Abstract
Background
Granulocyte-colony stimulating factor (G-CSF)-producing tumors can cause leukocytosis despite an absence of infection. G-CSF-producing tumors have been reported in various organs such as the lung, esophagus, and stomach but rarely in the breast. We report a case of G-CSF-producing malignant phyllodes tumor of the breast.
Case presentation
An 84-year-old woman visited our hospital complaining of a lump in her left breast without fever and pain. Laboratory tests revealed elevated white blood cell (WBC) count and G-CSF levels. A malignant tumor of the breast was diagnosed by core needle biopsy. We performed a total mastectomy and sentinel lymph node biopsy. The tumor was identified as a G-CSF-producing malignant phyllodes tumor. Within 7 days after surgery, the patient’s WBC count and G-CSF level had decreased to normal levels. She is alive without recurrence 13 months after surgery.
Conclusions
We encountered a rare case of G-CSF-producing malignant phyllodes tumor of the breast. PET–CT revealed diffuse accumulation of FDG in the bone. Phyllodes tumors need to be differentiated from bone metastasis, lymphoma, and leukemia. We must be careful to not mistake this type of tumor for bone marrow metastasis.
Similar content being viewed by others
Background
Granulocyte-colony stimulating factor (G-CSF)-producing tumors have been shown to cause leukocytosis despite the absence of infection. [1] G-CSF-producing tumors have been reported in various organs such as the lung, bladder, and stomach but rarely in the breast [2,3,4]. G-CSF-producing carcinomas progress rapidly and have poor prognosis. [5] We report a case of G-CSF-producing malignant phyllodes tumor of the breast.
Case presentation
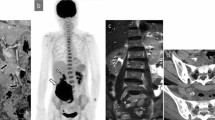
An 84-year-old woman suddenly noticed a lump in her left breast, so she visited our hospital. Physical examination revealed a large tumor with redness approximately 8 cm in the maximal dimension, which was occupying the entire left breast. Laboratory tests showed an elevated white blood cell (WBC) count (49,760 cells/μL with 87% neutrophils) without fever and a high G-CSF level (498 pg/mL). Tumor markers were normal (CEA 1.0 ng/mL, CA15-3 13.8 U/mL). Mammography revealed a large, high-density mass in her left breast (Fig. 1). Ultrasound examination revealed a lobulated, inhomogeneous, blood-rich mass. Contrast-enhanced magnetic resonance imaging revealed a large lobulated mass without apparent infiltration to the pectoral major muscle (Fig. 2). Computed tomography showed no enlarged axillary lymph nodes. Fluorodeoxyglucose (FDG) positron emission tomography–computed tomography (PET–CT) revealed no distant metastasis, although mild-to-moderate and homogeneous FDG uptake were detected in the spine, pelvic bone, and long bones, suggesting bone marrow hyperactivity (Fig. 3). She did not present splenomegaly. Histological diagnosis was difficult to confirm using core needle biopsy. These results suggested that this tumor was malignant, such as carcinoma, carcinosarcoma, or sarcoma of the breast. Bone marrow biopsy showed no evidence of bone metastasis. According to the standard treatment of breast cancer, we performed total mastectomy and sentinel lymph node biopsy. There was no lymph node metastasis. Macroscopic findings of excised specimens showed a huge and lobulated tumor, which was 8 cm in size (Fig. 4). The tumor cells showed no expression of the human epithelial growth factor receptor 2 (HER2). Histologic examination with hematoxylin and eosin (H&E) stain revealed many poorly differentiated and highly atypical cells (Fig. 5a). Immunostaining of the tumor cells was negative for AE1/AE3, EMA, and desmin. In addition, it was positive for G-CSF, indicating that the tumor was a G-CSF-producing malignant phyllodes tumor of the breast (Fig. 5a). After surgery, her WBC count had decreased to a normal level within 7 days. She was discharged 10 days after surgery and treated with no adjuvant therapy. She is alive without recurrence 13 months after surgery.
