanc MM: Dynamics of prostaglandin secretion, intrauterine fluid and uterine clearance in reproductively normal mares and mares with delayed uterine clearance. Theriogenology. 1999, 52: 1181-1192. 10.1016/S0093-691X(99)00210-1.
Card C: Post-breeding inflammation and endometrial cytology in mares. Theriogenology. 2005, 64: 580-588. 10.1016/j.theriogenology.2005.05.041.
Causey RC: Making sense of equine uterine infections: the many faces of physical clearance. Veterinary Journal. 2006, 172: 405-421. 10.1016/j.tvjl.2005.08.005.
Causey RC: Mucus and the mare: How little we know. Theriogenolgy. 2007, 68: 386-394. 10.1016/j.theriogenology.2007.04.011.
Combs GB, LeBlanc MM, Neuwirth L, Tran TQ: Effects of prostaglandin F2 [alpha], cloprostenol and fenprostalene on uterine clearance of radiocolloid in the mare. Theriogenology. 1996, 45: 1449-1455. 10.1016/0093-691X(96)00112-4.
Gutjahr S, Paccamonti DL, Pycock JF, Taverne MA, Dieleman SJ, van der Weijden GC: Effect of dose and day of treatment on uterine response to oxytocin in mares. Theriogenology. 2000, 54: 447-456. 10.1016/S0093-691X(00)00361-7.
Guevenc K, Reilas T, Katila T: Effect of insemination dose and site on uterine inflammatory response of mares. Theriogenology. 2005, 44: 2504-2512.
Hemberg E, Lundeheim N, Einarsson S: Retrospective study on vulvar conformation in relation to endometrial cytology and fertility in thoroughbred mares. Journal of Veterinary Medicine Series A. 2005, 52: 474-477. 10.1111/j.1439-0442.2005.00760.x.
Howe L, Wiggins R, Soothill PW, Millar MR, Horner PJ, Corfield AP: Mucinase and sialidase activity of the vaginal microflora: implications for the pathogenesis of preterm labour. International Journal of STD and AIDS. 1999, 10: 442-447. 10.1258/0956462991914438.
Hurtgen JP: Pathogenesis and treatment of endometritis in the mare: a review. Theriogenology. 2006, 66: 560-566. 10.1016/j.theriogenology.2006.04.006.
Katila T: Uterine defence mechanisms in the mare. Animal Reproduction Science. 1996, 42: 197-204. 10.1016/0378-4320(96)01507-2.
Lagow E, DeSouza MM, Carson DD: Mammalian reproductive tract mucins. Human Reproduction Update. 1999, 5: 280-292. 10.1093/humupd/5.4.280.
LeBlanc MM, Neuwirth L, Jones L, Cage C, Mauragis D: Differences in uterine position of reproductively normal mares and those with delayed uterine clearance detected by scintigraphy. Theriogenology. 1998, 50: 49-54. 10.1016/S0093-691X(98)00112-5.
LeBlanc MM: Persistent mating induced endometritis in the mare: pathogenesis, diagnosis and treatment. Recent Advances in Equine Reproduction. Edited by: Ball BA. 2003, New York: International Veterinary Information Service
Lyle SK, Ferrer MS: Low-dose insemination - Why, when and how. Theriogenolgy. 2005, 64: 572-579. 10.1016/j.theriogenology.2005.05.012.
Malschitzky E, Schilela A, Mattos ALG, Garbade P, Gregory RM, Mattos RC: Intrauterine fluid accumulation during foal heat increases embryonic death. Pferdeheilkunde. 2003, 19: 246-249.
Nie GJ, Johnson KE, Wenzel JG, Braden TD: Effect of administering oxytocin or cloprostenol in the periovulatory period on pregnancy outcome and luteal function in mares. Theriogenology. 2003, 60: 1111-1118. 10.1016/S0093-691X(03)00111-0.
Nielsen JM: Endometritis in the mare: a diagnostic study comparing cultures from swab and biopsy. Theriogenology. 2005, 64: 510-518. 10.1016/j.theriogenology.2005.05.034.
Nikolakopoulos E, Watson ED: Uterine contractility is necessary for the clearance of intrauterine fluid but not bacteria after bacterial infusion in the mare. Theriogenology. 1999, 52: 413-423. 10.1016/S0093-691X(99)00139-9.
Olmsted SS, Meyn LA, Rohan LC, Hillier SL: Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sexually Transmitted Diseases. 2003, 30: 257-260. 10.1097/00007435-200303000-00016.
Paccamonti DL, Lyle SK: Therapeutic considerations or pharmacological treatment of delayed uterine clearance. Pferdeheilkunde. 2003, 19: 656-659.
