Abstract
Background
Rapid emergence of Plasmodium resistance to anti-malarial drug mainstays has driven a continual effort to discover novel drugs that target different biochemical pathway (s) during infection. Plasma membrane Calcium + 2 ATPase (PMCA4), a novel plasma membrane protein that regulates Calcium levels in various cells, namely red blood cell (RBC), endothelial cell and platelets, represents a new biochemical pathway that may interfere with susceptibility to malaria and/or severe malaria.
Methods
This study identified several pharmacological inhibitors of PMCA4, namely ATA and Resveratrol, and tested for their anti-malarial activities in vitro and in vivo using the Plasmodium falciparum 3D7 strain, the Plasmodium berghei ANKA strain, and Plasmodium yoelii 17XL strain as model.
Results
In vitro propagation of P. falciparum 3D7 strain in the presence of a wide concentration range of the inhibitors revealed that the parasite growth was inhibited in a dose-dependent manner, with IC50s at 634 and 0.231 µM, respectively.
Results
The results confirmed that both compounds exhibit moderate to potent anti-malarial activities with the strongest parasite growth inhibition shown by resveratrol at 0.231 µM. In vivo models using P. berghei ANKA for experimental cerebral malaria and P. yoelii 17XL for the effect on parasite growth, showed that the highest dose of ATA, 30 mg/kg BW, increased survival of the mice. Likewise, resveratrol inhibited the parasite growth following 4 days intraperitoneal injection at the dose of 100 mg/kg BW.
Conclusion
The findings indicate that the PMCA4 of the human host may be a potential target for novel anti-malarials, either as single drug or in combination with the currently available effective anti-malarials.
Similar content being viewed by others
Background
Malaria is currently still a major public health problem around the globe. The World Health Organization (WHO) reported that an estimated 229 million cases of malaria occurred worldwide in 2020 [1] compared with 251 million cases in 2010 and 231 million cases in 2017. In malaria endemic country, such as Indonesia, around 5% of the total population reside in hyperendemic areas with annual parasite incidence (API) > 5. 29% live in mesoendemic areas (API between 1 and 5 per 1000), 56.1% live in hypoendemic areas (API < 1 per 1000) and 52% of the population live in malaria-free areas [2].
Strategies to control malaria include early diagnosis and prompt anti-malarial treatment of infected individuals, provision of long-lasting insecticidal net (LLIN) and indoor residual spraying (IRS) to reduce human contact with infected mosquitoes. However, the rapid development of drug resistance in the malarial parasite and insecticide-resistant anopheline mosquitoes have hampered large-scale efforts at malaria control [3], with cases of parasite resistance also reported in countries, such as Indonesia [2]. As many other infectious agents, the malarial parasite often takes advantage of its host’s protein machinery to proliferate and spread. Hence, targeting the host’s pathways involved in disease susceptibility is a possible approach to find novel treatments for malaria. Targeting the host’s molecules may also reduce the development of drug resistance in the parasite, because the modified biochemical events are beyond the parasite itself. Therefore, it is important to study new biochemical pathways both in the parasite and host cells to enable the identification of new therapeutic targets.
Recent genome-wide association studies have demonstrated that a common single nucleotide polymorphism (SNP) of the ATP2B4 gene encoding a calcium pump called the plasma membrane calcium ATPase 4 (PMCA4) has a very strong association with resistance against severe malaria [4]. PMCA is a family of ATP driven calcium pumps that ejects Ca2+ from the cytosol of most cell types [5]. PMCA is encoded by 4 different genes (named PMCA1–4) where the sequence differences are found mainly at the C and N terminal regions [6]. PMCA1 and PMCA4 appear to be the major isoforms that are ubiquitously expressed in most tissues/organs including red blood cells (RBCs) [7]. An expressed quantitative trait loci (eQTL) analysis has revealed that SNPs within the human PMCA4 gene are associated with lower PMCA4 expression and hence alteration of intra-cellular calcium within the RBCs [8]. It is known that a decrease in Ca2+ concentration in the parasitophorous vacuole within RBCs might impair parasite reproduction and maturation [8]. Thus, reduction of intra-cellular Ca2+ due to alteration of PMCA4 function or expression may affect the development and structure of intra-erythrocytic stages of the parasite [4]. Furthermore, changes in PMCA4 level or function might affect platelets and endothelial cell functions because these cells are also activated by intracellular Ca2+ [9, 10]. The activation of platelets and endothelial cells have been well known to have a crucial role in the pathogenesis of severe malaria by enhancing the sequestration and adherence of infected red blood cells within cerebral vasculature. These findings indicate that inhibiting PMCA4 function may be used as a potential alternative for anti-malarial treatment and the mitigation of malaria cerebral complication [8, 11,12,13,14,15].
