Abstract
Background
Premature death of Plasmodium-infected erythrocytes is considered to favourably influence the clinical course of malaria. Aurothiomalate has previously been shown to trigger erythrocyte death or eryptosis, which is characterized by cell membrane scrambling leading to phosphatidylserine exposure at the cell surface. Phosphatidylserine-exposing cells are rapidly cleared from circulating blood. The present study thus tested whether sodium aurothiomalate influences the intraerythrocytic parasite development in vitro and the clinical course of murine malaria in vivo.
Methods
Human erythrocytes were infected with Plasmodium falciparum BinH in vitro and mice were infected (intraperitoneal injection of 1 × 106 parasitized murine erythrocytes) with Plasmodium berghei ANKA in vivo.
Results
Exposure to aurothiomalate significantly decreased the in vitro parasitemia of P. falciparum-infected human erythrocytes without influencing the intraerythrocytic DNA/RNA content. Administration of sodium aurothiomalate in vivo (daily 10 mg/kg b.w. s.c. from the 8th day of infection) enhanced the percentage of phosphatidylserine-exposing infected and noninfected erythrocytes in blood. All nontreated mice died within 30 days of infection. Aurothiomalate-treatment delayed the lethal course of malaria leading to survival of more than 50% of the mice 30 days after infection.
Conclusions
Sodium aurothiomalate influences the survival of Plasmodium berghei-infected mice, an effect only partially explained by stimulation of eryptosis.
Similar content being viewed by others
Background
The malaria pathogen Plasmodium imposes oxidative stress on infected cells [1], which in turn elicits eryptosis, a form of erythrocyte death [2–4]. The signaling leading to cell membrane scrambling includes an increase in the cytosolic Ca2+ activity [5–10] and/or formation of ceramide and/or formation of ceramide [11]. Ca2+ further stimulates Ca2+-sensitive K+ channels [10, 12–18]. The Ca2+-permeable cation channels are activated by oxidative stress [19, 20]. Oxidative stress [21] and excessive cytosolic Ca2+ concentrations [22] are known to similarly trigger cell membrane scrambling or apoptosis in nucleated cells.
Phosphatidylserine-exposing cells are recognized by macrophages [23, 24], phagocytosed [25, 26] and thus rapidly cleared from circulating blood [27].
In malaria, accelerated clearance of infected erythrocytes [28] may counteract the development of parasitemia [29–31], in genetic erythrocyte disorders [9, 32–36], in iron deficiency [2], or following treatment with lead [3], chlorpromazine [37], azathioprine [38] or cyclosporine [39]. The erythrocyte cation channel is inhibited by erythropoietin [40] and may favourably influence the course of malaria [15]. Eryptosis is further inhibited by erythropoietin [41], caffeine [42] and thymol [43].
Eryptosis is stimulated by aurothiomalate, a gold-containing drug effective against rheumatoid arthritis [44]. Gold complexes have indeed been shown to counteract malaria [45–51]. They are considered to be effective through inhibition of heme aggregation, haemozoin formation and/or parasitic thioredoxin reductase as well as interaction with the DNA of the parasite [52–57].
The present study explored, whether sodium aurothiomalate augments the death of Plasmodium falciparum-infected human erythrocytes and/or Plasmodium berghei-infected mouse erythrocytes and whether this effect correlates with a favourable influence on parasitemia and host survival during murine malaria.
Methods
Human erythrocytes were drawn from healthy volunteers. The study was approved by the Ethical commission of the University of Tübingen.
Animal experiments were performed according to the German animal protection law and approved by the local authorities (registration number PY 4/09). Experiments were performed in healthy SV129/J wild type mice (aged 4 months, both male and female). The animals had free access to standard chow (C1310, Altromin, Lage, Germany) and drinking water. Blood was drawn by incision of the tail vein.
For infection of human erythrocytes the human pathogen Plasmodium falciparum (P. falciparum) strain BinH [58] was grown in vitro[37, 59]. Parasites were cultured as described earlier [60–62] at a hematocrit of 2% and a parasitemia of 2-10% in RPMI 1640 medium supplemented with Albumax II (0.5%; Gibco, Karlsruhe, Germany) in an atmosphere of 90% N2, 5% CO2 and 5% O2[62, 63].
To estimate the in vitro growth of Plasmodium falciparum the BinH strain was cultured and synchronized to the ring stage by sorbitol treatment as described previously [14, 63]. For the in vitro growth assay, synchronized parasitized erythrocytes were aliquoted in 96-well plates (200 μl aliquots, 1% hematocrit, 0.5-2% parasitemia) and grown for 48 h in the presence or absence of sodium aurothiomalate.
