Abstract
Background
Growing evidence suggests that epicardial adipose tissue (EAT) may contribute to the development of coronary artery disease (CAD). In this study, we explored gender disparities in EAT volume (EATV) and its impact on coronary atherosclerosis.
Methods
The study population consisted of 90 consecutive subjects (age: 63 ± 12 years; men: 47, women: 43) who underwent 256-slice multi-detector computed tomography (MDCT) coronary angiography. EATV was measured as the sum of cross-sectional epicardial fat area on CT images, from the lower surface of the left pulmonary artery origin to the apex. Subjects were segregated into the CAD group (coronary luminal narrowing > 50%) and non-CAD group.
Results
EATV/body surface area (BSA) was higher among men in the CAD group than in the non-CAD group (62 ± 13 vs. 33 ± 10 cm3/m2, p < 0.0001), but did not differ significantly among women in the 2 groups (49 ± 18 vs. 42 ± 9 cm3/m2, not significant). Multivariate logistic analysis showed that EATV/BSA was the single predictor for >50% coronary luminal narrowing in men (p < 0.0001). Predictors excluded were age, body mass index, hypertension, diabetes mellitus, and hyperlipidemia.
Conclusions
Increased EATV is strongly associated with coronary atherosclerosis in men.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Epicardial adipose tissue (EAT) is the visceral fat located between the outer layer of the myocardium and the visceral pericardium [1–4]. EAT volume (EATV) is correlated with various cardiovascular risk factors, independent of abdominal visceral adiposity, body mass index (BMI), hypertension, and diabetes mellitus [5–7]. Two population-based studies, the Multi-Ethnic Study of Atherosclerosis and the Framingham Heart Study, showed that EATV is an independent risk predictor for cardiovascular disease [5, 8, 9]. EAT is shown to be metabolically active and the source of pro-atherogenic mediators and adipocytokines [1–5]. Because EAT and the myocardium are located close anatomically, it is predicted that cytokines/adipocytokines produced by infiltrated macrophages or by adipocytes could locally modulate myocardial function or contribute to the pathogenesis of coronary atherosclerosis [1–5]. Recently, we [10, 11] and others [12] showed that proinflammatory cytokines and adipocytokines are expressed and secreted at a higher level in the adipose tissue of individuals with coronary artery disease (CAD) than in individuals without CAD.
Abdominal fat distribution is dissimilar between men and women: Visceral fat obesity is the dominant form in men, while subcutaneous fat obesity is the dominant form in women [13, 14]. However, gender differences in EATV distribution and the influence of EAT on coronary atherosclerosis has never been considered. In this study, we evaluated gender disparities in EATV and its impact on coronary atherosclerosis by using 256-slice multi-detector computed tomography (MDCT).
Subjects and methods
Subjects
We recruited 119 consecutive subjects who underwent 256-slice MDCT coronary angiography between October 2009 and April 2011 at the Kawashima Hospital, Tokushima, Japan. The subjects underwent MDCT if they had atherosclerotic risk factors such as age ≥65 years [15], hypertension, smoking, diabetes mellitus, or dyslipidemia or symptoms suggestive of angina pectoris. According to the 2010 Appropriate Use Criteria for Cardiac Computed Tomography [16] guidelines, cardiac CT is not necessarily recommended for asymptomatic individuals with low-to-moderate CAD risk. However, the prevalence of CAD was not negligible even in asymptomatic subgroups [17]; hence, we employed MDCT in subjects with moderate-to-high CAD risk after they were informed of the radiation-exposure related risk, and they provided written informed consent. Coronary CT angiography was performed using a 256-slice scanner (Brilliance iCT, Philips Healthcare, Amsterdam, Netherlands), and the diagnostic accuracy of the coronary CT angiography for coronary luminal narrowing was validated by routine invasive coronary angiography. Exclusion criteria included a history of cardiac surgery, iodine-based contrast allergy, or renal failure (creatinine, >1.5 mg/dL). Hypertension was defined as a systolic blood pressure of ≥140 mm Hg and/or diastolic blood pressure of ≥90 mm Hg or as the current use of antihypertensive treatment. Diabetes was defined as fasting plasma glucose of ≥6.99 mmol/L (126 mg/dL) or the current use of hypoglycemic treatment. Hyperlipidemia was defined as fasting serum LDL-cholesterol of ≥3.62 mmol/L (140 mg/dL), HDL-cholesterol of <1.03 mmol/L (40 mg/dL), triglyceride of ≥1.58 mmol/L (140 mg/dL), and/or the current use of antihyperlipidemic treatment. The subjects were segregated into 2 groups: CAD group (presence of plaques resulting in >50% luminal narrowing in the major coronary arteries) and non-CAD group (no plaque or plaques resulting in ≤50% luminal narrowing). All subjects in the CAD and non-CAD groups were further segregated into younger (age, <65 years) and older subgroup (age, ≥65 years).
