Abstract
Complex chromosomal rearrangements (CCRs) are balanced or unbalanced structural rearrangements involving three or more cytogenetic breakpoints on two or more chromosomal pairs. The phenotypic anomalies in such cases are attributed to gene disruption, superimposed cryptic imbalances in the genome, and/or position effects. We report a 14-year-old girl who presented with multiple congenital anomalies and developmental delay. Chromosome and FISH analysis indicated a highly complex chromosomal rearrangement involving three chromosomes (3, 7 and 12), seven breakpoints as a result of one inversion, two insertions, and two translocations forming three derivative chromosomes. Additionally, chromosomal microarray study (CMA) revealed two submicroscopic deletions at 3p12.3 (467 kb) and 12q13.12 (442 kb). We postulate that microdeletion within the ROBO1 gene at 3p12.3 may have played a role in the patient’s developmental delay, since it has potential activity-dependent role in neurons. Additionally, factors other than genomic deletions such as loss of function or position effects may also contribute to the abnormal phenotype in our patient.
Similar content being viewed by others
Background
Complex chromosomal rearrangements (CCRs) are balanced or unbalanced structural rearrangements involving three or more cytogenetic breakpoints on two or more chromosomes [1–5]. The apparently balanced CCRs range from simple three-way exchanges between three chromosomes to highly complex translocations involving many chromosomes and multiple breaks [6].
Chromosomal rearrangements may occur via several mechanisms [7], including non-allelic homologous recombination (NAHR) [8, 9] and nonhomologous end-joining (NHEJ), which both lead to deleted or duplicated genomic segments. However, a number of disease-associated rearrangements are not explained readily by either the NAHR or simple NHEJ recombination mechanisms. Fork stalling and template switching (FoSTeS) and microhomology-mediated break-induced replication (MMBIR) have been described as a mechanism associated with complex rearrangements caused by abnormal DNA replication [7, 10, 11]. More recently, Liber et al. and Tsai et al. proposed a mechanism in which simultaneous double-strand DNA breaks were induced by an unknown stimulus, such as free radicals or ionizing radiation. This is followed by joining of the break fragments in the wrong place due to the microhomology shared by these regions [12, 13].
Balanced and unbalanced CCRs are associated with a significant risk of mental retardation and phenotypic anomalies attributable to gene disruption, cryptic imbalances and/or from position effects [14–18]. Fluorescence in situ hybridization (FISH) and/or high resolution chromosomal microarray studies have identified cryptic CCRs as a cause of abnormal phenotype in a significant number of patients with apparently balanced chromosomal rearrangements [19–23].
We report a patient with multiple congenital anomalies and developmental delay who presented with a CCR involving three chromosomes 3, 7 and 12. G-banding, chromosomal microarray (CMA), and FISH were performed to clarify the nature of this complex abnormality.
Case presentation
Case report
The patient was a 14-year-old female who presented clinically with developmental delay and multiple congenital anomalies including abnormal teeth and abnormal faces. No further clinical information was available regarding this patient.