The parasitemia was assessed 0 h and 48 h after infection by flow cytometry of human erythrocytes and by counting of Giemsa-stained blood smears from infected mice. Parasitemia was defined as the percentage of erythrocytes stained with the DNA/RNA-specific fluorescence dye Syto16 or by identification of Giemsa-stained infected erythrocytes using light microscopy.
For Giemsa staining, the thick blood film was air-dried and fixed with methanol. 2% Giemsa solution (Sigma) was added for 30 min. The slide was rinsed with water and again dried. Then, the slides were analysed under a Leica CM E light microscope (100 ×, oil immersion).
To estimate DNA/RNA amplification of the intraerythrocytic parasite, the culture was ring stage-synchronized and re-synchronized after 6 h of culture (to narrow the developmental parasite stage), aliquoted (200 μl aliquots, 2% hematocrit and 10% parasitemia) and cultured for further 16 h in the presence or absence of sodium aurothiomalate. Thereafter, the DNA/RNA amount of the parasitized erythrocytes was determined by Syto16 fluorescence as a measure of intraerythrocytic parasite copies.
For infection of mice Plasmodium berghei ANKA-parasitized murine erythrocytes (1 × 106) were injected intraperitoneally [64, 65]. Where indicated, sodium aurothiomalate (10 mg/kg b.w. s.c) was administered from the 8th day of infection daily. Blood was collected from the mice daily starting 8 days after infection by incision of the tail. The hematocrit was determined by centrifugation in hematocrit capillaries. Parasitemia was determined by Syto16 staining in FACS analysis. In vitro experiments were performed at 37°C in Ringer solution containing (in mM) 125 NaCl, 5 KCl, 1 MgSO4, 32 N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES)/NaOH (pH 7.4), 5 glucose, 1 CaCl2[66]. Aurothiomalate was added to the NaCl Ringer at final concentrations varying from 0.1 to 100 μM (Sigma, Schnelldorf, Germany). For in vitro treatment, the final hematocrit was adjusted to 0.3%.
For determination of phosphatidylserine exposure, FACS analysis was performed as described [10]. After incubation in the presence or absence of sodium aurothiomalate, suspensions of Plasmodium falciparum- infected erythrocytes were stained with annexin V-APC (BD Biosciences Pharmingen, Heidelberg, Germany) and/or with Syto16 (Molecular Probes, Göttingen, Germany) to identify phosphatidylserine-exposing and infected erythrocytes, respectively. For annexin V-binding, erythrocytes were washed, resuspended in annexin V-binding buffer (Ringer solution containing 5 mM CaCl2. pH 7.4), stained with annexin V-APC (dilution 1:20), incubated for 20 min at room temperature, and diluted 1:5 with annexin V-binding buffer. Syto16 (final concentration of 20 nM) was added directly to the diluted erythrocyte suspension or co-incubated in the annexin V-containing buffer solution. Erythrocytes were analyzed by flow cytometry (FACS-Calibur, Becton Dickinson, Heidelberg, Germany) in fluorescence channel FL-1 for Syto16 (detected at 530 nm) and in FL-4 for annexin V-APC fluorescence intensity (detected at 660 nm).
Data are expressed as arithmetic means ± SEM, and statistical analysis was made by t-test or ANOVA using Tukey's test as post hoc test, as appropriate. p < 0.05 was considered as statistically significant.
Results and Discussion
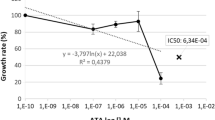
A first series of experiments explored the influence of aurothiomalate on the in vitro growth of Plasmodium falciparum in human erythrocytes. To this end, P. falciparum-infected erythrocytes were cultured in human erythrocytes and synchronized to the ring stage by sorbitol treatment. Within 48 hours the percentage of infected erythrocytes increased from 6.0% to 21.0% in the absence and to 9.8% in the presence of 100 μM aurothiomalate (Fig. 1A). Accordingly, aurothiomalate blunted the increase in the percentage of parasitized erythrocytes, an effect reaching statistical significance at ≥ 10 μM aurothiomalate (Fig. 1A). The halfmaximal inhibition (IC50) was achieved by 68 μM aurothiomalate. In contrast, at the concentrations tested, the presence of aurothiomalate did not influence the intraerythrocytic DNA amplification of the parasite (Fig. 1AB).
Effects of sodium aurothiomalate on intraerythrocytic amplification and in vitro parasitemia. A. In vitro parasitemia with P. falciparum (left panel) in human erythrocytes as a function of the aurothiomalate concentration (arithmetic means ± SEM, n = 16). *, *** indicate significant difference (p < 0.05, p < 0.001) from the absence of aurothiomalate. Intraerythrocytic DNA amplification (right panel) as a function of the aurothiomalate concentration (arithmetic means ± SEM, n = 12). B. Intraerythrocytic DNA amplification (right panel) as in B for different time periods (arithmetic means ± SEM, n = 8).