Multi-detector CT scan protocol
The Cardiac MDCT acquisition was performed with retrospective ECG-gated cardiac imaging. The MDCT scan was performed using the following parameters: detector collimation of 2 × 128 × 0.625 mm, creating 256 overlapping slices of 0.625-mm thickness via a dynamic z-flying focal spot, gantry rotation time of 0.27 s, and tube voltage 120 kVp. A current of 800–1050 mA (depending on patient habitus) was used for helical acquisitions and a current of 200 mA for axial acquisitions. Computed tomography dose index volume (CTDIV) was calculated as 74.1 ± 12.6 mGy (n = 50). The raw scan data were reconstructed with 75% of RR wave or particular optimal phase. A bolus dose of the contrast medium (Iohexol [Omnipaque; Daiichi-Sankyo Pharmaceutical, Tokyo, Japan], containing 350 mg iodine/mL) was injected at 0.7 mL/kg body weight within 9 s. Nitroglycerin (0.3 mg) was administered to all subjects immediately before CT imaging, and an oral β blocker (metoprolol, 60 or 120 mg) was administered 1 h before CT imaging to render heart rates <65 beats/min, if required.
Analysis of EATV
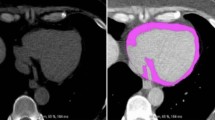
We performed volumetric quantification of EAT measured by the 256-slice MDCT, as described with some modifications [18–22] (Figure 1). Quantification of total EAT area (cm2) was performed at a workstation (Real-INTAGE, Kubota, Japan) with dedicated software. Volumetric measurements were performed on axial views of 0.625-mm slice thickness and number of slices ranging between 300 and 320. The superior border of the EATV measurements was the lower surface of the left pulmonary artery origin, while the inferior border was the left ventricular apex. The EAT area around the proximal, middle, and distal segment of the major coronary arteries was included in the volumetric measurements. The EAT area was calculated by tracing a region of interest (ROI), which included the heart and EAT. The ROI was manually placed outside the line of the visceral pericardium on a cross-sectional axial image (yellow line, Figure 1). The area outside the traced pericardium was excluded. A density range of -600 to -20 Hounsfield Units was used to isolate the adipose tissue. The EAT area of each slice was then summed and multiplied by the slice thickness and number of slices to determine the total EATV (cm3).
Total epicardial adipose tissue volume (EATV) measurements on 256-slice MDCT. (A)–(C): Axial images. A region of interest (ROI) was manually placed along the visceral pericardium (yellow line) (A) and EAT was extracted on an axial image (green) (B, C). (D) 3D image: EAT area was measured on each axial image from the lower surface of left pulmonary artery origin to the left ventricular apex and total EAT volume was obtained from multiplying EAT area and the slice thickness.
Assessment of coronary atherosclerosis
The presence of coronary atherosclerosis was estimated by CT angiographical data using a 256-slice Brilliance iCT (Philips Medical Systems). Coronary arteries were assessed for luminal narrowing by using 3D visualization tools after the axial images were reviewed for determination of anatomy, quality of the study, and appearance of the vessels. The coronary artery tree was segmented according to the modified American Heart Association classification [23]. Coronary vessel and diameter were assessed on 2D multiplanar reconstruction (MPR) and 2D thin slab maximum intensity projection (MIP) images. A 3D volume rendered (VR) image was used to display long segments of the vessels and their branches. To compare the possible gender differences in the calcified lesion, we compared the Agatston scores between the men and women in CAD and non-CAD [24].
Statistical analysis
Values are expressed as mean ± SD unless otherwise indicated. For comparison of the mean in the 2 groups, we used unpaired t-test when samples were normally distributed and non-parametric Mann-Whitney's U test when samples were not normally distributed. χ2 test was used to examine differences with categorical variables. Multigroup comparisons of variables were performed using one-way ANOVA followed by Tukey-Kramer HSD (honestly significant difference) test. Multiple logistic regression analysis was performed to adjust confounding factors. Variables were treated as continuous: one with a risk as 1 and one with no risk, i.e., as 0. We investigated the independent variables for detecting coronary artery luminal narrowing (>50%) by using unadjusted (univariate) and adjusted style (multivariate) for age, BMI, and other established risk factors (hypertension, hyperlipidemia, and diabetes mellitus). All analyses were performed using Jump version 9.0.2 software (SAS Institute Inc., Cary, NC). P values less than 0.05 were considered significant.
