Abstract
Background
Fermentation of xylose to ethanol has been achieved in S. cerevisiae by genetic engineering. Xylose utilization is however slow compared to glucose, and during anaerobic conditions addition of glucose has been necessary for cellular growth. In the current study, the xylose-utilizing strain TMB 3415 was employed to investigate differences between anaerobic utilization of glucose and xylose. This strain carried a xylose reductase (XYL1 K270R) engineered for increased NADH utilization and was capable of sustained anaerobic growth on xylose as sole carbon source. Metabolic and transcriptional characterization could thus for the first time be performed without addition of a co-substrate or oxygen.
Results
Analysis of metabolic fluxes showed that although the specific ethanol productivity was an order of magnitude lower on xylose than on glucose, product yields were similar for the two substrates. In addition, transcription analysis identified clear regulatory differences between glucose and xylose. Respiro-fermentative metabolism on glucose during aerobic conditions caused repression of cellular respiration, while metabolism on xylose under the same conditions was fully respiratory. During anaerobic conditions, xylose repressed respiratory pathways, although notably more weakly than glucose. It was also observed that anaerobic xylose growth caused up-regulation of the oxidative pentose phosphate pathway and gluconeogenesis, which may be driven by an increased demand for NADPH during anaerobic xylose catabolism.
Conclusion
Co-factor imbalance in the initial twp steps of xylose utilization may reduce ethanol productivity by increasing the need for NADP+ reduction and consequently increase reverse flux in glycolysis.
Similar content being viewed by others
Introduction
Production of fuel ethanol has increased several fold during the last decade due to increasing oil prices and environmental concerns [1]. The vast majority of this production comes from fermentation of agricultural products, primarily sugar cane and corn, by baker's yeast S. cerevisiae. Lignocellulose biomass from forest and agricultural residues is an alternative to sucrose (sugar cane) and starch (corn) based ethanol production [2, 3]. Next to glucose, the main component of lignocellulose is xylose, and the use of this substrate by S. cerevisiae has been enabled through expression of heterologous enzymes [4–6]. Xylose utilizing S. cerevisiae strains have been constructed by expressing a reduction/oxidation pathway involving xylose reductase (XR) and xylitol dehydrogenase (XDH) [7, 8] or a xylose isomerase (XI) pathway [9–11].
Successive cycles of metabolic engineering have improved xylose utilization in recombinant S. cerevisiae [12, 13]. Compared to glucose however the ethanol productivity from xylose is still low. Poor xylose utilization has been ascribed to potentially rate-controlling metabolic steps including: low substrate affinity of the recombinant enzymes [8]; cofactor imbalance in the XR-XDH reactions [7, 14]; low xylose transport capacity [15, 16]; and failure to recognize xylose as a fermentable carbon source [17, 18]. Among several experimental approaches, glucose and xylose metabolism have been investigated by transcriptional analysis to identify rate-controlling processes in xylose metabolism [17, 19–22]. Growing cells are needed to establish (pseudo) steady-state conditions for transcription analysis and determination of metabolic fluxes [23, 24]. The analysis of xylose utilizing strains has thus been hampered by poor anaerobic growth on xylose. Transcription analysis has consequently been conducted under aerobic conditions [17, 19, 20, 22] and/or with addition of glucose as a co-substrate [21]. Transcriptional characterization of anaerobic xylose metabolism has however remained elusive, regardless of the importance of this particular condition in a production setting.
For S. cerevisiae expressing the oxidoreductive xylose assimilating pathway, a recent accomplishment has been alteration of the cofactor specificity of XR through site directed mutagenesis [25–27]. By increasing the affinity of the P. stipitis XR for NADH, the objective has been to improve cofactor recycling in the XR-XDH coupled reactions. The current study utilized a S. cerevisiae strain harboring a mutated XR (K270R) with significantly improved substrate uptake rate and ethanol productivity [26]. The strain grew anaerobically on xylose as a sole carbon source which for the first time enabled quantitative metabolic flux determination and genome wide transcriptional analysis. The focus of the study was to compare metabolic fluxes during anaerobic glucose and xylose growth, and to analyze the observed differences on a transcriptional level.
Materials and methods
Strains and cultivation conditions
S. cerevisiae strains and plasmids used in this study are summarized in Table 1. Escherichia coli strain DH5α was used for sub-cloning and was grown on LB medium supplemented with 100 mg/L ampicillin. Defined mineral medium was used for S. cerevisiae cultivation and was composed of: xylose or glucose, 60 g/L; mineral salts ((NH4)2SO4, 5 g/L; KH2PO4, 3 g/L; MgSO4·7H2O, 0.5 g/L); buffer (potassium hydrogen phthalate, 50 mM pH 5.5); Tween 80, 0.4 g/L; ergosterol, 0.01 g/L [28]; vitamins and trace elements [29]. Identical medium was used for pre-cultures and batch fermentation in instrumented bioreactors with the exception that buffering agent was omitted in the latter case. At the start of each experiment, yeast strains were streaked from 15% (v/v) glycerol stocks and grown two days on Yeast Nitrogen Base (YNB) glucose plates. Pre-cultures were inoculated in baffled shake-flasks (10% liquid volume) at a predetermined cell density, OD620 nm = 0.5/0.025 (xylose/glucose), and grown for 20 hrs to yield cells in late exponential phase (OD620 nm~14). Cultivation of S. cerevisiae was performed at 30°C.