The present study was aimed at exploring the effects of treatment with PMCA inhibitors on malaria parasite growth in vitro and analysed whether treatment with specific PMCA4 inhibitor aurintricarboxylic acid (ATA) or pan-PMCA1/4 inhibitor resveratrol affected the growth of Plasmodium falciparum 3D7 in the RBC culture. This study found that both ATA and resveratrol have an inhibitory activity against P. falciparum growth in vitro, with resveratrol displaying strong inhibition at low concentration. The in vivo experiment on the effect of PMCA4 inhibitors on cerebral malaria showed a modest effect on parasite survival, while resveratrol showed significant inhibition of parasite growth at the concentration of 100 mg/kg BW.
Methods
Ethics approval
The study was approved by the Eijkman Institute Research Ethic Committee (EIREC).
PMCA4 pharmacological inhibitors
The PMCA4 specific inhibitor Aurintricarboxylic acid (ATA, C22H14O9) and general PMCA inhibitor Resveratrol (C14H12O3) were used in this study. ATA, purchased from Sigma-Aldrich (Saint Louis, MO, USA), has been identified as a potent specific pharmacological inhibitor of PMCA4 [16] with IC50 of 150 nM for PMCA4 inhibition. ATA has only a minor effect on the second isoform of PMCA expressed in the heart, PMCA1 [16]. Resveratrol (trans-Resveratrol, SRT501) was purchased from SelleckChem and was identified as a potent inhibitor of global PMCA family activity [15]. All compounds were dissolved in either double distillate water (ddH2O) or dimethyl sulfoxide (DMSO) for both in vitro and in vivo experiments, and further serially diluted into a working concentration using RMPI-1640 media for in vitro. Artemisinin (Sigma Aldrich, St. Louis, Mo, USA) and sulfadoxine were used as positive control on in vitro and in vivo experiment, respectively.
Parasite cultivation in vitro
The human parasite Plasmodium falciparum strain used in this study was chloroquine-sensitive 3D7. Initially, P. falciparum 3D7 strain, blood-stage parasites were cultivated in RPMI medium 1640 (Sigma Aldrich, St. Louis, Mo, USA) supplemented with 10% human serum, 0.005% hypoxanthine, 0.21% NaHCO3, 0.596% HEPES, 0.25% gentamicin and 3–5% human erythrocytes. The parasites were grown in Type AB+ human blood under controlled and placed in 25 mm3 flask and incubated in a candle jar at 37 °C as described by Trager and Jensen method [17] but with modification. All strains were synchronized once with d-Sorbitol 5% [18] 2 days before testing. Parasites was most suitable for drug assays when they were 1–2% parasitaemia, and mostly ring stages with no gametocytes.
Determination of anti-malarial activity of PMCA inhibitors in P. falciparum in vitro
When the P. falciparum parasite culture reached 1–2% parasitaemia, it was sub-cultured into 96- well plates (180 µl per well) and incubated in the presence of various concentrations of ATA and resveratrol, ranged from 0 to 10–9 nM, obtained through tenfold serial dilutions. Non-parasitized erythrocytes were used as a negative control and parasitized erythrocytes without any test PMCA4 inhibitors were used as positive control. The anti-malarial activity of the PMCA inhibitors was compared with the standard drug, artemisinin. After 48-h incubation, thin Giemsa blood smears were made for each well and the parasite levels were determined by manual counting of parasite under light microscope. All experiments were done in duplicate and the 50% inhibitory concentration (IC50) of each inhibitor was determined.