Results
General characteristics
Of the 119 subjects who underwent cardiac MDCT, 29 were excluded from analysis. Among the men, 11 were excluded because of differences in slice levels and 2 because of insufficient image quality; among the women, 11 were excluded because of differences in slice levels and 5 because of insufficient image quality. A total of 90 subjects (men: n = 47; age = 63 ± 12 years; women: n = 43, age = 64 ± 12 years) were analyzed (Table 1). By using MDCT, 22 men and 16 women were segregated into the CAD group (>50% luminal narrowing), while 25 men and 27 women were segregated into the non-CAD group. Among the 47 men, 36 (77%) had normal weight (BMI < 25 kg/m2), 8 (17%) were overweight (BMI 25–30 kg/m2), and 3 (6%) were obese (BMI > 30 kg/m2). Among the 43 women, 33 (77%) had normal weight (BMI < 25 kg/m2), 7 (16%) were overweight (BMI 25–30 kg/m2), and 3 (7%) were obese (BMI > 30 kg/m2). When the non-CAD and CAD groups were combined, we found that the EATV was higher in men than in women (80 ± 33 vs. 65 ± 21 cm3; p = 0.0089), but the mean EATV/height and EATV/BSA were comparable (Table 1).
Distribution of BMI and EATV/BSA in non-CAD and CAD subjects
EATV, EATV/height, and EATV/BSA were considerably higher among men in the CAD group than in those in the non-CAD group (Figure 2B-D) but not significantly different among women in the non-CAD and CAD groups (Figure 2B-D). BMI was not different in both groups (Figure 2A). Because age was higher in the CAD group than in the non-CAD group (Table 1), we tried to minimize the confounding effects of age by comparing patients in the younger (<65 years) and older (≥65 years) subgroups. EATV, EATV/height, and EATV/BSA were higher among men in the CAD group than in the non-CAD group both in younger (<65 years) and older (≥65 years) subgroups; however, in women, no significant difference was found in these values (Additional file 1 and Additional file 2).
Next, we examined the relationship between the degree of coronary artery stenosis and EATV. Subjects were divided into the following categories: grade 0 = no plaques in the major coronary branches; grade 1 = ≤25% luminal narrowing; grade 2 = ≤50% luminal narrowing; grade 3 = >50% luminal narrowing. EATV/BSA was larger among men in grade 2 and grade 3 than in grade 1 (Figure 3). In men and women, the Agatston score did not differ between the non-CAD and CAD groups (Figure 4, upper panel). There was no correlation between EATV/BSA and Agatston score in the non-CAD and CAD groups (Figure 4, lower panel).
EATV/BSA in subjects with degrees of coronary luminal stenosis. Subjects were segregated into the following categories based on the degree of coronary luminal stenosis determined using MDCT: grade 0 = no plaque in the major coronary arteries; grade 1 = ≤25% luminal narrowing; grade 2 = ≤50% luminal narrowing; grade 3 = >50% luminal narrowing. One-way ANOVA was performed followed by Tukey-Kramer HSD test. *p < 0.05 vs. grade 0.
Comparison of Agatston score in Non-CAD (○) and CAD (●) subjects and linear correlation between EATV/BSA and Agatston score in men and women. EATV, epicardial adipose tissue volume; BSA, body surface area. Coronary artery disease (CAD) was defined if one has plaque lesion(s) resulting in >50% luminal narrowing. Linear correlation between EATV/BSA and Agatston score in men and women.
Correlation between EATV/BSA and variables
When non-CAD and CAD groups were combined, EATV, EATV/height, and EATV/BSA were not correlated with BMI (data not shown). Even after segregation into non-CAD and CAD groups, EATV/height and EATV/BSA were not correlated with BMI (Figure 5). When non-CAD and CAD groups were combined, EATV/BSA correlated with age in men (r = 0.320; p = 0.032), and the correlation was lost when men were segregated into non-CAD and CAD groups (Figure 6). There was no correlation between EATV/BSA and age in women (Figure 6). EATV/BSA correlated with the presence of diabetes mellitus in women (r = 0.431; p = 0.009) but not in men (r = 0.029; p = 0.657). EATV/BSA was not correlated with the presence of hypertension and hyperlipidemia in either men or women.