In order to determine the effect of infection and of aurothiomalate on eryptosis, phosphatidylserine-exposing erythrocytes were identified by measurement of annexin V-binding in FACS analysis. Within 24 hours, the infection with P. falciparum markedly increased the annexin V-binding of infected and noninfected erythrocytes (Fig. 2). The percentage of annexin V-binding was, however, significantly higher in infected than in noninfected erythrocytes (Fig. 2). The phosphatidylserine exposure of infected erythrocytes was significantly increased in the presence of aurothiomalate (Fig. 2), an effect reaching statistical significance at 50 μM aurothiomalate.
Effects of aurothiomalate on phosphatidylserine exposure of infected and noninfected human erythrocytes. Arithmetic means ± SEM (n = 6) of the percentage of annexin V-binding infected (open bars) and non-infected (closed bars) erythrocytes following infection of human erythrocytes with P. falciparum in the presence of 0 - 100 μM aurothiomalate. ##, ### indicate significant difference (p < 0.01, p < 0.001) from non-infected erythrocytes, **, *** indicate significant difference (p < 0.01, p < 0.001) from the absence of aurothiomalate.
In a next series, mice were infected with P. berghei with or without sodium aurothiomalate treatment. Sodium aurothiomalate was administered daily from the 8th day of infection. Similar to the in vitro infection of human erythrocytes with P. falciparum, the infection of mice with P. berghei was followed by a marked increase in the percentage of phosphatidylserine-exposing erythrocytes. The phosphatidylserine exposure of infected erythrocytes was significantly more pronounced following treatment with sodium aurothiomalate than the phosphatidylserine exposure of noninfected erythrocytes (Fig. 3).
Effect of sodium aurothiomalate treatment on phosphatidylserine exposure of infected and non-infected erythrocytes from Plasmodium berghei -infected mice. Arithmetic means ± SEM (n = 6-8) of the percentage of phosphatidylserine-exposing infected (right bars) and non-infected (left bars) erythrocytes taken from animals without (black bars) and with (white bars) sodium aurothiomalate treatment (daily 10 mg/kg b.w. s.c.) on the 22nd day after infection. ### indicates significant difference (p < 0.001) from absence of sodium aurothiomalate. *** indicates significant difference (p < 0.001) from noninfected erythrocytes.
The parasitemia was still low on the 8th day of infection (Fig. 4B). The percentage of infected erythrocytes gradually increased with or without sodium aurothiomalate treatment. However, the percentage of parasitized erythrocytes was significantly lower in sodium aurothiomalate-treated animals than in animals without sodium aurothiomalate treatment (Fig. 4A, B).
Effect of sodium aurothiomalate treatment on the parasitemia and survival of Plasmodium berghei -infected mice. A: Original histograms of parasitemia-dependent Syto16 fluorescence in untreated animals (upper panels) and in animals treated daily from day 8 daily with 10 mg/kg b.w. sodium aurothiomalate s.c. (lower panels) 10 (left panels) and 20 (right panels) days after infection with P. berghei. B: Arithmetic means ± SEM of the parasitemia in mice without treatment (open circles, n = 8 mice) or with daily 10 mg/kg b.w. s.c. of sodium aurothiomalate (closed circles, n = 6 mice) as a function of days after infection with P. berghei. Significant difference (* p < 0.05, ** p < 0.01, *** p < 0.001; t-test) from the untreated animals on days 12 - 20. The results presented are one of three independent series. C: Survival of mice without treatment (open circles) or with daily 10 mg/kg b.w. sodium aurothiomalate s.c. (closed circles) as a function of days after infection with Plasmodium berghei. D-E: Arithmetic means ± SEM of the parasitemia in mice without treatment (open circles, n = 4 mice) or with daily 10 mg/kg b.w. s.c. of sodium aurothiomalate (closed circles, n = 4 mice) as a function of days after infection with P. berghei. The parasitemia was determined daily either by staining with Syto16 and subsequent FACS analysis as in B (D) or by daily Giemsa staining of blood smears and light microscopy-dependent analysis (E).
Since the FACS-dependent determination of parasitemia utilizes a DNA/RNA-specific dye, reticulocytes may also be counted as parasitized erythrocytes. Therefore, a second series of experiments was performed to compare the values for parasitemia determined by FACS analysis to those obtained from Giemsa staining. As shown in Fig. 4D, E, parasitemia was lower in the aurothiomalate-treated group of mice, irrespective of the methods applied.
