Abstract
Background
While paraneoplastic leukocytosis is a common phenomenon in solid tumors, extreme elevations of white blood counts (WBC) in the range of more than 100,000/μl are uncommon in patients with non-hematologic malignancies. Leukocytosis with mature neutrophils due to a granulocyte colony-stimulating factor (G-CSF) producing tumor is only seen on rare occasions.
Case presentation
Massive neutrophil leukocytosis of approximately 100,000/μl was diagnosed in a 57-year-old Caucasian woman with metastatic undifferentiated endometrial sarcoma. A bone marrow trephine biopsy revealed massively increased granulopoiesis, but no evidence of monoclonal myeloproliferative disease. After the primary tumor had been resected, white blood count (WBC) plummeted and went back to nearly normal levels within one week. With progressive metastatic disease, granulocyte colony-stimulating factor (G-CSF) plasma levels were found to be increased by 10-fold. White blood count (WBC) strictly correlated with tumor burden and response to chemotherapy. In the final stage of treatment resistent disease, white blood count (WBC) approximated 300,000/μl.
Conclusion
We report on a granulocyte colony-stimulating factor (G-CSF) secreting undifferentiated endometrial sarcoma, which was associated with extreme neutrophil counts. White blood count (WBC) were closely correlated with tumor burden and associated with an aggressive clinical course. We suggest that paraneoplastic neutrophilia represents a poor prognostic sign in soft tissue sarcoma. In patients with similar constellations, antitumor therapy must not be delayed.
Similar content being viewed by others

Background
Paraneoplastic leukocytosis is a common phenomenon in several different solid tumors. Affected patients typically have total white blood counts (WBC) between 12,000 and 30,000/μl with mainly mature neutrophil granulocytes [1].
Idiopathic extreme leukocytosis, also known as leukemoid reaction and defined as WBC ≥ 40,000/μl is rare in patients with non-hematologic malignancies: in the largest retrospective analysis of these uncommon patients, only 10% of cases were paraneoplastic in origin. Ninety-six percent of these patients had neutrophilia, and the mean WBC was 53,000/μl [2]. A severe left shift, i.e. the presence of immature granulocytes such as myelocytes, promyelocytes, and blasts suggests myeloproliferative disorders, while paraneoplastic neutrophilia typically presents with mature neutrophils only. Leukocytosis with non-clonally derived mature neutrophils, due to paraneoplastic production of granulocyte colony-stimulating factor (G-CSF) is a rare phenomenon and has been described in a variety of different tumors [2, 3]. Most cases are associated with gastrointestinal, lung, or genitourinary carcinomas [4–8]. G-CSF producing tumors are typically poorly differentiated and patients are diagnosed in an advanced stage with a large tumor burden [9, 10]. Accordingly, disease outcomes are poor, which has been postulated to represent autocrine stimulation of tumor growth by G-CSF [11, 12]. In this article we report on a patient with a G-CSF producing undifferentiated endometrial sarcoma with massive neutrophil leukocytosis and an aggressive clinical course.
Case presentation
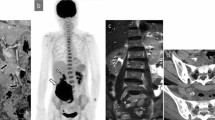
Herein, we report on a 57-year-old Caucasian woman, who presented to our emergency department on July 30th 2011 with severe abdominal pain and a palpable lower abdominal tumor. A computed tomography (CT) scan revealed a 14 × 10 × 12 cm mass with close relation to the uterus (Figure 1). Multiple pulmonary and peritoneal nodules consistent with metastatic disease were seen. Blood counts revealed massive leukocytosis (WBC 96,910/μl) and anemia with a hemoglobin concentration of 8 g/dl. A differential hemogram revealed immature precursor cells such as promyelocytes and myelocytes as well as pronounced neutrophilia with 88% neutrophils. Lactate dehydrogenase (LDH) was slightly elevated (260 U/l), but the remaining laboratory values were within normal limits (Table 1). A bone marrow biopsy was performed at the same day. Figure 2A shows the trephine biopsy at 400x magnification stained with hematoxylin & eosin (H&E). Figure 2B reveals marked hypercellularity due to granulocytic proliferation (naphthol-ASD-chloroacetate esterase stain, 400x). Immunohistochemistry with anti-CD34 stained vascular endothelium as positive control, but there was no detecteable increase in CD34+ cells (Figure 2C, 200x). Moreover, there were no signs of myelodysplasia. Cytogenetic and molecular analyses revealed neither translocation t(9;22) nor a BCR/ABL fusion gene. In addition, analysis of JAK2-V617F mutation was performed and showed a negative result.