Linear correlation between BMI and EATV/height (upper panel) and EATV/BSA (lower panel) in men and women. Lines were plotted in non-CAD (○) and CAD subjects (●). EATV, epicardial adipose tissue volume; BSA, body surface area. Coronary artery disease (CAD) was defined if one has plaque lesion(s) resulting in >50% luminal narrowing.
Linear correlation between age and EATV/BSA in men and women. Lines were plotted in Non-CAD (○) and CAD subjects (●), respectively. EATV, epicardial adipose tissue volume; BSA, body surface area. Coronary artery disease (CAD) was defined if one has plaque lesion(s) resulting in >50% luminal narrowing.
Predictors for coronary atherosclerotic lesions
By univariate logistic regression analysis, presence of coronary atherosclerotic lesions, defined by >50% luminal narrowing, correlated with EATV, EATV/BSA, EATV/height, and age in men (Table 2). On the other hand, the presence of coronary atherosclerotic lesions correlated with EATV and EATV/BSA in women (Table 2). Body weight, BMI, systolic and diastolic blood pressure, HbA1c, triglycerides, LDL- and HDL-cholesterol, and the presence of hypertension and diabetes mellitus were not associated with the presence of coronary atherosclerotic lesions. Multivariate logistic regression analysis indicated that EATV/BSA was detected as an independent risk factor for >50% luminal narrowing only in men (Table 3). BMI, age, presence of hypertension, diabetes mellitus, and hyperlipidemia were not associated with the presence of coronary atherosclerotic lesions.
Discussion
In the present study, we employed a method for assessing total EATV by using 256-slice MDCT and found that EATV/BSA was comparable between men and women. We discovered that EATV/BSA was correlated with the presence of coronary atherosclerosis only in men. To our knowledge, this is the first study to report gender disparity in EATV and that EATV is a determinant for coronary atherosclerosis only in men.
Volumetric quantification of EAT
EAT is distributed asymmetrically around the heart and located mainly at the atrioventricular and interventricular grooves, around major coronary arteries and the free wall of the right ventricle, and at the apex of the left ventricle. In this study, we determined the volume of EAT by using a volumetric measurement [18–22] and sought a precise estimate of the EAT volume with an increased slice range of 300–320 (0.625-mm/slice thickness). The superior border for EATV measurements was the lower surface of the left pulmonary artery origin, while the inferior border was the left ventricular apex. All epicardial fat surrounding the proximal, middle, and distal segment of major coronary arteries was included in the volumetric measurement. It is assumed that our measure employed reliable volumetric quantification of total EAT volume.
Gender and age difference in EATV
Mean EAT volume was higher in men than in women, while mean EATV/BSA did not differ significantly between them. Although EATV is used as the sole marker of epicardial adiposity in most previous studies [18, 19] barring a few [7], EATV corrected by BSA can serve as a more accurate and reliable marker for assuming atherosclerotic risk among various types of constitution samples. In our study, EATV/BSA was not correlated with BMI in men and women (Figure 5), but correlated with age in men (r = 0.320; p = 0.030) (Figure 6) and with the presence of diabetes mellitus in women (r = 0.431; p = 0.009). EATV/BSA was not correlated with the presence of hypertension nor hyperlipidemia in men and women. The above data suggests that EATV is determined by different factors in men and women.
Association of EATV and Coronary Atherosclerosis
Several studies have already shown that total EAT volume measured by CT is associated with the presence of coronary atherosclerosis [20–22]. Although the comparison of EAT volume in men and women has been performed previously [24], no studies have reported the gender-differentiated impact of EAT volume on coronary atherosclerosis. We found that EATV, EATV/height, and EATV/BSA were clearly higher in CAD than in non-CAD group in men (Table 1, Figure 2). Higher EATV, EATV/height, and EATV/BSA were observed both in younger (<65 years) and older (≥65 years) CAD groups in men (Additional file 1 and Additional file 2); however, in women, these values did not differ significantly between non-CAD and CAD groups. Multivariate regression analysis indicated that EATV/BSA was strongly correlated with the presence of atherosclerotic lesions in men. These findings suggest that EAT deposition may be more strongly involved than total body fat compartments in coronary atherogenesis, and that epicardial fat deposition plays a role mainly in men.