The treatment with sodium aurothiomalate further resulted in enhanced survival of P. berghei-infected mice. As shown in Fig. 4C, all untreated animals died within 30 days after the infection. In contrast, 57% of the sodium aurothiomalate-treated animals were still alive 30 days after infection. All treated mice died, however, until day 44 after infection.
To investigate whether aurothiomalate treatment influences inflammation, the plasma levels of the inflammatory mediator TNFα, were determined on the 16th day of infection. As a result, the TNFα concentration in non-treated mice was 53.4 ± 29.7 pg/ml whereas the TNFα was below the detection limit in mice treated with sodium aurothiomalate (both n = 4).
As shown earlier, TNF-α may exert an antiparasitic effect in animal models [67–69], and high TNF production is associated with more rapid clinical and parasitologic recovery in humans [70]. Even though aurothiomalate does not seem to affect induction of TNF-alpha in phagocytic cell cultures [71], the present observations clearly demonstrate an effect of the drug on TNF production in vivo.
Malaria is paralleled by loss of erythrocytes leading to anemia. As shown in Fig. 5, the hematocrit of aurothiomalate-treated mice was significantly reduced. The effect could have been due to enhanced eryptosis or hemolysis. In noninfected erythrocytes aurothiomalate has previously been shown to trigger eryptosis rather than hemolysis [44].
Effect of sodium aurothiomalate treatment on the hematocrit of Plasmodium berghei -infected mice. Arithmetic means ± SEM of packed cell volume (hematocrit) in mice without treatment (open circles, n = 8 mice) or with daily 10 mg/kg b.w. s.c. of sodium aurothiomalate (closed circles, n = 8 mice) as a function of days after infection with P. berghei. * indicates significant difference (p < 0.05; t-test).
The present study demonstrates that aurothiomalate had only mild effects on the parasite burden and moderately delayed the lethal course of malaria following infection of mice with P. berghei. Similar to what has been observed earlier [64], the infection of mice with P. berghei was followed by an invariably lethal course of malaria without aurothiomalate treatment. More than 50% of the sodium aurothiomalate-treated animals survived the infection for 30 days, even though they all died until day 44.
The effect of sodium aurothiomalate treatment may in part be due to a toxic effect on the pathogen, which compromises the intraerythrocyte growth of the parasite. As a matter of fact, gold-containing drugs have previously been shown to be toxic for Plasmodia[45–57]. Drugs could specifically enter infected erythrocytes, as the pathogen dramatically enhances the permeability of the erythrocyte membrane [1].
Alternatively, sodium aurothiomalate may exert a protective effect by accelerating the death of infected erythrocytes. Phosphatidylserine-exposing erythrocytes are engulfed by macrophages [25, 26] and are thus rapidly cleared from circulating blood [27]. As eryptosis mainly affects infected erythrocytes, accelerated eryptosis should decrease the parasitemia and thus favourably influence the course of the disease [29]. On the other hand, eryptosis has been suggested to foster vascular derangements of metabolic syndrome [72].
The discrepancy between the moderate influence of aurothiomalate on parasitemia and the effect on survival of the infected host is suggestive for an additional effect of the drug on mouse survival. Possibly it is in part the anti-inflammatory effect of the drug, which accounts for at least part of the effect on host survival and the stimulation of eryptosis. As a matter of fact, aurothiomalate treatment virtually abolished the increase in TNFα plasma concentration following infection.
Conclusion
In mice, sodium aurothiomalate delays the lethal course of malaria. Presumably, the effect is not only due to the toxicity for the pathogen and due to stimulation of eryptosis, but may involve the anti-inflammatory activity of the drug.
Abbreviations
- ANOVA:
-
(analysis of variance)
- APC:
-
(allophycocyanin)
- FACS:
-
(fluorescence-activated cell sorter)
- FL:
-
(fluorescence channel)
- Hb:
-
(hemoglobin)
- TNF:
-
(tumor necrosis factor).
References
Kirk K: Membrane transport in the malaria-infected erythrocyte. Physiol Rev. 2001, 81: 495-537.
Koka S, Foller M, Lamprecht G, Boini KM, Lang C, Huber SM, Lang F: Iron deficiency influences the course of malaria in Plasmodium berghei infected mice. Biochem Biophys Res Commun. 2007, 357: 608-614. 10.1016/j.bbrc.2007.03.175.
Koka S, Huber SM, Boini KM, Lang C, Foller M, Lang F: Lead decreases parasitemia and enhances survival of Plasmodium berghei-infected mice. Biochem Biophys Res Commun. 2007, 363: 484-489. 10.1016/j.bbrc.2007.08.173.