Histologic examination of the excised tumor. a H&E stain reveals many poorly differentiated and highly atypical cells. Tumors mainly consist of spindle-shaped to polygonal atypical high-grade stromal cells that grow into sheets while forming multinucleated giant cells. b Immunostaining of the tumor cells is positive for G-CSF
Discussion
In 1951, Fahey presented the possibility that tumors themselves can produce substances capable of stimulating the bone marrow [6]. Asano et al. first reported the production of G-CSF by a carcinoma in 1977 [7]. Since then, G-CSF-producing tumors have been reported mainly in lung, bladder, and gastric cancers [2,3,4]. We searched PubMed and Google Scholar using the keywords “G-CSF production” and “breast cancer”. As a result, there were only two well-reported cases of G-CSF-producing breast cancer [5, 8]. According to Anano’s report, the diagnostic criteria for a G-CSF-producing tumor are extreme leukocytosis, elevated G-CSF activity, a decrease in the WBC count after tumor resection, and proof of G-CSF production in the tumor [7]. In our patient, laboratory tests showed an elevated WBC count. However, there was no redness and swelling in her left breast, and she was afebrile. Serum G-CSF level was high (498 pg/mL). Her WBC count and G-CSF had declined to a normal level within 7 days. Thus, our patient met the diagnostic criteria.
Histologically, G-CSF-producing tumors are often poorly differentiated or undifferentiated [8]. Currently, there are no clear guidelines for the therapy of G-CSF-producing tumors [9] and the therapeutic strategy is usually selected based on the primary organ that is affected [10]. However, the prognosis of G-CSF-producing tumors is poor, regardless of the primary organ affected, and average survival time is only a few months [8].
PET–CT showed multiple uptakes in the primary tumor, brain, urinary system, and bones in the patient. Unlike bone metastasis, uptakes in the bones were diffusely distributed within the spine, pelvic, and long bones. This is a characteristic finding of G-CSF-producing tumors. In 1998, Sugawara reported FDG uptake in the bone marrow when G-CSF preparations were administered to patients undergoing chemotherapy [12]. The accumulation of FDG in PET–CT reflects the glucose metabolism of cells. It has been suggested that G-CSF increases the hematopoietic activity of the granulocyte system of the bone marrow and enhances glucose metabolism [13]. In our patient, we performed a bone marrow biopsy to rule out metastasis and leukemia, which was confirmed.
Breast tumors mainly consist of spindle-shaped to polygonal atypical high-grade stromal cells that grow into sheets while forming multinucleated giant cells. The sarcoma component of the tumor was negative for AE1/AE3 and EMA; therefore, a diagnosis of metaplastic carcinoma was negative. The tumor had atypical ductile hyperplasia showing hyperplasia at the same time as mesenchymal tumor cells. We, therefore, determined it to be a phyllodes tumor. In addition, the nucleoli were clearly visible in the tumor cells. The patient was diagnosed with a malignant phyllodes tumor. Neutrophil infiltration was observed between the tumor cells, which was consistent with the diagnosis of a G-CFS-producing tumor.
Phyllodes tumors account for 0.3–1.0% of breast tumors [14]. They are classified into benign, borderline, and malignant according to histopathologic features [12]. It is often difficult to distinguish benign from malignant phyllodes tumors from other benign tumors such as fibroadenomas before surgery [15]. Approximately 10–15% of phyllodes tumors are malignant [10]. Malignant phyllodes tumors are characterized by a typical rapid growth and reported to cause local recurrence at a rate of 20–65% [15, 16]. Rapid surgery with proper margin to determine an accurate diagnosis and careful follow-up after surgery are important [17].
Conclusions
We treated a patient with G-CSF-producing malignant phyllodes tumor of the breast. It was a triple-negative type malignant tumor that was mainly composed of atypical stromal cells as in the past two reported cases [5, 11]. Including this case, a G-CSF-producing tumor has presented as a malignant tumor mainly composed of stromal cells. In addition, PET–CT revealed diffuse accumulation of FDG in the bone. Phyllodes tumors need to be differentiated from bone metastasis, lymphoma, and leukemia. We must be careful to not mistake this type of tumor for bone marrow metastasis.
Availability of data and materials
The data supporting the conclusions of this article are included within the article.
Abbreviations
- FDG:
-
Fluorodeoxyglucose
- G-CSF:
-
Granulocyte-colony stimulating factor
- H&E:
-
Hematoxylin and eosin
- HER2:
-
Human epithelial growth factor receptor 2
- PET–CT:
-
Positron emission tomography–computed tomography
- WBC:
-
White blood cell
References
Imawari Y, Kamio M, Nogi H, Kawase K, Toriumi Y, Uchida K, et al. A case of granulocyte colony-stimulating factor producing metastatic breast cancer. J Jpn Surg Assoc. 2011;72:2512–5 (in Japanese with English abstract).