Anaerobic batch cultivation was performed in an instrumented bioreactor (Applikon Biotechnology, AC Schiedam, the Netherlands) with 1.5 L working volume and a starting OD620 nm of 0.2. The medium contained 60 g/L of glucose or xylose and was prepared as described above with antifoam (Dow Corning, Midland, USA) added to the reactor at a final concentration of 0.2 mL/L. Temperature was maintained at 30°C and the pH was controlled at 5.5 through addition of 3 M KOH. Cultures were grown under aerobic conditions until the cell density reached OD620 nm = 1.0, upon which conditions were changed to anaerobiosis for the remainder of the experiment (Figure 1). During the aerobic phase, the culture was sparged with air at a flow rate of 0.4 L/min and the stirring was set to 500 rpm. During the anaerobic phase, oxygen free conditions were maintained by nitrogen (> 99.995%) sparging at a flow rate of 0.2 L/min and the stirring rate was reduced to 200 rpm. Dissolved oxygen (DO) was monitored using a DO probe and CO2 production was detected online by an INNOVA 1313 fermentation monitor (LumaSense Technologies, Ballerup, Denmark). Cultures were sampled for HPLC, OD620 nm and dry cell weight measurements. Samples were collected for transcriptome analysis during exponential aerobic (OD620 nm~1.0) and anaerobic growth (OD620 nm~4.0). Transcriptome samples (50 mL) were collected from the bioreactor into a pre-chilled (-80°C) glass bottle. Samples were immediately centrifuged (5000*g, 2 min, 4°C) and the biomass pellet was frozen in liquid nitrogen. Experiments were performed in biological duplicate.
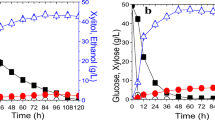
Fermentation profiles of glucose and xylose growth. The dashed vertical line indicates switch between aerobic and anaerobic conditions. Samples for transcription analysis were collected at OD620 nm ~ 1 and OD620 nm ~ 4, which corresponds ln OD ~ 0 and ln OD ~ 1.4 in the figure. A. Biomass production during glucose cultivation. B. Biomass production during xylose cultivation C. Substrate consumption and metabolite formation during glucose cultivation. D. Substrate consumption and metabolite formation during xylose cultivation. Symbols: glucose/xylose, "squares"; ethanol, "diamonds"; glycerol, "circles"; xylitol, "triangles" and biomass, "stars".
Strain construction
S. cerevisiae strain TMB 3415 was constructed from the TMB 3043 (Table 1) parent strain [13]. This genetic background encompasses genetic changes previously identified as beneficial for xylose utilization. Genes for the non-oxidative pentose phosphate pathway [12] and xylulokinase XKS1 [30] have been over-expressed, and the non-specific aldose reductase gene GRE3 has been deleted [31]. Genes encoding Pichia stipitis mutated xylose reductase (XYL1 K270R) and xylitol dehydrogenase (XYL2) were integrated in TMB 3043 by transformation with YIpOB9 (Table 1) linearized with Eco RV, using the Lithium Acetate method [32]. The resulting strain, TMB 3662, was rendered prototrophic by integration of the linearized (Eco RV) vector YIplac128 (Table 1), yielding strain TMB 3415. Standard molecular biology techniques were employed [33] and Fermentas GeneJet plasmid miniprep kit (Fermentas, Vilnius, Lithuania) was used for plasmid extraction.
Calculation of metabolic fluxes
Exponential anaerobic growth was confirmed by linear regression of the natural logarithm of cell concentration against time. Specific rates of product formation and substrate consumption were calculated in Matlab (Matlab R2007b, The MathWorks Inc., MA, USA) using Equation 1 & 2 and measured metabolite and cell concentrations. A pseudo-steady state assumption was validated by observing constant specific production- and consumption rates within 2-3 cell duplications, as well as good agreement of measured values between biological replicates.


Where: X = biomass (g/L); Met = metabolite concentration (g/L); μ = specific growth rate (h-1); r = specific production rate (g/h×gDW).
Microarray analysis
Microarray analysis was performed on cell samples collected from aerobic and anaerobic batch cultivation on glucose and xylose as described above. RNA from two independent biological cultivations was analyzed. Total RNA was extracted from frozen cell pellets using a bead-beater (Biospecs products, Bartlesville, OK, USA) and Trizol reagent (Invitrogen, CA, USA). All extractions where performed on the same total amount of cells (approximately 10 mg dry weight). RNA was further purified using the RNeasy mini kit (Qiagen, Hilden, Germany). RNA quality and concentration were measured using an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and Nanodrop ND-1000 (Thermo Fisher Scientific, Waltham, MA, USA) respectively. Total RNA was processed using the GeneChip® Expression 3'-Amplification Reagents One-cycle cDNA synthesis kit (Affymetrix Inc, Santa Clara, CA, USA) to produce double-stranded cDNA. This was used as a template to generate biotin-targeted cRNA following the manufacturer's specifications. Fifteen μg of the biotin labeled cRNA was fragmented to strands between 35 and 200 bases in length, 10 μg of which was hybridised onto the GeneChip® genome array overnight in the GeneChip® Hybridisation oven6400 using standard procedures. The arrays were washed and then stained in a GeneChip® Fluidics Station 450. Scanning was performed with the GeneChip® Scanner 3000 and image analysis was performed using GeneChip® Operating Software. The RMA algorithm [34] was used for normalization and scaling of the raw signal data. Student's t-test was used to identify genes with significantly (p ≤ 0.05) altered gene expression. All array data is presented as fold changes, i.e. the log2 ratio of expression signals.