Methods and results
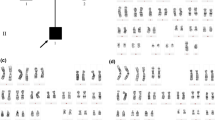
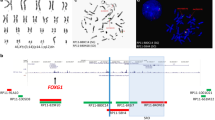
Peripheral blood sample from the patient was referred to our laboratory for chromosome analysis. Metaphase chromosomes were prepared according to standard procedures [24–27]. Analysis of the GTG-banded metaphase chromosomes at the resolution level of 400 bands revealed highly complex rearrangements including one inversion, two insertions, and two translocations involving seven breakpoints at chromosomes 3, 7, and 12 (Figure 1). All of the rearrangements were confirmed by FISH studies using whole-chromosome painting (WCP) probes for chromosomes 3, 7, and 12 (Abbott Molecular, VYSIS, Chicago, IL and Cytocell, Cambridge, UK) and subtelomere 7p/7q probes (Abbott Molecular, VYSIS) (Weise et al. 2008) (Figure 2). WCP FISH results confirmed the ins (3;7), the t (3;12), and the cryptic der (7) t (7;12). The subtelomere 7p probe present on der (3) qter further characterized the translocation between chromosome 7 and chromosome 12. This portion of 7p (7pter) had been translocated to chromosome 12 before being translocated jointly with a segment of chromosome 12 to chromosome 3. This type of CCR has been classified as type III [6], since the number of breaks was greater than the number of affected chromosomes and it included one insertion. SNP-microarray study was performed in order to rule out cryptic copy number variations. Genomic DNA was extracted from whole blood using the Gentra Puregene kit (Qiagen-Sciences, Maryland, USA). Microdeletion/microduplication screening was performed for the proband and his mother, and available half-brothers using an SNP-array platform (CytoScan HD; Affymetrix, Santa Clara, CA), following the manufacturer’s instructions. The CytoScan HD array has 2.67 million probes, including 1.9 million copy number probes and 0.75 million SNP probes. Array data were analyzed using the Chromosome Analysis Suite (ChAS) (Affymetrix, Inc.) software v 2.0. CMA testing revealed two genomic segments in the proband consistent with deletions of approximately 467 kb at 3p12.3 and 442 kb at 12q13.12 (Figure 3A, B). Although, we could not confirm, the deletion at 3p12.3 is likely to encompass the breakpoint on the derivative chromosome 3, where the insertion of chromosome 7 material occurred. This microdeletion encompassed two exons of ROBO1 gene, extended from 78,952,028 to 79,418,897 bp (UCSC genome Browser; http://genome.ucsc.edu/; hg19 release). Similarly, the deletion at 12q13.12 is likely resulted from the translocation between chromosomes 3 and 12. This microdeletion extended from 49,988,357 to 50,429,906 bp and encompassed thirteen genes. CMA testing of the mother detected no dosage abnormality (gain or loss). The father was not available for follow up.
FISH results with whole-chromosome painting (WCP) for chromosomes 3 and 7 (A/B) and 7 and 12 (C/D), and subtelomeric FISH for chromosome 7(E/F). The bottom row of images are the same FISH images shown in the top row but with inverted DAPI stain. The chromosomes involved in the CCR are depicted by red arrows. A/B: WCP3 (green) paints the normal chromosome 3 part of the der (3) and der (12); WCP7 (red) paints the normal chromosome 7, part of der (7), and the inserted part in der (3). C/D: WCP12 (green) paints the normal chromosome 12, part of der (12), translocated part to der (3), and translocated part to der (7); WCP7 (red) paints the normal chromosome 7 and part of der (7) and inserted part to der (3). E/F: Subtelomeric 7p (green) and 7q (red) are shown in the normal chromosome 7. The subtelomeric 7p probe of the other chromosome 7 is located on der (3) after translocation to chromosome 12.
The combination of cytogenetic, FISH, and array analysis revealed a complex rearrangement with nine breakpoints:
46,××, der (3) (3pter → 3p12::7q11.2 → 7q22::3q27 → 3p12::12q13 → 12q24.3::7p22 → 7pter), der (7) (12qter → 2q24.3::7p22 → 7q11.2::7q22 → 7qter), der (12) (12pter → 12q13::3q27 → 3qter).arr [hg19] 3p12.3(78,952,028-79,418,897) ×1, 12q13.12 (49,988,357-50,429,906)×1.