Potential mechanisms
Our study showed that EAT volume was the most strong predictor for detecting coronary atherosclerosis. In other words, EAT could be more closely linked to the development and pathogenesis of CAD than other traditional risk factors such as hypertension, dyslipidemia, obesity, and diabetes mellitus. Currently, the mechanism by which EAT volume is associated with coronary atherosclerosis only in men is unclear. Three possible mechanisms are discussed.
Gender differences in EAT adipocytokine
Numerous studies suggest that the role of coronary risk factors in the progression of coronary atherosclerosis differ in men and women [25–27]. EAT volume is reported to be associated with visceral adipose tissue (VAT) volume but not with subcutaneous adipose tissue volume [2, 3]. Greater VAT volume is more prevalent in men and is strongly associated with metabolic syndromes [13, 14, 27, 28], suggesting that the gender differences in this syndrome may contribute to gender differences in CAD [19]. Park et al. [29] reported that echocardiographically determined EAT thickness, a surrogate marker of total EAT volume [30, 31], was significantly increased in patients with metabolic syndrome and CAD, but the power of EAT thickness to predict metabolic syndrome and CAD was stronger in patients with less BMI (<27 kg/m2). Associations between whole body fat distribution and EAT volume, in men and women, need to be clarified in future studies. EAT volume can be a mere marker of metabolic syndrome or a local stimulator of atherosclerotic lesions [1–4, 12, 32]. Although EATV accounts for ~1% of the total body fat mass, EAT volume accounts for 15–20% of the total cardiac volume and covers ~80% of the total cardiac surface [2–4]. Products secreted by EAT can be delivered to atherosclerotic plaques via the vasa vasorum [12, 32]. It has been reported that the expression of pro- and anti-inflammatory cytokines was attenuated in EAT near coronary atherosclerotic lesions [10, 11, 33, 34]. Zhou et al. reported that decreased adiponectin mRNA expression was associated with enhanced expression of cytokines IL-6, TNF-α, or TLR4 in EAT [33]. Gao et al. reported that mRNA and protein expression of chemerin, a novel adipocytokine regulating immune responses and glucose and lipid metabolism, are higher in EAT of Chinese patients with CAD [34]. They also showed that the severity of coronary atherosclerosis is positively correlated with the chemerin mRNA level in EAT rather than its circulating level [34]. We previously reported that the expression of pro-inflammatory cytokines was positively correlated, and the expression of anti-inflammatory cytokines was negatively correlated, with the ratio of M1/M2 macrophages in epicardial adipose tissue of CAD patients [10, 11]. Thus, locally produced adipocytokine could enhance the progression of coronary atherosclerosis in men.
Gender differences in coronary plaque burden and composition
Greater CAD event rates in men than in women could be a result of the differences in coronary artery plaque burden and composition [35, 36]. The coronary plaque burden is lower in women, and plaque morphology differs by age and gender [35]. Plaque rupture with a large necrotic core and disrupted fibrous cap infiltrated by macrophage and lymphocytes is common in men and older women, while plaque erosion with no fibrous cap but with intima consist of smooth muscle and proteoglycan is common in younger women. Women have relatively less calcified plaques and mixed plaques than men [36]. Taken together, coronary plaque lesions can be more strongly activated in men through inflammatory process which is enhanced in EAT with or without the underlying metabolic syndrome [10, 11]. Because no correlation was found between EATV/BSA and Agatston score in non-CAD and CAD groups (Figure 4, lower panel), gender difference in coronary calcified lesion may not be related to the impact of EATV/BSA on coronary atherosclerosis in our subjects.
Gender differences in coronary microvascular function
Angina-like chest pain with angiographically normal coronary arteries affects women more frequently than men [37]. These individuals often show microvascular dysfunction as detected by reduced coronary blood flow reserve (CFR) [38]. In women with microvascular dysfunction, multivariate regression analysis revealed epicardial fat thickness as the only independent predictor of microvascular dysfunction (p < 0.0001), although traditional atherosclerotic risk factors such as age, hypertension, HOMA-IR, and menopause did not predict women with abnormal microvascular function [39]. The impact of EATV on coronary microvascular dysfunction in men and women should also be studied in future studies.
Limitations
The major limitation of this study is that the results are not conclusive; The number of patients was very small to derive definite conclusions, especially when the study population is divided in subgroups. The number of female subjects in particular was very small. There is no power analysis. Furthermore, some of the results of the study were not as expected, i.e., the absence of association of traditional cardiovascular risk factors with coronary artery disease. It appears that more subjects should be included in order to achieve adequate statistical power.