Analysis of substrate and products
Metabolite concentrations were determined by HPLC using a Waters HPLC system (Milford, MA, USA). An Aminex HPX-87H ion-exchange column (Bio-Rad, Hercules, CA, USA) and a refractive index detector (RID-6a, Shimadzu, Kyoto, Japan) were used for separation and detection, respectively. The column temperature was 45°C and 5 mM H2SO4 was used as a mobile phase at a flow rate of 0.6 mL/min. Cell dry weight was determined by filtering a known sample volume through a dried and pre-weighed 0.45-μm pore membrane (Pall Corporation, New York, USA), washing with distilled water and drying in a microwave oven for 8 min at 350 W.
Results
Aerobic and anaerobic batch cultivation
TMB 3415 was cultivated in synthetic medium containing either glucose or xylose as the sole carbon source, under aerobic and anaerobic conditions. Aerobic growth was maintained for approximately 2 cell divisions, after which anaerobiosis was established by switching the sparging gas from air to nitrogen. The fermentation profiles of glucose and xylose growth in this setup are presented in Figure 1. Balanced exponential cell growth was seen for glucose and xylose under both aerobic and anaerobic conditions, allowing for the assumption of pseudo steady-state conditions [24]. Growth rates and metabolic fluxes were calculated for the anaerobic growth phase (Table 2).
In glucose medium, growth rates were similar regardless of oxygenation (0.43 h-1 vs. 0.33 h-1) (Figure 1A) and comparable to previous reports [35]. During xylose consumption on the other hand, the growth rate decreased from 0.20 h-1 to 0.025 h-1 under anaerobic conditions (Figure 1C). Specific rates of substrate uptake and metabolite production were also different during anaerobic glucose and xylose fermentation (Table 2). The substrate uptake rate and the ethanol production rate were approximately an order of magnitude lower during xylose utilization than during glucose utilization (Table 2). The productivity, 0.13 g/gDW×h, and yield, 0.43 g/g, of ethanol from xylose (Table 2) was however significantly higher compared to several recently investigated xylose-utilizing strains [20, 26, 36, 37]. It is reasonable to assume that anaerobic growth of strain TMB 3415 results from increased ethanol productivity and low xylitol yield, which in turn can be ascribed to the K270R mutation in XR [26]. Compared to strains expressing a xylose isomerase based pathway, the ethanol productivity in TMB 3415 was higher than in non-growing strains [10, 11, 38], whereas it was similar to a strain growing at μ = 0.03 h-1 [39] and lower than a strain growing at μ = 0.09 h-1 [40].
Topography of microarray data
The present study aimed at highlighting regulatory differences between anaerobic glucose and xylose growth by xylose-utilizing S. cerevisiae. Specifically, differences in growth rate, substrate consumption and ethanol productivity were analyzed on a transcriptional level. Transcriptional characterization have not previously been performed under anaerobic conditions due to the inability of recombinant S. cerevisiae strains to grow on xylose in the absence of oxygen [17, 20–22]. A few strains expressing Piromyces XI are able to grow anaerobically on xylose [39, 40], however to the best of our knowledge transcription analysis of these strains has not been reported.
The overall structure of the microarray data was examined using Principle Component Analysis (PCA) [41]. PCA has been used to reduce the dimensionality of microarray data and to identify features of experimental conditions that best explain the observed variance in gene expression [42]. The projection of the two principle components, oxygen availability and carbon source, segregated the individual samples in a two dimensional space (Figure 2A). The four conditions, glucose aerobic (GA), xylose aerobic (XA), glucose anaerobic (GAnA) and xylose anaerobic (XAnA), were separated along the axis of the first and second principle component. The first principal component, which was responsible for the most variance in the data set, separated samples according to oxygen availability (aerobic or anaerobic) (Figure 2A). The second principal component separated samples according to carbon source (glucose or xylose). The PCA projection of the transcription data singled out anaerobic xylose growth as the most unique group in the data set (Figure 2A).
A PCA projection of individual microarray samples. The dashed lines separate samples in 4 quadrants depending on the experimental condition. Symbols: Aerobic glucose, "empty circle"; Anaerobic glucose, "filled circle"; Aerobic xylose, "empty square"; Anaerobic xylose, "filled square". B The number of differently expressed genes (95% confidence interval) is indicated for pairwise comparisons of experimental conditions. Abreviations: GA, glucose aerobic; GAnA, glucose anaerobic; XA, xylose aerobic; XAnA, xylose anaerobic. C1-C4 designate specific pairwise comparisons, e.g. C1 = glucose aerobic-glucose anaerobic.
Next, the microarray data was organized into four relevant pairwise comparisons: GA vs. GAnA (Comparison 1 = C1); GA vs. XA (Comparison 2 = C2); XA vs. XAnA (Comparison 3 = C3) and GAnA vs. XAnA (Comparison 4 = C4) (Figure 2B). Comparisons C1 and C2 have previously been reported in slightly different experimental setups [17, 20, 23]. However, comparisons C3 and C4 allowed for the first time analysis of transcription during anaerobic growth on xylose as a sole carbon source. Globally, the transition from aerobic to anaerobic growth changed the expression level of more genes on xylose (C3, 809 genes) than on glucose (C1, 546 genes) (Figure 2B). This difference is presumably due to that aerobic metabolism on xylose is fully respiratory, while it is respiro-fermentative on glucose. Likewise a higher number of genes displayed different expression levels between glucose and xylose utilization under aerobic conditions (C2, 1059 genes) than under anaerobic conditions (C4, 641 genes) (Figure 2B).