Parasite cultivation in vivo
Plasmodium berghei strain ANKA and Plasmodium yoelii strain 17XL parasites (from Prof. Laurent Renia, Agency for Science, Technology and Research (A*STAR), Singapore) from cryopreserved stock was thawed and passaged into C57BL/6 strain mouse as donor and monitored until the parasitaemia level between 1 and 3% [16]. Approximately 1 × 104 infected erythrocytes from the donor [19] were inoculated to the experimental C57BL/6 mice.
Determination of anti-malarial activity of PMCA inhibitors Aurintricarboxyclic Acid (ATA) against Plasmodium berghei in experimental cerebral malaria (ECM) model on in vivo
Plasmodium berghei-infected mice were treated intraperitoneally with Aurintricarboxylic Acid (ATA) drug in various concentration (30 mg/kg BW, 10 mg/kg BW, 1 mg/kg BW) daily, started from one day before infection until 9 days post infection. In addition, anti-malarial drug sulfadoxine was used as positive control for this experiment and administered 25 mg/kg BW as its therapeutic dosage. The sulfadoxine treatment was done at 6–9 days post infection. A group without any treatment was also used as a comparison in this experiment. The survival rates of all experimental mice were observed as well as the concentration of red blood cells (RBCs) and body weight during the course of infection. The amount of RBC was examined under microscope using Niewbauer-Chamber. All the observation was followed until day 14 post infection.
Determination of PMCA4 inhibitor activity in vivo
Plasmodium yoelii were inoculated intraperitoneally into BALB/c mice and monitored daily until parasitaemia level of 1–2% with Giemsa-stained blood smear [20]. The PMCA4 inhibitor, resveratrol was given ip at various concentration daily for 4 days, generally started at days 5 post inoculation. In addition, anti-malarial drug sulfadoxine was used as positive control for this experiment and administered 25 mg/kg BW as its therapeutic dosage. The IC50 of each drug were analyzed based on parasitaemia level following 4-day treatment. The data was statistically calculated using a probit analysis.
Results
Anti-malarial activity of PMCA4 inhibitor, aurintricarboxylic acid (ATA), in vitro
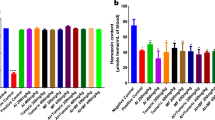
Plasmodium falciparum parasite growth in various concentrations of ATA is shown in Table 1. Cultivation of the parasite in the presence of a wide concentration range of ATA exhibited a dose-dependent pattern with a weak growth inhibition at the lower concentrations (0.1 nM–10–1 mM) but marked inhibition at higher doses 100 µM (Fig. 1). No obvious effect of the inhibitors on the amount of RBC observed. Using a probit analysis, the IC50 of ATA was found at the 634 µM. In comparison, the IC50 of artemisinin, which was used as control positive compound, was 2.34 nM (Fig. 2).
Anti-malarial activity of PMCA4 inhibitor, resveratrol, in vitro
The parasitaemia of the parasite culture in the presence of various concentrations of resveratrol is shown in Table 1. Cultivation of P. falciparum in the presence of resveratrol at the concentration ranges of 1 nM–1 mM exhibited a dose-dependent growth inhibition pattern with IC50 at 0.231 µM (Fig. 3). Compared to ATA, resveratrol showed stronger inhibition against P. falciparum growth in vitro.
Effect PMCA4 inhibitor Aurintricarboxyclic Acid (ATA) in vivo against Plasmodium berghei in experimental cerebral malaria (ECM) model
All mice in the untreated group succumbed to CM on day 7–9 post infection whilst all mice in the sulfadoxine group survived until the end of the experiment. Treatment with various concentrations of ATA yielded similar survival rates with the untreated groups except for the highest dose (30 mg/kg BW) that showed a significantly higher survival rate (Fig. 4). When compared to sulfadoxine, however, all ATA-treated groups including that with the highest dose had significantly lower survival rates. These suggest that ATA 30 mg/kg BW may increase survival after infection with P. berghei, but was not as effective as sulfadoxine.