Bone marrow trephine biopsy performed on admission. A) Hematoxylin & eosin staining, 400x magnification. B) Naphthol-ASD-chloroacetate esterase stain, 400x magnification, showing marked hypercellularity due to granulocytic hyperproliferation (black arrows). C) Anti-CD34 immunohistochemistry reveals no detectable increase in CD34+ cells, 200x magnification.
Simultaneously, the general condition of the patient rapidly worsened over night and a further hemoglobin decline was diagnosed. Another CT scan on the following day confirmed abdominal bleeding (white arrows, Figure 3). An emergency operation including hysterectomy and bilateral adnexectomy was performed. Intraoperatively, a grossly enlarged, partly ruptured uterus with spontaneous intraperitoneal bleeding was found. A concurrently causative bleeding disturbance was excluded by routine laboratory examinations. Histology revealed undifferentiated endometrial sarcoma (FNCLCC grade 3).
Only few hours after tumor resection, leukocyte and platelet counts decreased rapidly. Within one week, blood counts returned to near normal levels. Figure 4 depicts the rapid decline of WBC immediately after surgery.
Unfortunately, the first postoperative CT scan documented rapid progression of bilateral disseminated pulmonary metastases (Figure 5). In parallel, WBC increased, and chemotherapy with gemcitabine and docetaxel was initiated [13]. Temporary stabilization was achievable, and WBC correlated strictly with tumor burden, showing a significant increase after 5 cycles of chemotherapy. At that time, another CT scan confirmed considerable growth of all preexisting and multiple new tumor manifestations. Because paraneoplastic G-CSF secretion was suspected, G-CSF plasma levels were analyzed by enzyme linked immunosorbent assay (ELISA, RnD Systems) and found to be enormously increased to 1,270 pg/ml, corresponding to about 10 times the upper limit of normal (range 12,5 – 142 pg/ml). At the same day, WBC had reached almost 200,000/μl (Figure 4). The performance status of the patient worsened rapidly, certainly due to the massive tumor growth with organ infiltration but also due to a developing leukostasis syndrome. Second-line chemotherapy with doxorubicin was initiated, but proved to be ineffective in controlling the disease. After 2 cycles, chemotherapy was discontinued. The patient passed away within few weeks due to fulminant disease progression.
Conclusion
In summary, this case demonstrates severe leukemoid reaction with pathologic left shift caused by a G-CSF secreting advanced undifferentiated endometrial sarcoma imitating hematologic malignancy.
Consistent with previously reported cases of paraneoplastic G-CSF secretion, leukemoid reaction was closely correlated with tumor burden and associated with an aggressive clinical course. To our knowledge, neutrophilic leukocytosis up to 200,000/μl and a severe left shift with massively increased G-CSF levels has not been reported before as a paraneoplastic phenomenon in sarcoma. We suggest not to delay antitumor treatment in patients with a similar constellation, but rather aggressively treat the underlying solid malignancy utilizing a response-inducing chemotherapy regimen.
Consent
Written informed consent was obtained from the patient herself before blood was drawn for G-CSF analysis. The patient agreed to publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
Shoenfeld Y, Tal A, Berliner S, Pinkhas J: Leukocytosis in non hematological malignancies - a possible tumor-associated marker. J Cancer Res Clin Oncol. 1986, 111 (1): 54-58. 10.1007/BF00402777.