Finally, subsets of genes were isolated according to the following criteria: (i) genes that changed expression levels between aerobic and anaerobic conditions regardless of carbon source (C1&C3, Figure 3A) and (ii) genes that changed expression levels on glucose and xylose regardless of oxygenation level (C2&C4, Figure 3B). Relatively few genes, 113, changed expression on both glucose and xylose during transition from aerobic to anaerobic conditions (Figure 3A). Likewise, only 130 genes changed expression level on xylose compared to glucose irrespective of oxygenation level (Figure 3B).
Venn diagram showing the fraction of genes common for: (a) transition between aerobic-anaerobic conditions regardless of carbon source; or (b) transition between glucose and xylose regardless of oxygenation level. The total number inside each circle represents the number of genes with significantly changed expression levels in that particular comparison, e.g. 546 genes for the glucose aerobic - glucose anaerobic transition (C1, Figure 2B).
Gene ontology (GO) terms
Within a group of genes, up- and down-regulated pathways and processes can be identified by searching for over-represented gene ontology (GO) terms http://www.geneontology.org/. Each annotated gene in the S. cerevisiae genome is associated with one or several GO terms that describe the corresponding biological process, e.g. amino acid synthesis. Significantly enriched gene ontology terms (p < 0.01) were identified within the previously described groups (C1, C2, C3, C4, C1&C3 and C2&C4) (Table 3). If more than one GO term in the same "family" were identified, only the most significant term was listed.
Comparing aerobic and anaerobic metabolism on glucose (C1, Figure 2B), unilateral down-regulation of respiratory processes was identified (Table 3). Down-regulation of respiratory genes in response to anaerobiosis has previously been reported for glucose grown cells in batch culture [17] and C- and N-limited chemostat culture [42, 43]. In contrast, respiratory pathways were not down-regulated between aerobic and anaerobic xylose metabolism (C3, Figure 2B), whereas processes related to protein synthesis were significantly repressed (Table 3). Down-regulation of ribosome biogenesis and amino acid synthesis [42, 44] reflects the ten fold reduction of growth rate between aerobic and anaerobic conditions on xylose (Figure 1B). In addition, the GO terms "alcohol metabolism" and "hexose catabolism" were up-regulated under anaerobic conditions on xylose (Table 3). In the group of genes that changed expression in response to anaerobiosis irrespective of carbon source (C1&C3, Figure 3A), repression of cellular respiration was identified (Table 3).
Aerobic glucose metabolism was respiro-fermentative, while aerobic xylose metabolism was completely respiratory with absent ethanol production (Figure 1). On a transcription level, this difference was visible in higher expression of several respiration related GO terms on xylose compared to glucose during aerobic conditions (C2, Table 3) [17, 20]. During anaerobic conditions, protein synthesis was down-regulated on xylose compared to glucose, while expression of respiratory processes and the GO term "hexose metabolism" was up-regulated (C4, Table 3). The lower expression of amino acid biosynthesis [42, 44] is related to the lower growth rate on xylose compared to glucose under anaerobic conditions. Oxidative phosphorylation was up-regulated on xylose in the group of genes that were differently expressed on glucose and xylose under both aerobic and anaerobic conditions (C4&C2, Table 3).
Respiration was not repressed between aerobic and anaerobic growth on xylose (C3, Table 3), and higher expression of respiratory processes was observed during both aerobic and anaerobic conditions compared to glucose (C4&C2, Table3). Certain processes in cellular respiration were however down-regulated both on glucose and xylose in response to anaerobiosis (C1&C3, Table 3). This indicates that although oxidative metabolism was not unilaterally down-regulated under anaerobic conditions, some processes were still repressed.
Regulation of the central carbon metabolism
Expression of the GO term "hexose metabolism" increased specifically under anaerobiosis on xylose, both in comparison to anaerobic glucose utilization (C4) and to xylose under aerobic conditions (C3) (Table 3). This observation was dependent on anaerobic xylose growth and has thus not previously been reported. The GO term "hexose metabolism" encompasses several aspects of the central carbon metabolism (glycolysis, pentose phosphate pathway, gluconeogenesis etc.), which is responsible for the cell's energy metabolism. As such, regulation of central carbon metabolism is linked to growth rate, protein synthesis and ethanol production rate [44, 45]. Several reaction steps in the central carbon metabolism are catalyzed by more than one isozyme, which enable the cell to regulate the direction/flux of the pathway in response to the energetic state of the cell. Therefore, expression of genes in the central carbon metabolism was investigated in greater detail to pinpoint differences between glucose and xylose anaerobic growth (Figure 4).
Regulation of central metabolism under aerobic/anaerobic growth on glucose/xylose. The fold change (log2) of expression is presented for the C1, C2, C3 and C4 comparisons. Cofactor utilization is only depicted for relevant reactions. Standard three letter code is used for all genes names http://www.yeastgenome.org/.
Increased reversed flux in glycolysis during anaerobic xylose utilization was indicated by expression of several isozymes specific for gluconeogenesis. The hexokinase gene HXK1 was expressed higher on xylose than on glucose irrespective of aeration (Figure 4). Previously, high HXK1 expression has been reported under aerobic xylose growth [17, 20] and during metabolism of non-fermentable carbon sources [46]. Further down the pathway, the exclusively gluconeogenetic enzyme fructose-1,6-bisphosphatase FBP1 was up-regulated specifically during anaerobic xylose utilization (Figure 4). Expression of glyceraldehyde-3-phosphate dehydrogenase isozyme TDH1 is linked to stationary growth and gluconeogenesis [47] and was similarly up-regulated on xylose during anaerobic conditions (Figure 4). Also the minor isoform of phosphoglycerate mutase GPM2 was up-regulated on xylose during anaerobic conditions (Figure 4) but the function of this enzyme is largely unknown [48].