Priedkalns J, Leiser R: Female Reproductive System. Textbook of Veterinary Histology. Edited by: Dellmann HD, Eurell JA. 1998, Baltimore: Lippincottt, Williams and Wilkins, 247-261. Fifth
Pycock JF: How to maximize the chances of breeding successfully from the older maiden mare. Proceedings American Association of Equine Practitioners. 2006, 52: 245-249.
Rambags BPB: Early pregnancy loss in aged mares: probable causes and cures. Pferdeheilkunde. 2003, 19: 653-656.
Rigby S, Hill J, Miller C, Thompson J, Varner D, Blanchard D: Administration of oxytocin immediately after insemination does not improve pregnancy rates in mares bred by fertile or subfertile stallions. Theriogenology. 1999, 51: 1143-1150. 10.1016/S0093-691X(99)80017-X.
Rigby SL, Barhoumi R, Burghardt RC, Colleran P, Thompson JA, Varner DD, Blanchard TL, Brinsko SP, Taylor T, Wilkerson MK, Delp MD: Mares with delayed uterine clearance have an intrinsic defect in myometrial function. Biology of Reproduction. 2001, 65: 740-747. 10.1095/biolreprod65.3.740.
Samper JC: Breeding mares with frozen semen in private practice. Proceedings American Association of Equine Practitioners. 2001, 47: 314-318.
Sieme H, Bonk A, Ratjen J, Klug E, Rath D: Effect of number and site/technique of insemination on pregnancy in mares. Pferdeheilkunde. 2003, 19: 677-683.
Traub-Dargatz JL, Salman MD, Voss JL: Medical problems of adult horses, as ranked by equine practitioners. Journal of American Veterinary Association. 1991, 198: 1745-1747.
Troedsson MH, Liu IK, Ing M, Thurmond M: Multiple site electromyography recordings of uterine activity following an intrauterine bacterial challenge in mares susceptible and resistant to chronic uterine infection. Journal of Reproduction and Fertility. 1993, 99: 307-313. 10.1530/jrf.0.0990307.
Troedsson MH: Uterine clearance and resistance to persistent endometritis in the mare. Theriogenology. 1999, 52: 461-471. 10.1016/S0093-691X(99)00143-0.
Troedsson MH, Loset K, Alghamdi AM, Dahms B, Crabo BG: Interaction between equine semen and the endometrium: the inflammatory response to semen. Animal Reproduction Science. 2001, 68: 273-278. 10.1016/S0378-4320(01)00164-6.
Troedsson MH, Desvousges A, Alghamdi AS, Dahms B, Dow CA, Hayna J, Valesco R, Collahan PT, Macpherson ML, Pozor M, Buhi WC: Components in seminal plasma regulating sperm transport and elimination. Animal Reproduction Science. 2005, 89: 171-186. 10.1016/j.anireprosci.2005.07.005.
Veronesi MC, Carluccio A, Kindahl H, Faustini M, Battocchio M, Cairoli F: Oxytocin-induced PGF2 alpha release in mares with and without post-breeding delayed uterine clearance. Journal of Veterinary Medicine Series A. 2006, 53: 259-262. 10.1111/j.1439-0442.2006.00820.x.
Vidament M, Dupere AM, Julienne P, Evain A, Noue P, Palmer E: Equine frozen semen: Freezability and fertility field results. Theriogenology. 1997, 48: 907-917. 10.1016/S0093-691X(97)00319-1.
Watson ED: Post-breeding endometritis in the mare. Animal Reproduction Science. 2000, 60-61: 221-232. 10.1016/S0378-4320(00)00110-X.
Watson ED, Barbacini S, Berrocal B, Sheerin O, Marchi V, Zavaglia G, Necchi D: Effect of insemination time of frozen semen on incidence of uterine fluid in mares. Theriogenology. 2001, 56: 123-131. 10.1016/S0093-691X(01)00548-9.
Wiggins R, Hicks SJ, Soothill PW, Millar MR, Corfield AP: Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sexually Transmitted Infections. 2001, 77: 402-408. 10.1136/sti.77.6.402.
Zent WW, Troedsson MHT, Xue J-L: Postbreeding uterine fluid accumulation in a normal population of thoroughbred mares: a field study. Proceedings of American Association of Equine Practitioners. 1998, 44: 64-65.
Acknowledgements
The authors are currently funded under the National Development Plan, through the Research Stimulus Fund, administered by the Department of Agriculture, Fisheries and Food. The authors would like to thank Mary Gallagher, Eamonn Fitzpatrick, Carolyn Cummins and Wilfried Schneeweiss for all their help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Maischberger, E., Irwin, J., Carrington, S. et al. Equine post-breeding endometritis: A review. Ir Vet J 61, 163 (2008). https://doi.org/10.1186/2046-0481-61-3-163
Published:
DOI: https://doi.org/10.1186/2046-0481-61-3-163