Granger JM, Kontoyiannis DP: Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study. Cancer. 2009, 115 (17): 3919-3923. 10.1002/cncr.24480.
Sakka V, Tsiodras S, Giamarellos-Bourboulis EJ, Giamarellou H: An update on the etiology and diagnostic evaluation of a leukemoid reaction. Eur J Intern Med. 2006, 17 (6): 394-398. 10.1016/j.ejim.2006.04.004.
Nakamura A, Tanaka S, Takayama H, Sakamoto M, Ishii H, Kusano M, Onizuka Y, Ota S, Mitamura K: A mesenteric liposarcoma with production of granulocyte colony-stimulating factor. Intern Med. 1998, 37 (10): 884-890. 10.2169/internalmedicine.37.884.
Marui T, Yamamoto T, Akisue T, Hitora T, Yoshyia S, Kurosaka M: Granulocyte colony-stimulating factor-producing undifferentiated sarcoma occurring in previously fractured femur. A case report and review of the literature. Arch Pathol Lab Med. 2003, 127 (4): e186-189.
Nasser SM, Choudry UH, Nielsen GP, Ott MJ: A leukemoid reaction in a patient with a dedifferentiated liposarcoma. Surgery. 2001, 129 (6): 765-767. 10.1067/msy.2001.109498.
Nara T, Hayakawa A, Ikeuchi A, Katoh N, Kishimoto S: Granulocyte colony-stimulating factor-producing cutaneous angiosarcoma with leukaemoid reaction arising on a burn scar. Br J Dermatol. 2003, 149 (6): 1273-1275. 10.1111/j.1365-2133.2003.05684.x.
Ishii T, Sakamoto A, Matsuda S, Matsumoto Y, Harimaya K, Takahashi Y, Oda Y, Iwamoto Y: Leiomyosarcoma in the humerus with leukocytosis and elevation of serum G-CSF. Skeletal Radiol. 2012, 41 (6): 719-723. 10.1007/s00256-011-1337-6.
Kasuga I, Makino S, Kiyokawa H, Katoh H, Ebihara Y, Ohyashiki K: Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001, 92 (9): 2399-2405. 10.1002/1097-0142(20011101)92:9<2399::AID-CNCR1588>3.0.CO;2-W.
Kitamura H, Kodama F, Odagiri S, Negahara N, Inoue T, Kanisawa M: Granulocytosis associated with malignant neoplasms: a clinicopathologic study and demonstration of colony-stimulating activity in tumor extracts. Hum Pathol. 1989, 20 (9): 878-885. 10.1016/0046-8177(89)90100-7.
Berdel WE, Danhauser-Riedl S, Steinhauser G, Winton EF: Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood. 1989, 73 (1): 80-83.
Nakayama S, Yokote T, Iwaki K, Hirata Y, Akioka T, Miyoshi T, Takayama A, Nishiwaki U, Masuda Y, Tsuji M, Hanafusa T: Co-expression of multiple cytokines and their receptors in primary clear cell sarcoma of the pubic bone with features of a small round cell tumor. Int J Immunopathol Pharmacol. 2012, 25 (3): 799-804.
Tanner EJ, Garg K, Leitao MM, Soslow RA, Hensley ML: High grade undifferentiated uterine sarcoma: surgery, treatment, and survival outcomes. Gynecol Oncol. 2012, 127 (1): 27-31. 10.1016/j.ygyno.2012.06.030.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CD and HGK wrote the manuscript. SB and EM performed laboratory analyses. MRM, KCW, and LK revised and corrected the manuscript. UV has processed tumor tissue inclusive immunohistochemistry. MH provided CT-imaging. All authors read and approved the final manuscript.
Christiane Dorn, Stefanie Bugl contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Dorn, C., Bugl, S., Malenke, E. et al. Paraneoplastic granulocyte colony-stimulating factor secretion in soft tissue sarcoma mimicking myeloproliferative neoplasia: a case report. BMC Res Notes 7, 313 (2014). https://doi.org/10.1186/1756-0500-7-313
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-0500-7-313