While reversed flux in glycolysis was indicated during anaerobic xylose growth, increased activity of the oxidative pentose phosphate pathway was observed through expression of 6-phosphogluconate dehydrogenase(GND2) and glucose-6-phosphate dehydrogenase (ZWF1) (Figure 4). Both GND2 and ZWF1 catalyze the reduction of NADP+ and were specifically up-regulated under anaerobic conditions on xylose (Figure 4). During anaerobic conditions, NADP+ reduction in the oxidative pentose phosphate pathway controls the rate of xylose utilization as a consequence of the cofactor imbalance in the XR-XDH reaction [49]. Increased flux in the oxidative pentose phosphate pathway (PPP) must however be supported by proportionally increased reversed flux in glycolysis, which indeed was seen in the current study.
Discussion
In the present study, metabolic fluxes and genome-wide transcription analysis were investigated in recombinant S. cerevisiae under strictly anaerobic conditions with xylose as a sole carbon source. The currently employed strain is the first strain utilizing an oxidoreductive xylose-assimilating pathway capable of sustained anaerobic xylose growth. Anaerobic growth on xylose has previously been described for strains expressing an isomerase based pathway [39, 40], however to the best of our knowledge, transcription analysis have not been reported for these strains. In previous transcription studies, steady state condition on xylose has been achieved by addition of oxygen or glucose to support growth [17, 20–22]. The difference between aerobic utilization of glucose and xylose is thus well described [17, 20], whereas the current study represents the first complete characterization of anaerobic xylose growth.
Calculated metabolic fluxes showed that substrate uptake rate (2.6 g/gDW×h vs. 0.29 g/gDW×h) and ethanol productivity (1.2 g/gDW×h vs. 0.13 g/gDW×h) on glucose and xylose were proportional to the growth rates (0.33 h-1 vs. 0.025 h-1) (Table 2). Transcription data likewise verified that the low anaerobic growth rate on xylose correlated directly to reduced expression of genes for amino acid synthesis and protein synthesis (Table 3), which has previously been shown for glucose grown cultures [42]. The ethanol yield from consumed substrate (0.43 g/g) on the other hand was identical in anaerobic glucose and xylose fermentation. On xylose, 20% substrate was lost as xylitol, however on glucose this was approximately balanced by almost two times higher glycerol yield compared to xylose (Table 2). The lower glycerol yield during xylose utilization is most likely due to that xylitol acts as a redox sink for anabolic reactions analogously to glycerol [50]. Supporting this argument, it has previously been seen that addition of an external redox acceptor reduced both glycerol and xylitol formation in cultivation of xylose utilizing S. cerevisiae [51, 52].
Transcription analysis showed that oxidative phosphorylation was de-repressed on xylose compared to glucose under aerobic conditions, which correlates with the absence of respiro-fermentative metabolism on xylose (Table 3) [17, 18, 20]. During anaerobic conditions however, most respiratory genes continued to be highly expressed on xylose while they were unilaterally repressed on glucose (Table 3). The maintenance of unrepressed oxidative metabolism during anaerobic xylose growth can not be completely explained by lack of catabolite repression [53] since exclusively oxygen dependent repression was seen on glucose. It is however possible that on xylose, expression of "oxidative metabolism" was partly maintained as compensatory response to the cofactor imbalance during anaerobic conditions. Altered redox metabolism and up-regulation of genes for NADPH formation and NADH oxidation was previously seen during oxygen-limited xylose growth [17] and in an evolutionary engineered xylose-utilizing strain [21]. In the current study, the NAD+/NADH dehydrogenase shuttle (NDI1, NDE1 and NDE2) and NADP+ linked glutamate dehydrogenase (GDH3) were down-regulated on glucose under anaerobic conditions but up-regulated on xylose (data not shown).
During xylose utilization, up-regulation of gluconeogenesis and the oxidative pentose phosphate pathway coincided with anaerobiosis (Figure 4). On xylose, increased flux in the oxidative PPP is explained by need for NADP+ reduction in anerobic co-factor recycling [49]. Increased reversed flux in upper glycolysis follows consequently from mass balance at the glucose-6-phosphate node (Figure 4). As such, it has previously been seen that high flux in the oxidative PPP, and consequently gluconeogenesis, lowered net glycolytic flux and ethanol productivity [54]. The connection between cofactor recycling, gene expression and metabolic flux offers an explanation to the low ethanol productivity on xylose compared to glucose, despite similar ethanol yields (Table 2). Similarly it is possible that reduced back-flow in glycolysis is the primary reason for the increased ethanol productivity and anaerobic growth rate in the mutant XR (K270R) utilized in the current study [26]. Further improvement of cofactor imbalance in the initial two steps of xylose utilization by protein engineering is expected to improve performance of S. cerevisiae strains utilizing an oxido-reductive xylose assimilating pathway.
Conclusion
The present work describes the metabolic and transcriptional characterization of the xylose utilizing strain TMB 3415 under aerobic and anaerobic conditions. Under anaerobic conditions, metabolism and growth rate on xylose were proportionally reduced compared to glucose, which was reflected in terms of repressed protein synthesis. In addition, cofactor imbalance during anaerobic xylose growth may have caused up-regulation of oxidoreductive metabolism, pentose phosphate pathway and gluconeogenesis. To further investigate regulation of xylose metabolism in anaerobic conditions, strains using oxidoreductive and isomerase based xylose assimilating pathways should be compared at the transcriptional level. The regulatory effect of cofactor imbalance could thus be assayed.