Kaira K, Ishizuka T, Tanaka H, et al. Lung cancer producing granulocyte colony-stimulating factor and rapid spreading to peritoneal cavity. J Thorac Oncol. 2008;3:1054–5.
Sato K, Terada K, Sugiyama T, Masuda H, Kakinuma H, Kato T, et al. Granulocyte colony-stimulating factor produced by bladder carcinoma of a patient with leukemoid reaction did not affect proliferation of the tumor cells. J Urol. 1994;151:1687–90.
Kawaguchi M, Asada Y, Terada T, et al. Aggressive recurrence of gastric cancer as a granulocyte-colony-stimulating factor-producing tumor. Int J Clin Oncol. 2010;15:191–5.
Fukui Y, Kawashima M, Kawaguchi K, Takeuchi M, Hirata M, Kataoka T, et al. Granulocyte-colony-stimulating factor-producing metaplastic carcinoma of the breast with significant elevation of serum interleukin-17 and vascular endothelial growth factor levels. Int Cancer Conf J. 2018;7:107–13.
Fahey RJ. Usual leukocyte responses in primary carcinoma of the lung. Cancer. 1951;4:930–5.
Asano S, Urabe A, Okabe T, Sato N, Kondo Y, Ueyama Y, et al. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977;49:845–52.
Inoue T, Okumura F, Mizushima T, Nishi Y, Nishie H. A case of granulocyte colony-stimulating factor producing Intrahepatic cholangiocarcinoma. Jpn Biliary Assoc. 2015;29:138–44.
Wu H, Li L, Yang J, Guo C, Zhang W, Wang H. Radiotherapy with apatinib for recurrence of malignant phyllodes tumor of the breast. Medicine. 2020;3:1–4.
Ogura T, Takii M, Arisaka Y, Masuda D, Kuwabara H. A case of adenosquamous cell carcinoma of the gallbladder producing G-CSF with diffusely uptake in the spine by FDG-PET. Jpn Biliary Assoc. 2011;25:759–67.
Suzuki K, Ota D, Nishi T, Mori M, Kato T, Takeuchi M, et al. A case of granulocyte colony-stimulating factor-producing spindle cell carcinoma of the breast. Breast Cancer. 2015;15:213–7.
Sugawara Y, Fisher SJ, Zasadny KR, et al. Preclinical and clinical studies of bone marrow uptake of fluorine-1-fluorodeoxyglucose with or without granulocyte colony-stimulating factor during chemotherapy. J Clin Oncol. 1998;16:173–80.
Murata Y, Kubota K, Yukihiro M, et al. Correlations between 18F-FDG uptake by bone marrow and hematological parameters: measurements by PET/CT. Nucl Med Biol. 2006;33:999–1004.
Nguyen N, Maciolek L, Qiu S, Sadruddin S, Nguyen Q. Malignant phyllodes tumor of the breast in a 26-year-old woman. Cureus. 2020;1:1–14.
Lisa R. Malignant phyllodes breast tumor. Radiology Case Reports. 2017;12:645–7.
Asoglu O, Ugurlu M, Blanchard K, et al. Risk factors for recurrence and death after primary surgical treatment of malignant phyllodes tumors. Ann Surg Oncol. 2004;11:1011–7.
Goto W, Kashiwagi S, Takada K, Asano Y, Morisaki T, Noda S, et al. A case of a malignant phyllodes tumor that was difficult to distinguish from stromal sarcoma. J Jpn Cancer Chemother. 2018;45:2429–31 (in Japanese with English abstract).
Acknowledgements
The authors thank Hitoshi Tsuda, an expert on pathology at National Cancer Center, for suggesting the present case.
Funding
No funding was received in support of this work.
Author information
Authors and Affiliations
Contributions
KK and KM performed the surgical treatment for this patient. KH and YK diagnosed the patient. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mizoguchi, K., Kaneshiro, K., Kubo, M. et al. Granulocyte-colony stimulating factor-producing malignant phyllodes tumor of the breast: a rare case. surg case rep 7, 24 (2021). https://doi.org/10.1186/s40792-021-01113-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-021-01113-x