Analysis on the body weight changes and the red blood cells concentrations on the untreated and ATA-treated groups revealed a non-significant pattern in which both groups exhibit a decrease in bodyweight and red blood cell concentrations during the course of the experiment. Similar pattern was also found on the degree of parasitaemia in treated and untreated groups before the mice succumbed (Table 2).
Anti-malarial activity of PMCA4 inhibitor, resveratrol, in vivo
Treatment of the P. yoelii-infected mice with intraperitoneal resveratrol at the concentrations of 1 mg-100 mg/kg BW revealed a non-significant inhibition of the growth rate at day 4, except at the concentration of 100 mg/kg BW where a 37% growth inhibition was shown (Fig. 5).
Discussion
The identification of PMCA4 SNPs as significant genetic determinants for malaria severity has prompted the idea that PMCA4 may be a novel target for malaria treatment. PMCA4 is coded for by the Atp2b4 gene located on chromosome 1 (Iq32) and several single nucleotide polymorphisms (SNPs), namely rs10900585; rs1541254; rs1541255; and rs4951074, have been implicated in susceptibility to severe malaria in genome wide association [4] and epidemiologic studies in Africa and Oceania [19, 20]. The molecular basis for the association of PMCA4 and severe malarial disease is currently still unclear but owing to the position of the SNPs in the promoter region of the gene, the effect might be mediated by the altered expression of the PMCA4, as shown by Zambo et al. [21]. Indeed, an expressed quantitative trait loci (eQTL) analysis has shown that PMCA4 SNPs are correlated with the level of PMCA4 expression in RBCs [8].
The findings of this study show that ATA and Resveratrol exhibited a potent anti-malarial activity on P. falciparum growth in the in vitro RBC cultures. The anti-malarial properties of the two PMCA4 inhibitors may be linked to the role of this protein in RBCs [14]. Inhibition of PMCA4 results in excess intracellular calcium which then activates a calcium-activated potassium channel (the Gardos channel), resulting in potassium efflux, RBC volume loss, and elevated mean corpuscular haemoglobin concentration (MCHC). Hydration of RBC has been linked with clinical severity of blood-stage malaria in the haemoglobin disorder sickle cell disease [22], and with infectivity of P. falciparum merozoites into RBCs [23].
At the in vivo experiment, particularly to determine the effect of ATA on cerebral malaria, the effect on the mice survival was noted at higher dose. Similar pattern was also observed with resveratrol on the parasite growth. The findings are in accordance with previous finding whereby ablation of PMCA4 confer a slight protection against cerebral malaria and did not significantly alter peripheral parasite burdens [8, 23].
Aurintricarboxylic acid (ATA) was identified as a potent PMCA4 inhibitor previously with IC50 at 150 nM at cardiac fibroblast [24]. ATA also inhibits other enzymes, such as nucleases, calpain and influenza virus neuraminidase at higher concentrations [25,26,27,28]. This study found that the anti-malarial property of ATA in vitro was relatively potent with IC50 at 634 mM, but in in vivo experiment, particularly to determine its activity against cerebral malaria, its activity was modest and was only observed at higher dose. Therefore, it is suggested that either the anti-malarial property of ATA in vivo is not mediated through the PMCA4 inhibition or their different pharmacokinetic property of the compound when given intraperitoneally. For the ATA activity in vivo, delivery through oral instead of intraperitoneal injection may be considered.
Likewise, resveratrol which exhibits a potent anti-malarial property in vitro has currently been available at the market as antioxidant formula indicated for a wide range of diseases. In addition to its property as pan PMCA inhibitor, the compound also demonstrated efficacy on various diseases in different dosage [29]. The resveratrol PMCA inhibition property was attained at micromolar range in various cells [13, 30]. Nevertheless, this anti-malarial property was observed at higher dose (100 mg/kg BW) through the intraperitoneal prescription in vivo using dimethyl sulfoxide as solvent. Therefore, effort to increase its solubility in water as well as the possibility for oral prescription might be worthy to pursue. Overall, it is suggested that the anti-malarial property of resveratrol is mediated through its property as PMCA4 inhibitor. As to the ATA modest effect in vivo, this phenomenon may relate to the pharmacokinetic property of the compound when given intraperitoneally or may also indicate other unspecific targets as reported before [15, 31]. The intrinsic antioxidant capacity of the resveratrol molecule and its ability to trigger the activation/repression of a wide range of membrane receptors, kinases and other enzymes have turned the quest for a molecular mechanism of action into an epic task [31]. To our knowledge, this is the first report of the anti-malarial activity of resveratrol and certainly deserves further exploration to circumvent the everlasting problem of anti-malarial drug resistance.