Discussion
The disease-associated CCRs are frequently used to establish the genotype-phenotype relationship [28, 29]. A combination of several different approaches, including karyotype, FISH, and CMA studies, has been useful in identifying several disease-associated genes and regions [28–35]. The phenotype of our patient with multiple congenital abnormalities and developmental delay with the apparently balanced CCR led us to perform CMA testing to rule out cryptic copy number variations (CNVs). According to the NCBI Map Viewer (http://www.ncbi.nlm.nih.gov/mapview/), the 3p12.3 deletion was within the ROBO1 gene, while the deletion at 12q13.12 involved thirteen genes (FAM186B, PRPF40B, FMNL3, TMBIM6, NCKAP5L, BCDIN3D-AS1, BCDIN3D, FAIM2, LOC283332, AQP2, AQP5, AQP6, RACGAP1). It is not clear if these copy number losses are de novo or paternally inherited, since the patient’s father was not available for follow up studies. The ROBO1 gene encodes a receptor that is a member of the neural cell adhesion molecule (NCAM; 116930) family of receptors, acting as an axon guidance receptor. ROBO1 may play a role in neuronal development, and its disruption may predispose humans to developmental dyslexia [36, 37]. Among the genes deleted at 12q13 three are OMIM genes including NCKAP5L (OMIM 615104), AQP2 (OMIM 107777) and AQP5 (OMIM 600442). NCKAP5L gene encodes a protein involved in proteolysis, GTPase-mediated signaling, cytoskeletal organization, and other pathways. Furthermore, neuronal depolarization regulates the transcription of these genes, suggesting potential activity-dependent roles in neurons [38]. Mutation in AQP2 is associated with diabetes insipidus and in AQP5 with palmoplantar keratoderma, Bothnian type. However, copy number losses or gains at these loci have not yet been associated with a clinical phenotype. We propose that microdeletion within ROBO1 may play a role in our patient’s developmental delay.
Since the exact genomic location of at least five out of seven breakpoints in our patient is unknown, we can only speculate as to the disruption of genes resulting in loss of function [21, 39] or position effects [40] in these chromosomal breakpoints. For example, inversion of 3p12q27 may interrupt the DYX5 gene (OMIM 606896) at 3p12, which is associated with neurofunctional disorder, developmental dyslexia [41], or speech sound disorder [42]. Furthermore, interruption in MASP1 (OMIM 257920) located at the distal end (3q27) of the inversion 3 may have resulted in 3MC syndrome, which encompasses four rare autosomal recessive disorders previously designated as the Carnevale, Mingarelli, Malpuech, and Michels syndromes, respectively. The main features of these syndromes are facial dysmorphism, cleft lip and palate, postnatal growth deficiency, cognitive impairment, and craniosynostosis [43]. The other gene interruption may have occurred in the complex der (3) ins (3;7), where one of the breakpoints maps to 7q11.21. This was previously suggested as a candidate region for ectrodactyly, ectodermal dysplasia, and cleft lip/palate syndrome (designated EEC1) [44]. Alternatively, fusion of these genes at the breakpoints where insertion has occurred may generate a gain-of-function mutation [45]. Additional molecular studies are needed in order to determine whether any interruption or disruption of the genes caused by the chromosomal rearrangements.
Conclusion
Two sub-microscopic deletions resulted from this apparently balanced CCR. Microdeletion within the ROBO1 gene with potential activity-dependent roles in neurons may have played a role in our patient’s developmental delay. Furthermore, gene disruptions or position effects altering gene regulation by chromosomal rearrangements due to interference with some gene regulatory elements, may have also contributed to our patient’s abnormal phenotype.
Consent
These studies were performed on anonymized samples received in the clinical laboratory and thus were exempted from the requirement for consent by an opinion for the Western Institutional review Board.
Abbreviations
- CCR:
-
Complex chromosomal rearrangement
- SNP-microarray:
-
Single nucleotide polymorphism.
References
Houge G, Liehr T, Schoumans J, Ness GO, Solland K, Starke H, Claussen U, Strømme P, Akre B, Vermeulen S: Ten years follow up of a boy with a complex chromosomal rearrangement: going from a > 5 to 15-breakpoint CCR. Am J Med Genet A 2003,118 A(3):235–240.
Weise A, Rittinger O, Starke H, Ziegler M, Claussen U, Liehr T: De novo 9-break-event in one chromosome 21 combined with a microdeletion in 21q22.11 in a mentally retarded boy with short stature. Cytogenet Genome Res 2003,103(1–2):14–16.
de Vree PJ, Simon ME, van Dooren MF, Stoevelaar GH, Hilkmann JT, Rongen MA, Huijbregts GC, Verkerk AJ, Poddighe PJ: Application of molecular cytogenetic techniques to clarify apparently balanced complex chromosomal rearrangements in two patients with an abnormal phenotype:case report. Mol Cytogenet 2011, 2: 15.