Abbreviations
- 1:
-
3-DPG: 1,3-bisphosphoglycerate
- CoA:
-
coenzyme A
- DHAP:
-
dihydroxyacetone-phosphate
- G3P:
-
3-phosphoglycerate
- G2P:
-
2-phosphoglycerate
- GAP:
-
glyceraldehyde 3-phosphate
- fru-6P:
-
fructose 6-phosphate
- fru-1:
-
6-bisP: fructose 1,6-bisphosphate
- glu-6P:
-
glucose 6-phosphate
- HPLC:
-
high performance liquid chromatography
- NADH:
-
nicotinamide adenine dinucleotide
- NADPH:
-
nicotinamide adenine dinucleotide phosphate
- OD:
-
optical density
- - P:
-
phosphate
- PEP:
-
phosphoenolpyruvate
- PPP:
-
pentose phosphate pathway
- XDH:
-
xylitol dehydrogenase
- XI:
-
xylose isomerase
- XK:
-
xylulokinase
- XR:
-
xylose reductase
- YNB:
-
yeast nitrogen base.
References
Otero JM, Panagiotou G, Olsson L: Fueling industrial biotechnology growth with bioethanol. Adv Biochem Eng/Biotechnol. 2007, 108: 1-40. full_text. full_text.
Hahn-Hägerdal B, Galbe M, Gorwa-Grauslund MF, Liden G, Zacchi G: Bio-ethanol--the fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24: 549-556. 10.1016/j.tibtech.2006.10.004.
Galbe M, Sassner P, Wingren A, Zacchi G: Process engineering economics of bioethanol production. Adv Biochem Eng Biotechnol. 2007, 108: 303-327.
Hahn-Hägerdal B, Karhumaa K, Jeppsson M, Gorwa-Grauslund MF: Metabolic engineering for pentose utilization in Saccharomyces cerevisiae. Adv Biochem Eng Biotechnol. 2007, 108: 147-177.
Jeffries TW: Engineering yeasts for xylose metabolism. Curr Opin Biotechnol. 2006, 17: 320-326. 10.1016/j.copbio.2006.05.008.
van Maris AJ, Winkler AA, Kuyper M, de Laat WT, van Dijken JP, Pronk JT: Development of efficient xylose fermentation in Saccharomyces cerevisiae: xylose isomerase as a key component. Adv Biochem Eng Biotechnol. 2007, 108: 179-204.
Eliasson A, Christensson C, Wahlbom CF, Hahn-Hägerdal B: Anaerobic xylose fermentation by recombinant Saccharomyces cerevisiae carrying XYL1, XYL2, and XKS1 in mineral medium chemostat cultures. Appl Environ Microbiol. 2000, 66: 3381-3386. 10.1128/AEM.66.8.3381-3386.2000.
Kötter P, Ciriacy M: Xylose fermentation by Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1993, 38: 776-783. 10.1007/BF00167144. 10.1007/BF00167144.
Kuyper M, Harhangi HR, Stave AK, Winkler AA, Jetten MS, de Laat W, den Ridder JJ, Op den Camp HJ, van Dijken JP, Pronk JT: High-level functional expression of a fungal xylose isomerase: the key to efficient ethanolic fermentation of xylose by Saccharomyces cerevisiae?. FEMS Yeast Res. 2003, 4: 69-78. 10.1016/S1567-1356(03)00141-7.
Brat D, Boles E, Wiedemann B: Functional expression of a bacterial xylose isomerase in Saccharomyces cerevisiae. Appl Environ Microbiol. 2009, 75: 2304-2311. 10.1128/AEM.02522-08.
Walfridsson M, Bao X, Anderlund M, Lilius G, Bulow L, Hahn-Hägerdal B: Ethanolic fermentation of xylose with Saccharomyces cerevisiae harboring the Thermus thermophilus xylA gene, which expresses an active xylose (glucose) isomerase. Appl Environ Microbiol. 1996, 62: 4648-4651.
Johansson B, Hahn-Hägerdal B: The non-oxidative pentose phosphate pathway controls the fermentation rate of xylulose but not of xylose in Saccharomyces cerevisiae TMB3001. FEMS Yeast Res. 2002, 2: 277-282.
Karhumaa K, Hahn-Hägerdal B, Gorwa-Grauslund MF: Investigation of limiting metabolic steps in the utilization of xylose by recombinant Saccharomyces cerevisiae using metabolic engineering. Yeast. 2005, 22: 359-368. 10.1002/yea.1216.
Bruinenberg PM, Debot PH, van Dijken JP, Scheffers WA: The role of redox balances in the anaerobic fermentation of xylose by yeasts. Eur J Appl Microbiol Biotechnol. 1983, 18: 287-292. 10.1007/BF00500493. 10.1007/BF00500493.
Runquist D, Fonseca C, Radstrom P, Spencer-Martins I, Hahn-Hägerdal B: Expression of the Gxf1 transporter from Candida intermedia improves fermentation performance in recombinant xylose-utilizing Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2009, 82: 123-130. 10.1007/s00253-008-1773-y.
Saloheimo A, Rauta J, Stasyk OV, Sibirny AA, Penttila M, Ruohonen L: Xylose transport studies with xylose-utilizing Saccharomyces cerevisiae strains expressing heterologous and homologous permeases. Appl Microbiol Biotechnol. 2007, 74: 1041-1052. 10.1007/s00253-006-0747-1.