Lang F, Gulbins E, Lerche H, Huber SM, Kempe DS, Foller M: Eryptosis, a window to systemic disease. Cell Physiol Biochem. 2008, 22: 373-380. 10.1159/000185448.
Berg CP, Engels IH, Rothbart A, Lauber K, Renz A, Schlosser SF, Schulze-Osthoff K, Wesselborg S: Human mature red blood cells express caspase-3 and caspase-8, but are devoid of mitochondrial regulators of apoptosis. Cell Death Differ. 2001, 8: 1197-1206. 10.1038/sj.cdd.4400905.
Brand VB, Sandu CD, Duranton C, Tanneur V, Lang KS, Huber SM, Lang F: Dependence of Plasmodium falciparum in vitro growth on the cation permeability of the human host erythrocyte. Cell Physiol Biochem. 2003, 13: 347-356. 10.1159/000075122.
Bratosin D, Estaquier J, Petit F, Arnoult D, Quatannens B, Tissier JP, Slomianny C, Sartiaux C, Alonso C, Huart JJ, Montreuil J, Ameisen JC: Programmed cell death in mature erythrocytes: a model for investigating death effector pathways operating in the absence of mitochondria. Cell Death Differ. 2001, 8: 1143-1156. 10.1038/sj.cdd.4400946.
Daugas E, Cande C, Kroemer G: Erythrocytes: death of a mummy. Cell Death Differ. 2001, 8: 1131-1133. 10.1038/sj.cdd.4400953.
Lang KS, Roll B, Myssina S, Schittenhelm M, Scheel-Walter HG, Kanz L, Fritz J, Lang F, Huber SM, Wieder T: Enhanced erythrocyte apoptosis in sickle cell anemia, thalassemia and glucose-6-phosphate dehydrogenase deficiency. Cell Physiol Biochem. 2002, 12: 365-372. 10.1159/000067907.
Lang KS, Duranton C, Poehlmann H, Myssina S, Bauer C, Lang F, Wieder T, Huber SM: Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ. 2003, 10: 249-256. 10.1038/sj.cdd.4401144.
Lang KS, Myssina S, Brand V, Sandu C, Lang PA, Berchtold S, Huber SM, Lang F, Wieder T: Involvement of ceramide in hyperosmotic shock-induced death of erythrocytes. Cell Death Differ. 2004, 11: 231-243. 10.1038/sj.cdd.4401311.
Bernhardt I, Hall AC, Ellory JC: Effects of low ionic strength media on passive human red cell monovalent cation transport. J Physiol. 1991, 434: 489-506.
Bernhardt I, Weiss E, Robinson HC, Wilkins R, Bennekou P: Differential effect of HOE642 on two separate monovalent cation transporters in the human red cell membrane. Cell Physiol Biochem. 2007, 20: 601-606. 10.1159/000107543.
Duranton C, Huber SM, Lang F: Oxidation induces a Cl(-)-dependent cation conductance in human red blood cells. J Physiol. 2002, 539: 847-855. 10.1113/jphysiol.2001.013040.
Foller M, Kasinathan RS, Koka S, Lang C, Shumilina E, Birnbaumer L, Lang F, Huber SM: TRPC6 contributes to the Ca(2+) leak of human erythrocytes. Cell Physiol Biochem. 2008, 21: 183-192. 10.1159/000113760.
Huber SM, Gamper N, Lang F: Chloride conductance and volume-regulatory nonselective cation conductance in human red blood cell ghosts. Pflugers Arch. 2001, 441: 551-558. 10.1007/s004240000456.
Bookchin RM, Ortiz OE, Lew VL: Activation of calcium-dependent potassium channels in deoxygenated sickled red cells. Prog Clin Biol Res. 1987, 240: 193-200.
Brugnara C, de Franceschi L, Alper SL: Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J Clin Invest. 1993, 92: 520-526. 10.1172/JCI116597.
Lang PA, Kaiser S, Myssina S, Wieder T, Lang F, Huber SM: Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am J Physiol Cell Physiol. 2003, 285: C1553-C1560.
Duranton C, Huber S, Tanneur V, Lang K, Brand V, Sandu C, Lang F: Electrophysiological properties of the Plasmodium Falciparum-induced cation conductance of human erythrocytes. Cell Physiol Biochem. 2003, 13: 189-198. 10.1159/000072421.
Tyurina YY, Tyurin VA, Zhao Q, Djukic M, Quinn PJ, Pitt BR, Kagan VE: Oxidation of phosphatidylserine: a mechanism for plasma membrane phospholipid scrambling during apoptosis?. Biochem Biophys Res Commun. 2004, 324: 1059-1064. 10.1016/j.bbrc.2004.09.102.