In conclusion, we have explored the anti-malarial activities of two compounds that are known as PMCA4 inhibitors. The results indicated that PMCA4 represents a promising novel target for anti-malarial development. Further study to determine biochemical pathways affected by the PMCA4 inhibitors to exert its anti-malarial property and drug delivery is currently on going.
Data availability
All relevant data are within the manuscript.
References
WHO. World Malaria Report 2020: 20 years of global progress and challenges. Geneva: World Health Organization; 2020.
Ministry of Health, Republic of Indonesia. Informasi Malaria Indonesia. 2022. https://www.malaria.id/
WHO. Global report on antimalarial drug efficacy and drug resistance. Geneva: World Health Organization; 2010.
Timmann C, Thye T, Vens M, Evans J, May J, Ehmen C, et al. Genome-wide association study indicates two novel resistance loci for severe malaria. Nature. 2012;489:443–6.
Stafford N, Wilson C, Oceandy D, Neyses L, Cartwright EJ. The plasma membrane calcium ATPases and their role as major new players in human disease. Physiol Rev. 2017;97:1089–125.
Strehler EE. The ATP2B plasma membrane Ca2+ ATPase family: regulation in response to changing demands of cellular calcium transport. In: Chakraborti S, Dhalla NS, editors. Regulation of Ca2+-ATPases, V-ATPases and F-ATPases. Berlin: Springer; 2016. p. 63–80.
Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50.
Lessard S, Gatof ES, Beaudoin M, Schupp PG, Sher F, Ali A, et al. An erythroid-specific ATP2B4 enhancer mediates red blood cell hydration and malaria susceptibility. J Clin Invest. 2017;127:3065–74.
Oceandy D, Mohammed TMA, Cartwright EJ, Neyses L. Local signals with global impacts and clinical implications: lessons from the plasma membrane calcium pump (PMCA4). Biochim Biophys Acta. 2011;1813:974–8.
Stafford N, Wilson C, Ocenady D, Neyses L, Cartwright EJ. The plasma membrane Calcium ATPases and their role as major new players in human disease. Physiol Rev. 2017;97:1089–125.
McMorran BJ, Marshall VM, de Graaf C, Drysdale KE, Shabbar M, Smyth GK, et al. Platelets kill intra erythrocytic malarial parasites and mediate survival to infection. Science. 2009;323:797–800.
Network MGE. Insight into malaria susceptibility using genome-wide data on 17,000 individuals from Africa, Asia and Oceania. Nat Commun. 2019;10:5732.
Mohammed TMA, Abou-Leisa R, Baudoin F, Stafford N, Neyses L, Cartwright EJ, et al. Development and characterization of a novel fluorescent indicator protein PMCA4-GcaMP2 in cardiomyocytes. J Mol Cell Cardiol. 2013;63:57–8.
Mohamed TMA, Zakeri SA, Baudoin F, Wolf M, Oceandy D, Cartwright EJ, et al. Optimisation and validation of a high throughput screening compatible assay to identify inhibitors of the plasma membrane calcium ATPase pump–a novel therapeutic target for contraception and malaria. J Pharm Sci. 2013;16:217–30.
Peterson JA, Oblad RV, Mecham JC, Kenealey JD. Resveratrol inhibits plasma membrane Ca2+-ATPase inducing an increase in cytoplasmic calcium. Biochem Biophys Rep. 2016;7:253–8.