Pellestor F, Anahory T, Lefort G, Puechberty J, Liehr T, Hedon B, Sarda P: Complex chromosomal rearrangements: origin and meiotic behavior. Hum Reprod Update 2011,17(4):476–494.
Zhang F, Khajavi M, Connolly AM, Towne CF, Batish SD, Lupski JR: The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat Genet 2009,41(7):849–853.
Madan K: Balanced complex chromosome rearrangements: reproductive aspects. A review. Am J Med Genet 2012,158 A(4):947–963.
Gu W, Zhang F, Lupski JR: Mechanisms for human genomic rearrangements. Pathogenetics 2008,1(1):4.
Emanuel BS, Shaikh TH: Segmental duplications: an ‘expanding’ role in genomic instability and disease. Nat Rev Genet 2001,2(10):791–800.
Lupski JR: Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet 1998,14(10):417–422.
Lee JA, Carvalho CM, Lupski JR: A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 2007,131(7):1235–1247.
Hastings PJ, Ira G, Lupski JR: A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet 2009,5(1):e1000327.
Lieber MR: The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 2010, 79: 181–211.
Tsai AG, Lieber MR: Mechanisms of chromosomal rearrangement in the human genome. BMC Genomics 2010,11(Suppl 1):S1.
Batista DA, Pai GS, Stetten G: Molecular analysis of a complex chromosomal rearrangement and a review of familial cases. Am J Med Genet 1994,53(3):255–263.
Madan K, Nieuwint AW, Van Bever Y: Recombination in a balanced complex translocation of a mother leading to a balanced reciprocal translocation in the child. Review of 60 cases of balanced complex translocations. Hum Genet 1997,99(6):806–815.
Warburton D: De novo balanced chromosome rearrangements and extra marker chromosomes identified at prenatal diagnosis: clinical significance and distribution of breakpoints. Am J Hum Genet 1991,49(5):995–1013.
Chandley AC: Chromosome anomalies and Y chromosome microdeletions as causal factors in male infertility. Hum Reprod 1998,13(Suppl 1):45–50.
Rosenberg C, Knijnenburg J, Chauffaille Mde L, Brunoni D, Catelani AL, Sloos W, Szuhai K, Tanke HJ: Array CGH detection of a cryptic deletion in a complex chromosome rearrangement. Hum Genet 2005,116(5):390–394.
Colovati ME, Da Silva LR, Takeno SS, Mancini TI, AR ND, Guilherme RS, De Mello CB, Melaragno MI, Perez AB A: Marfan syndrome with a complex chromosomal rearrangement including deletion of the FBN1 gene. Mol Cytogenet 2012, 5: 5.
De Gregori M, Ciccone R, Magini P, Pramparo T, Gimelli S, Messa J, Novara F, Vetro A, Rossi E, Maraschio P, Bonaglia MC, Anichini C, Ferrero GB, Silengo M, Fazzi E, Zatterale A, Fischetto R, Previderé C, Belli S, Turci A, Calabrese G, Bernardi F, Meneghelli E, Riegel M, Rocchi M, Guerneri S, Lalatta F, Zelante L, Romano C, Fichera M, et al.: Cryptic deletions are a common finding in “balanced” reciprocal and complex chromosome rearrangements: a study of 59 patients. J Med Genet 2007,44(12):750–762.
Griesi-Oliveira K, Moreira Dde P, Davis-Wright N, Sanders S, Mason C, Orabona GM, Vadasz E, Bertola DR, State MW, Passos-Bueno MR: A complex chromosomal rearrangement involving chromosomes 2, 5, and X in autism spectrum disorder. Am J Med Genet B Neuropsychiatr Genet 2012,159 B(5):529–536.