McConkey DJ, Orrenius S: The role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun. 1997, 239: 357-366. 10.1006/bbrc.1997.7409.
Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM: A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000, 405: 85-90. 10.1038/35011084.
Henson PM, Bratton DL, Fadok VA: The phosphatidylserine receptor: a crucial molecular switch?. Nat Rev Mol Cell Biol. 2001, 2: 627-633. 10.1038/35085094.
Boas FE, Forman L, Beutler E: Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci USA. 1998, 95: 3077-3081. 10.1073/pnas.95.6.3077.
Yamanaka M, Eda S, Beppu M: Carbohydrate chains and phosphatidylserine successively work as signals for apoptotic cell removal. Biochem Biophys Res Commun. 2005, 328: 273-280. 10.1016/j.bbrc.2004.12.171.
Kempe DS, Lang PA, Duranton C, Akel A, Lang KS, Huber SM, Wieder T, Lang F: Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 2006, 20: 368-370.
Schwarzer E, Turrini F, Ulliers D, Giribaldi G, Ginsburg H, Arese P: Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med. 1992, 176: 1033-1041. 10.1084/jem.176.4.1033.
Foller M, Bobbala D, Koka S, Huber SM, Gulbins E, Lang F: Suicide for survival--death of infected erythrocytes as a host mechanism to survive malaria. Cell Physiol Biochem. 2009, 24: 133-140. 10.1159/000233238.
Koka S, Bobbala D, Lang C, Boini KM, Huber SM, Lang F: Influence of paclitaxel on parasitemia and survival of Plasmodium berghei infected mice. Cell Physiol Biochem. 2009, 23: 191-198. 10.1159/000204107.
Lang PA, Kasinathan RS, Brand VB, Duranton C, Lang C, Koka S, Shumilina E, Kempe DS, Tanneur V, Akel A, Lang KS, Foller M, Kun JF, Kremsner PG, Wesselborg S, Laufer S, Clemen CS, Herr C, Noegel AA, Wieder T, Gulbins E, Lang F, Huber SM: Accelerated clearance of Plasmodium-infected erythrocytes in sickle cell trait and annexin-A7 deficiency. Cell Physiol Biochem. 2009, 24: 415-428. 10.1159/000257529.
Ayi K, Turrini F, Piga A, Arese P: Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004, 104: 3364-3371. 10.1182/blood-2003-11-3820.
Cappadoro M, Giribaldi G, O'Brien E, Turrini F, Mannu F, Ulliers D, Simula G, Luzzatto L, Arese P: Early phagocytosis of glucose-6-phosphate dehydrogenase (G6PD)-deficient erythrocytes parasitized by Plasmodium falciparum may explain malaria protection in G6PD deficiency. Blood. 1998, 92: 2527-2534.
de Jong K, Emerson RK, Butler J, Bastacky J, Mohandas N, Kuypers FA: Short survival of phosphatidylserine-exposing red blood cells in murine sickle cell anemia. Blood. 2001, 98: 1577-1584. 10.1182/blood.V98.5.1577.
Kean LS, Brown LE, Nichols JW, Mohandas N, Archer DR, Hsu LL: Comparison of mechanisms of anemia in mice with sickle cell disease and beta-thalassemia: peripheral destruction, ineffective erythropoiesis, and phospholipid scramblase-mediated phosphatidylserine exposure. Exp Hematol. 2002, 30: 394-402. 10.1016/S0301-472X(02)00780-4.
Kuypers FA, Yuan J, Lewis RA, Snyder LM, Kiefer CR, Bunyaratvej A, Fucharoen S, Ma L, Styles L, de Jong K, Schrier SL: Membrane phospholipid asymmetry in human thalassemia. Blood. 1998, 91: 3044-3051.
Koka S, Lang C, Boini KM, Bobbala D, Huber SM, Lang F: Influence of chlorpromazine on eryptosis, parasitemia and survival of Plasmodium berghe infected mice. Cell Physiol Biochem. 2008, 22: 261-268. 10.1159/000149804.
Bobbala D, Koka S, Geiger C, Foller M, Huber SM, Lang F: Azathioprine favourably influences the course of malaria. Malar J. 2009, 8: 102-10.1186/1475-2875-8-102.
Bobbala D, Koka S, Lang C, Boini KM, Huber SM, Lang F: Effect of cyclosporine on parasitemia and survival of Plasmodium berghei infected mice. Biochem Biophys Res Commun. 2008, 376: 494-498. 10.1016/j.bbrc.2008.09.005.