Jin YS, Laplaza JM, Jeffries TW: Saccharomyces cerevisiae engineered for xylose metabolism exhibits a respiratory response. Appl Environ Microbiol. 2004, 70: 6816-6825. 10.1128/AEM.70.11.6816-6825.2004.
Souto-Maior AM, Runquist D, Hahn-Hägerdal B: Crabtree-negative characteristics of recombinant xylose-utilizing Saccharomyces cerevisiae. J Biotechnol. 2009, 143: 119-123. 10.1016/j.jbiotec.2009.06.022.
Bengtsson O, Jeppsson M, Sonderegger M, Parachin NS, Sauer U, Hahn-Hägerdal B, Gorwa-Grauslund MF: Identification of common traits in improved xylose-growing Saccharomyces cerevisiae for inverse metabolic engineering. Yeast. 2008, 25: 835-847. 10.1002/yea.1638.
Salusjärvi L, Kankainen M, Soliymani R, Pitkänen JP, Penttilä M, Ruohonen L: Regulation of xylose metabolism in recombinant Saccharomyces cerevisiae. Microb Cell Fact. 2008, 7: 16- 10.1186/1475-2859-7-18.
Sonderegger M, Jeppsson M, Hahn-Hägerdal B, Sauer U: Molecular basis for anaerobic growth of Saccharomyces cerevisiae on xylose, investigated by global gene expression and metabolic flux analysis. Appl Environ Microbiol. 2004, 70: 2307-2317. 10.1128/AEM.70.4.2307-2317.2004.
Wahlbom CF, Otero RRC, van Zyl WH, Hahn-Hägerdal B, Jonsson LJ: Molecular analysis of a Saccharomyces cerevisiae mutant with improved ability to utilize xylose shows enhanced expression of proteins involved in transport, initial xylose metabolism, and the pentose phosphate pathway. Appl Environ Microbiol. 2003, 69: 740-746. 10.1128/AEM.69.2.740-746.2003.
DeRisi JL, Iyer VR, Brown PO: Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997, 278: 680-686. 10.1126/science.278.5338.680.
Nielsen J, Villadsen J, Liden G: Bioreaction engineering principles. 2003, New York: Kluwer Academic/Plenum Publishers, 2
Kostrzynska M, Sopher CR, Lee H: Mutational analysis of the role of the conserved lysine-270 in the Pichia stipitis xylose reductase. FEMS Microbiol Lett. 1998, 159: 107-112. 10.1111/j.1574-6968.1998.tb12848.x.
Bengtsson O, Hahn-Hägerdal B, Gorwa-Grauslund MF: Xylose reductase from Pichia stipitis with altered coenzyme preference improves ethanolic xylose fermentation by recombinant Saccharomyces cerevisiae. Biotechnol Biofuels. 2009, 2: 9- 10.1186/1754-6834-2-9.
Petschacher B, Leitgeb S, Kavanagh KL, Wilson DK, Nidetzky B: The coenzyme specificity of Candida tenuis xylose reductase (AKR2B5) explored by site-directed mutagenesis and X-ray crystallography. Biochem J. 2005, 385: 75-83. 10.1042/BJ20040363.
Andreasen AA, Stier TJ: Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953, 41: 23-36. 10.1002/jcp.1030410103.
Verduyn C, Postma E, Scheffers WA, van Dijken JP: Effect of benzoic acid on metabolic fluxes in yeasts - a continuous culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992, 8: 501-517. 10.1002/yea.320080703.
Xue XD, Ho NWY: Xylulokinase activity in various yeasts including Saccharomyces cerevisiae containing the cloned xylulokinase gene. Appl Biochem Biotechnol. 1990, 24-5: 193-199.
Träff KL, Cordero RR, van Zyl WH, Hahn-Hägerdal B: Deletion of the GRE3 aldose reductase gene and its influence on xylose metabolism in recombinant strains of Saccharomyces cerevisiae expressing the xylA and XKS1 genes. Appl Environ Microbiol. 2001, 67: 5668-5674. 10.1128/AEM.67.12.5668-5674.2001.
Gietz RD, Schiestl RH, Willems AR, Woods RA: Studies on the transformation of intact yeast cells by the Liac/S-DNA/PEG procedure. Yeast. 1995, 11: 355-360. 10.1002/yea.320110408.
Sambrook J, Fritsch EF, Maniatis T: Molecular cloning: a laboratory manual. 1989, Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 2
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP: Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003, 4: 249-264. 10.1093/biostatistics/4.2.249.
Nissen TL, Hamann CW, Kielland-Brandt MC, Nielsen J, Villadsen J: Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis. Yeast. 2000, 16: 463-474. 10.1002/(SICI)1097-0061(20000330)16:5<463::AID-YEA535>3.0.CO;2-3.
Ruohonen L, Aristidou A, Frey AD, Penttilä M, Kallio PT: Expression of Vitreoscilla hemoglobin improves the metabolism of xylose in recombinant yeast Saccharomyces cerevisiae under low oxygen conditions. Enzyme Microb Technol. 2006, 39: 6-14. 10.1016/j.enzmictec.2005.06.024.
Karhumaa K, Fromanger R, Hahn-Hägerdal B, Gorwa-Grauslund MF: High activity of xylose reductase and xylitol dehydrogenase improves xylose fermentation by recombinant Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2007, 73: 1039-1046. 10.1007/s00253-006-0575-3.