Papadopoulou E, Sismani C, Christodoulou C, Ioannides M, Kalmanti M, Patsalis P: Phenotype-genotype correlation of a patient with a “balanced” translocation 9;15 and cryptic 9q34 duplication and 15q21q25 deletion. Am J Med Genet 2010,152 A(6):1515–1522.
Schluth-Bolard C, Delobel B, Sanlaville D, Boute O, Cuisset JM, Sukno S, Labalme A, Duban-Bedu B, Plessis G, Jaillard S, Dubourg C, Henry C, Lucas J, Odent S, Pasquier L, Copin H, Latour P, Cordier MP, Nadeau G, Till M, Edery P, Andrieux J: Cryptic genomic imbalances in de novo and inherited apparently balanced chromosomal rearrangements: array CGH study of 47 unrelated cases. Eur J Med Genet 2009,52(5):291–296.
Wang BT, Hemmat M, Jayakar P, Boyar F, Chan P, El Naggar M, Anguiano A: Paternal mosaic inv (20) resulting in a recombinant chromosome 20 in two siblings. Pediatr Int 2010,52(3):492–495.
Hemmat M, Wang BT, Warburton PE, Yang X, Boyar FZ, El Naggar M, Anguiano A: Neocentric X-chromosome in a girl with Turner-like syndrome. Mol Cytogenet 2012,5(1):29.
Hemmat M, Hemmat O, Anguiano A, Boyar FZ, El Naggar M, Wang JC, Wang BT, Sahoo T, Owen R, Haddadin M: Genotype-phenotype analysis of recombinant chromosome 4 syndrome: an array-CGH study and literature review. Mol Cytogenet 2013,6(1):17.
Hemmat M, Hemmat O, Boyar FZ: Isochromosome Yp and jumping translocation of Yq resulting in five cell lines in an infertile male: a case report and review of the literature. Mol Cytogenet 2013,6(1):36.
Wirth J, Nothwang HG, van der Maarel S, Menzel C, Borck G, Lopez-Pajares I, Brøndum-Nielsen K, Tommerup N, Bugge M, Ropers HH, Haaf T: Systematic characterisation of disease associated balanced chromosome rearrangements by FISH: cytogenetically and genetically anchored YACs identify microdeletions and candidate regions for mental retardation genes. J Med Genet 1999, 36: 271–278.
Bugge M, Bruun-Petersen G, Brondum-Nielsen K, Friedrich U, Hansen J, Jensen G, Jensen PK, Kristoffersson U, Lundsteen C, Niebuhr E, Rasmussen KR, Rasmussen K, Tommerup N: Disease associated balanced chromosome rearrangements: a resource for large scale genotypephenotype delineation in man. J Med Genet 2000,37(11):858–865.
Higgins JJ, Pucilowska J, Lombardi RQ, Rooney JP: Candidate genes for recessive non-syndromic mental retardation on chromosome 3p (MRT2A). Clin Genet 2004, 65: 496–500.
David D, Cardoso J, Marques B, Marques R, Silva ED, Santos H, Boavida MG: Molecular characterization of a familial translocation implicates disruption of HDAC9 and possible position effect on TGFbeta2 in the pathogenesis of Peters’ anomaly. Genomics 2003, 81: 489–503.
Endris V, Wogatzky B, Leimer U, Bartsch D, Zatyka M, Latif F, Maher ER, Tariverdian G, Kirsch S, Karch D, Rappold GA: The novel Rho-GTPase activating gene MEGAP/srGAP3 has a putative role in severe mental retardation. Proc Natl Acad Sci 2002, 99: 11754–11759.
Lledo B, Ortiz JA, Morales R, Manchon I, Galan F, Bernabeu A, Bernabeu R: Characterization of a balanced complex chromosomal rearrangement carrier ascertained through a fetus with dup15q26.3 and del5p15.33: case report. Hum Fertil (Camb) 2013,16(3):215–217.
Dutta UR, Pidugu VK, Goud CV, Hoefers C, Hagemann M, Dalal A: Identification and molecular cytogenetic characterization of a novel complex Y chromosome rearrangement in a boy with disorder of sexual development. Gene 2013,519(2):374–380.