Myssina S, Huber SM, Birka C, Lang PA, Lang KS, Friedrich B, Risler T, Wieder T, Lang F: Inhibition of erythrocyte cation channels by erythropoietin. J Am Soc Nephrol. 2003, 14: 2750-2757. 10.1097/01.ASN.0000093253.42641.C1.
Wiese L, Hempel C, Penkowa M, Kirkby N, Kurtzhals JA: Recombinant human erythropoietin increases survival and reduces neuronal apoptosis in a murine model of cerebral malaria. Malar J. 2008, 7: 3-10.1186/1475-2875-7-3.
Floride E, Foller M, Ritter M, Lang F: Caffeine inhibits suicidal erythrocyte death. Cell Physiol Biochem. 2008, 22: 253-260. 10.1159/000149803.
Mahmud H, Mauro D, Foller M, Lang F: Inhibitory effect of thymol on suicidal erythrocyte death. Cell Physiol Biochem. 2009, 24: 407-414. 10.1159/000257433.
Sopjani M, Foller M, Lang F: Gold stimulates Ca2+ entry into and subsequent suicidal death of erythrocytes. Toxicology. 2008, 244: 271-279. 10.1016/j.tox.2007.12.001.
Blackie MA, Beagley P, Chibale K, Clarkson C, Moss JR, Smith PJ: Synthesis and antimalarial activity in vitro of new heterobimetallic complexes: Rh and Au derivatives of chloroquine and a series of ferrocenyl-4-amino-7-chloroquinolines. Organometal Chem. 2003, 688: 144-152. 10.1016/j.jorganchem.2003.07.026.
Navarro M: Gold complexes as potential anti-parasitic agents. Coordination Chemistry Reviews. 2009, 253: 1619-1626. 10.1016/j.ccr.2008.12.003.
Navarro M, Perez H, Sanchez-Delgado RA: Toward a novel metal-based chemotherapy against tropical diseases. 3. Synthesis and antimalarial activity in vitro and in vivo of the new gold-chloroquine complex [Au(PPh3)(CQ)]PF6. J Med Chem. 1997, 40: 1937-1939. 10.1021/jm9607358.
Navarro M, Vasquez F, Sanchez-Delgado RA, Perez H, Sinou V, Schrevel J: Toward a novel metal-based chemotherapy against tropical diseases. 7. Synthesis and in vitro antimalarial activity of new gold-chloroquine complexes. J Med Chem. 2004, 47: 5204-5209. 10.1021/jm049792o.
Sanchez-Delgado RA, Navarro M, Perez H, Urbina JA: Toward a novel metal-based chemotherapy against tropical diseases. 2. Synthesis and antimalarial activity in vitro and in vivo of new ruthenium- and rhodium-chloroquine complexes. J Med Chem. 1996, 39: 1095-1099. 10.1021/jm950729w.
Sannella AR, Casini A, Gabbiani C, Messori L, Bilia AR, Vincieri FF, Majori G, Severini C: New uses for old drugs. Auranofin, a clinically established antiarthritic metallodrug, exhibits potent antimalarial effects in vitro: Mechanistic and pharmacological implications. FEBS Lett. 2008, 582: 844-847. 10.1016/j.febslet.2008.02.028.
Wasi N, Singh HB, Gajanana A, Raichowdary AN: Synthesis of metal complexes of antimalarial drugs and in vitro evaluation of their activity against plasmodium falciparum. Inorg Chim Acta. 1987, 135: 133-137. 10.1016/S0020-1693(00)83277-6.
Cohen SN, Yielding KL: Inhibition of DNA and RNA polymerase reactions by chloroquine. Proc Natl Acad Sci USA. 1965, 54: 521-527. 10.1073/pnas.54.2.521.
Egan TJ: Haemozoin (malaria pigment): a unique crystalline drug target. Targets. 2003, 2: 115-124. 10.1016/S1477-3627(03)02310-9.
Egan TJ: Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. J Inorg Biochem. 2008, 102: 1288-1299. 10.1016/j.jinorgbio.2007.12.004.
Martinez A, Rajapakse CS, Naoulou B, Kopkalli Y, Davenport L, Sanchez-Delgado RA: The mechanism of antimalarial action of the ruthenium(II)-chloroquine complex [RuCl(2)(CQ)] (2). J Biol Inorg Chem. 2008, 13: 703-712. 10.1007/s00775-008-0356-9.
Slater AF, Cerami A: Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992, 355: 167-169. 10.1038/355167a0.
Ziegler J, Linck R, Wright DW: Heme Aggregation inhibitors: antimalarial drugs targeting an essential biomineralization process. Curr Med Chem. 2001, 8: 171-189.