Karhumaa K, Garcia Sanchez R, Hahn-Hägerdal B, Gorwa-Grauslund MF: Comparison of the xylose reductase-xylitol dehydrogenase and the xylose isomerase pathways for xylose fermentation by recombinant Saccharomyces cerevisiae. Microb Cell Fact. 2007, 6: 5- 10.1186/1475-2859-6-5.
Kuyper M, Winkler AA, van Dijken JP, Pronk JT: Minimal metabolic engineering of Saccharomyces cerevisiae for efficient anaerobic xylose fermentation: a proof of principle. FEMS Yeast Res. 2004, 4: 655-664. 10.1016/j.femsyr.2004.01.003.
Kuyper M, Hartog MM, Toirkens MJ, Almering MJ, Winkler AA, van Dijken JP, Pronk JT: Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res. 2005, 5: 399-409. 10.1016/j.femsyr.2004.09.010.
Raychaudhuri S, Stuart JM, Altman RB: Principal components analysis to summarize microarray experiments: application to sporulation time series. Pac Symp Biocomput. 2000, 455-466.
Fazio A, Jewett MC, Daran-Lapujade P, Mustacchi R, Usaite R, Pronk JT, Workman CT, Nielsen J: Transcription factor control of growth rate dependent genes in Saccharomyces cerevisiae: A three factor design. BMC Genomics. 2008, 9: 14- 10.1186/1471-2164-9-341.
Tai SL, Boer VM, Daran-Lapujade P, Walsh MC, de Winde JH, Daran JM, Pronk JT: Two-dimensional transcriptome analysis in chemostat cultures. Combinatorial effects of oxygen availability and macronutrient limitation in Saccharomyces cerevisiae. J Biol Chem. 2005, 280: 437-447. 10.1074/jbc.M501243200.
Cipollina C, Brink van den J, Daran-Lapujade P, Pronk JT, Porro D, de Winde JH: Saccharomyces cerevisiae SFP1: at the crossroads of central metabolism and ribosome biogenesis. Microbiology. 2008, 154: 1686-1699. 10.1099/mic.0.2008/017392-0.
Moss T: At the crossroads of growth control; making ribosomal RNA. Curr Opin Genet Dev. 2004, 14: 210-217. 10.1016/j.gde.2004.02.005.
Rodriguez A, de la Cera T, Herrero P, Moreno F: The hexokinase 2 protein regulates the expression of the GLK1, HXK1 and HXK2 genes of Saccharomyces cerevisiae. Biochem J. 2001, 355: 625-631.
McAlister L, Holland MJ: Differential expression of the three yeast glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem. 1985, 260: 15019-15027.
Heinisch JJ, Muller S, Schluter E, Jacoby J, Rodicio R: Investigation of two yeast genes encoding putative isoenzymes of phosphoglycerate mutase. Yeast. 1998, 14: 203-213. 10.1002/(SICI)1097-0061(199802)14:3<203::AID-YEA205>3.0.CO;2-8.
Jeppsson M, Johansson B, Hahn-Hägerdal B, Gorwa-Grauslund MF: Reduced oxidative pentose phosphate pathway flux in recombinant xylose-utilizing Saccharomyces cerevisiae strains improves the ethanol yield from xylose. Appl Environ Microbiol. 2002, 68: 1604-1609. 10.1128/AEM.68.4.1604-1609.2002.
Vemuri GN, Eiteman MA, McEwen JE, Olsson L, Nielsen J: Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proc Nat Acad Sci USA. 2007, 104: 2402-2407. 10.1073/pnas.0607469104.
Almeida JR, Bertilsson M, Hahn-Hägerdal B, Lidén G, Gorwa-Grauslund MF: Carbon fluxes of xylose-consuming Saccharomyces cerevisiae strains are affected differently by NADH and NADPH usage in HMF reduction. Appl Microbiol Biotechnol. 2009, 10.1007/s00253-00009-02053-00251.
Wahlbom CF, Hahn-Hägerdal B: Furfural, 5-hydroxymethyl furfural, and acetoin act as external electron acceptors during anaerobic fermentation of xylose in recombinant Saccharomyces cerevisiae. Biotechnol Bioeng. 2002, 78: 172-178. 10.1002/bit.10188.
Gancedo JM: Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998, 62 (2): 334-61.
Eliasson A, Boles E, Johansson B, Osterberg M, Thevelein JM, Spencer-Martins I, Juhnke H, Hahn-Hägerdal B: Xylulose fermentation by mutant and wild-type strains of Zygosaccharomyces and Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2000, 53: 376-382. 10.1007/s002530051629.
Bettiga M, Bengtsson O, Hahn-Hägerdal B, Gorwa-Grauslund MF: Arabinose and xylose fermentation by recombinant Saccharomyces cerevisiae expressing a fungal pentose utilization pathway. Microb Cell Fact. 2009, 8: 40- 10.1186/1475-2859-8-40.
Gietz RD, Sugino A: New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking 6-base pair restriction sites. Gene. 1988, 74: 527-534. 10.1016/0378-1119(88)90185-0.
Acknowledgements
This work was supported by the Swedish Energy Agency (Energimyndigheten).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DR participated in the design of the study, performed the experimental work and wrote the manuscript. BHH participated in the design of the study and commented on the manuscript. MB participated in the design of the study, performed the experimental work and commented on the manuscript. All the authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Runquist, D., Hahn-Hägerdal, B. & Bettiga, M. Increased expression of the oxidative pentose phosphate pathway and gluconeogenesis in anaerobically growing xylose-utilizing Saccharomyces cerevisiae. Microb Cell Fact 8, 49 (2009). https://doi.org/10.1186/1475-2859-8-49
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2859-8-49