Morteza H, Rumple MJ, Mahon LW, Maryam T, Bryant N, Boyar FZ: Short stature, digit anomalies and dysmorphic facial features caused by duplication of miR-17~92 cluster. Molecular cytogenet 2014. In Press
Hannula-Jouppi K, Kaminen-Ahola N, Taipale M, Eklund R, Nopola-Hemmi J, Kaariainen H, Kere J: The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet 2005, 1: e50. Note: Electronic Article
Anthoni H, Sucheston LE, Lewis BA, Tapia-Páez I, Fan X, Zucchelli M, Taipale M, Stein CM, Hokkanen ME, Castrén E, Pennington BF, Smith SD, Olson RK, Tomblin JB, Schulte-Körne G, Nöthen M, Schumacher J, Müller-Myhsok B, Hoffmann P, Gilger JW, Hynd GW, Nopola-Hemmi J, Leppanen PH, Lyytinen H, Schoumans J, Nordenskjöld M, Spencer J, Stanic D, Boon WC, Simpson E, et al.: The aromatase gene CYP19A1: several genetic and functional lines of evidence supporting a role in reading, speech and language. Behav Genet 2012,42(4):509–527.
Chahrour MH, Yu TW, Lim ET, Ataman B, Coulter ME, Hill RS, Stevens CR, Schubert CR, Autism Sequencing Collaboration ARRA, Greenberg ME, Gabriel SB, Walsh CA: Whole-exome sequencing and homozygosity analysis implicate depolarization-regulated neuronal genes in autism. PLoS Genet 2012,8(4):e1002635. doi:10.1371/journal.pgen.1002635. Epub 2012 Apr 12
Baptista J, Mercer C, Prigmore E, Gribble SM, Carter NP, Maloney V, Thomas NS, Jacobs PA, Crolla JA: Breakpoint mapping and array CGH in translocations: comparison of a phenotypically normal and an abnormalcohort. Am J Hum Genet 2008,82(4):927–936.
Noonan JP, McCallion AS: Genomics of long-range regulatory elements. Annu Rev Genomics Hum Genet 2010, 11: 1–23.
Nopola-Hemmi J, Myllyluoma B, Haltia T, Taipale M, Ollikainen V, Ahonen T, Voutilainen A, Kere J, Widen E: A dominant gene for developmental dyslexia on chromosome 3. J Med Genet 2001, 38: 658–664.
Stein CM, Schick JH, Taylor HG, Shriberg LD, Millard C, Kundtz-Kluge A, Russo K, Minich N, Hansen A, Freebairn LA, Elston RC, Lewis BA, Iyengar SK: Pleiotropic effects of a chromosome 3 locus on speech-sound disorder and reading. Am J Hum Genet 2004, 74: 283–297.
Rooryck C, Diaz-Font A, Osborn DPS, Chabchoub E, Hernandez-Hernandez V, Shamseldin H, Kenny J, Waters A, Jenkins D, Al Kaissi A, Leal GF, Dallapiccola B: And 9 others: mutations in lectin complement pathway genes COLEC11 and MASP1 cause 3MC syndrome. Nature Genet 2011, 43: 197–203.
Buss PW, Hughes HE, Clarke A: Twenty-four cases of the EEC syndrome: clinical presentation and management. J Med Genet 1995, 32: 716–723.
Lupski JR, Stankiewicz P: Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet 2005,1(6):e49.
Acknowledgment
The authors would like to express their thanks to Jeff Radcliff (Quest Diagnostics) for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MH, First authors; performed analysis, interpretation of the results, drafting and finalizing the manuscript. XY, participated in writing the FISH results. PC, RM and LR Performed the analysis and literature review. FZB reviewed the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hemmat, M., Yang, X., Chan, P. et al. Characterization of a complex chromosomal rearrangement using chromosome, FISH, and microarray assays in a girl with multiple congenital abnormalities and developmental delay. Mol Cytogenet 7, 50 (2014). https://doi.org/10.1186/1755-8166-7-50
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1755-8166-7-50