Binh VQ, Luty AJ, Kremsner PG: Differential effects of human serum and cells on the growth of Plasmodium falciparum adapted to serum-free in vitro culture conditions. Am J Trop Med Hyg. 1997, 57: 594-600.
Huber SM, Uhlemann AC, Gamper NL, Duranton C, Kremsner PG, Lang F: Plasmodium falciparum activates endogenous Cl(-) channels of human erythrocytes by membrane oxidation. EMBO J. 2002, 21: 22-30. 10.1093/emboj/21.1.22.
Jensen JB, Trager W: Plasmodium falciparum in culture: establishment of additional strains. Am J Trop Med Hyg. 1978, 27: 743-746.
Trager W, Jensen JB: Human malaria parasites in continuous culture. Science. 1976, 193: 673-675. 10.1126/science.781840.
Koka S, Lang C, Niemoeller OM, Boini KM, Nicolay JP, Huber SM, Lang F: Influence of NO synthase inhibitor L-NAME on parasitemia and survival of Plasmodium berghei infected mice. Cell Physiol Biochem. 2008, 21: 481-488. 10.1159/000129641.
Brand V, Koka S, Lang C, Jendrossek V, Huber SM, Gulbins E, Lang F: Influence of amitriptyline on eryptosis, parasitemia and survival of Plasmodium berghei-infected mice. Cell Physiol Biochem. 2008, 22: 405-412. 10.1159/000185482.
Huber SM, Duranton C, Henke G, Van De SC, Heussler V, Shumilina E, Sandu CD, Tanneur V, Brand V, Kasinathan RS, Lang KS, Kremsner PG, Hubner CA, Rust MB, Dedek K, Jentsch TJ, Lang F: Plasmodium induces swelling-activated ClC-2 anion channels in the host erythrocyte. J Biol Chem. 2004, 279: 41444-41452. 10.1074/jbc.M407618200.
Lackner P, Hametner C, Beer R, Burger C, Broessner G, Helbok R, Speth C, Schmutzhard E: Complement factors C1q, C3 and C5 in brain and serum of mice with cerebral malaria. Malar J. 2008, 7: 207-10.1186/1475-2875-7-207.
Duranton C, Tanneur V, Lang C, Brand VB, Koka S, Kasinathan RS, Dorsch M, Hedrich HJ, Baumeister S, Lingelbach K, Lang F, Huber SM: A high specificity and affinity interaction with serum albumin stimulates an anion conductance in malaria-infected erythrocytes. Cell Physiol Biochem. 2008, 22: 395-404. 10.1159/000185483.
Clark IA, Cowden WB, Butcher GA, Hunt NH: Possible roles of tumor necrosis factor in the pathology of malaria. Am J Pathol. 1987, 129: 192-199.
Neifer S, Kremsner PG, Bienzle U: Application of anti-TNF to Plasmodium vinckei-infected mice is followed by an increase of parasitaemia. Acta Trop. 1989, 46: 273-275. 10.1016/0001-706X(89)90029-6.
Taverne J, Tavernier J, Fiers W, Playfair JH: Recombinant tumour necrosis factor inhibits malaria parasites in vivo but not in vitro. Clin Exp Immunol. 1987, 67: 1-4.
Kremsner PG, Winkler S, Brandts C, Wildling E, Jenne L, Graninger W, Prada J, Bienzle U, Juillard P, Grau GE: Prediction of accelerated cure in Plasmodium falciparum malaria by the elevated capacity of tumor necrosis factor production. Am J Trop Med Hyg. 1995, 53: 532-538.
Bondeson J: The mechanisms of action of disease-modifying antirheumatic drugs: a review with emphasis on macrophage signal transduction and the induction of proinflammatory cytokines. Gen Pharmacol. 1997, 29: 127-150.
Zappulla D: Environmental stress, erythrocyte dysfunctions, inflammation, and the metabolic syndrome: adaptations to CO2 increases?. J Cardiometab Syndr. 2008, 3: 30-34. 10.1111/j.1559-4572.2008.07263.x.
Acknowledgements
This study was supported by the Deutsche Forschungsgemeinschaft (La 315/6-1 and La 315/13-1) and the Carl-Zeiss-Stiftung. The authors acknowledge the meticulous preparation of the manuscript by Sari Rübe and Tanja Loch.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
IA carried out the flow cytometry analysis, DB participated in the in vivo experiments, SMQ analyzed the TNFα plasma levels, AE maintained the malaria parasite culture. MF and FL conceived the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Alesutan, I., Bobbala, D., Qadri, S.M. et al. Beneficial effect of aurothiomalate on murine malaria. Malar J 9, 118 (2010). https://doi.org/10.1186/1475-2875-9-118
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-9-118