Kurusamy S, López-Maderuelo D, Little R, Cadagan D, Savage AM, Ihugba JC, et al. Selective inhibition of plasma membrane calcium ATPase 4 improves angiogenesis and vascular reperfusion. J Mol Cell Cardiol. 2017;109:38–47.
Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5.
Moll K. Freezing and thawing asexual Plasmodium spp. In: Moll K, Kaneko A, Scherf A, Wahlgren M, Eds. 6th Edn. Methods in Malaria Research. Malaria Research and Reference Reagent Resource Centre (MR4), 2013; 17–18.
Ndila CM, Uyoga S, Macharia AW, Nyutu G, Peshu N, Ojal J, et al. Human candidate gene polymorphism and risk of severe malaria in children in Kilifi, Kenya: a case-control association study. Lancet Haematol. 2018;5:333–45.
Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’Connor KT, Neumann JC, et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem. 2004;279:33742–50.
Prasad V, Okunade GW, Miller ML, Shull GE. Phenotypes of SERCA and PMCA knockout mice. Biochem Biophys Res Commun. 2004;322:1192–203.
Bedu-Addo G, Meese S, Mockenhaup FP. An ATP2B4 polymorphism protects against malaria in pregnancy. J Infect Dis. 2013;207:1600–3.
Tiffert T, Lew VL, Ginsburg H, Krugliak M, Croisille L, Mohandas N. The hydration state of human red blood cells and their susceptibility to invasion by Plasmodium falciparum. Blood. 2005;105:4853–948.
Villegas-Mendez A, Stafford N, Haley MJ, Pravitasari NE, Baudoin F, Ali A, et al. The plasma membrane calcium ATPase 4 does not influence parasite levels but partially promotes experimental cerebral malaria during murine blood stage malaria. Malar J. 2021;20:297.
Mohammed TMA, Abou-Leisa R, Baudoin F, Stafford N, Neyses L, Cartwright EJ, et al. The plasma membrane calcium ATPase 4 signalling in cardiac fibroblasts mediates cardiomyocyte hypertrophy. Nature Comm. 2016;7:11074.
Hallick RB, Chelm BK, Gray PW, Orozco EM Jr. Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977;4:3055–64.
Posner A, Raser KJ, Hajimohammadreza I, Yuen PW, Wang KK. Aurintricarboxylic acid is an inhibitor of mu- and m-calpain. Biochem Mol Biol Int. 1995;36:291–9.
Hashem AM, Flaman AS, Farnsworth A, Brown EG, Domseelar GV, He R, Li X. Aurintricarboxylic acid is a potent inhibitor of influenza A and B virus neuraminidases. PLoS ONE. 2009;2009(4): e8350.
Mukherjee S, Dudley J, Das DK. Dose-dependency of Resveratrol in providing health benefits. Dose-Response. 2010;8:478–500.
Santofimia-Castano P, Salido GM, Gonzalez A. Interferences of Resveratrol with fura-2-derived fluorescence in intracellular free-Ca2+ concentration determinations. Cytotechnology. 2016;68:1369–80.
Kulkarni SS, Cantó C. The molecular targets of resveratrol. Biochim Biophys Acta. 2015;852:1114–23.
Acknowledgements
The authors wish to thank the Chairman of the Eijkman Institute for Molecular Biology (EIMB) and Executive Director and team from the Indonesian Science Fund (Dana Ilmu Pengetahuan Indonesia/DIPI) for their support in this activity.
Funding
This study was supported by Research Grant Number MR/P015816/1 from The Indonesia Science Fund-Indonesia Endowment Fund for Education (Dana Ilmu Pengetahuan Indonesia/DIPI- Lembaga Pengelola Dana Pendidikan/LPDP), Indonesia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
PBSA, JS, DS, KNC and DO design the study, data analysis, responsible for management and manuscript writing. FKD, RR, IER, NEP, AFMK data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Asih, P.B.S., Siregar, J.E., Dewayanti, F.K. et al. Treatment with specific and pan-plasma membrane calcium ATPase (PMCA) inhibitors reduces malaria parasite growth in vitro and in vivo. Malar J 21, 206 (2022). https://doi.org/10.1186/s12936-022-04228-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04228-0