Conclusions
By assessing EAT volume using 256-slice MDCT, we found that EATV/BSA was comparable in men and women, but EATV/BSA was an independent risk factor of significant coronary atherosclerosis in men. Gender disparity in EAT volume for detection of coronary atherosclerosis prompts us to evaluate new diagnostic strategy and the underlying mechanisms.
Abbreviations
- EAT:
-
Epicardial adipose tissue
- EATV:
-
Epicardial adipose tissue volume
- MDCT:
-
Multi-detector computed tomography
- CAD:
-
Coronary artery disease
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- HbA1c:
-
Glycosylated hemoglobin
- ROI:
-
Region of interest
- CTDIV:
-
Computed tomography dose index volume
- MPR:
-
Multiplanar reconstruction
- MIP:
-
Maximum intensity projection
- VR:
-
Volume rendered
- ANOVA:
-
Analysis of variance
- HSD:
-
Honestly significant difference.
References
Mazurek T, Zhang L, Zalewski A, et al: Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003, 108: 2460-2466. 10.1161/01.CIR.0000099542.57313.C5.
Iacobellis G, Corradi D, Sharma AM: Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Prac Cardiovasc Med. 2005, 2: 536-543. 10.1038/ncpcardio0319.
Sacks HS, Fain JN: Human epicardial adipose tissue: A review. Am Heart. 2007, 153: 907-917. 10.1016/j.ahj.2007.03.019.
Rabkin SW: Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007, 8: 253-261. 10.1111/j.1467-789X.2006.00293.x.
Rosito GA, Massaro JM, Hoffman U, et al: Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation. 2008, 117: 605-613. 10.1161/CIRCULATIONAHA.107.743062.
Taguchi R, Takasu J, Itani Y, et al: Pericardial fat accumulation in male as a risk factor for coronary artery disease. Atherosclerosis. 2001, 157: 203-209. 10.1016/S0021-9150(00)00709-7.
Ueno K, Anzai T, Jinzaki M, et al: Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J. 2009, 73: 1927-1933. 10.1253/circj.CJ-09-0266.
Mahabadi AA, Massaro JM, Rosito GA, et al: Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: The Framingham Heart Study. Eur Heart J. 2009, 30: 850-856.
Ding J, Hsu FC, Harris TB, et al: The association of pericardial fat with incident coronary heart disease. The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009, 90: 499-504. 10.3945/ajcn.2008.27358.
Hirata Y, Kurobe H, Akaike M, et al: Enhanced inflammation in epicardial fat in patients with coronary artery disease. Int Heart J. 2011, 52: 139-142. 10.1536/ihj.52.139.
Hirata Y, Tabata M, Kurobe H, et al: Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol. 2011, 58: 248-55. 10.1016/j.jacc.2011.01.048.
Verhagen SN, Visseren FJL: Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011, 214: 3-10. 10.1016/j.atherosclerosis.2010.05.034.
Kotani K, Tokunaga K, Fujioka S, et al: Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994, 18: 207-212.
Camhi SM, Bray GA, Bouchard C, et al: The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race difference. Obesity (Silver Spring). 2011, 19: 402-408. 10.1038/oby.2010.248.
Cassel CK, Neugarten BL: A forecast of women's health and longevity: Implications for an aging America. West J Med. 1988, 149: 712-717.
Taylor AJ, Cerqueira M, Hodgson JM, et al: ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. J Am Coll Cardiol. 2010, 56: 1864-1894. 10.1016/j.jacc.2010.07.005.
Hwang Y, Kim Y, Chung IM, Ryu J, Park H: Coronary heart disease risk assessment and characterization of coronary artery disease using coronary CT angiography: comparison of asymptomatic and symptomatic groups. Clin Radiol. 2010, 65: 601-608. 10.1016/j.crad.2010.04.009.
Nichols JH, Samy B, Nasir K, et al: Volumetric measurement of pericardial adipose tissue from contrast-enhanced coronary computed tomography angiography: a reproducibility study. J Cardiovasc Comput Tomogr. 2008, 2: 288-295. 10.1016/j.jcct.2008.08.008.
Gorter PM, van Lindert AS, de Vos AM, et al: Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008, 197: 896-903. 10.1016/j.atherosclerosis.2007.08.016.
Greif M, Becker A, von Ziegler F, et al: Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009, 29: 781-786. 10.1161/ATVBAHA.108.180653.
Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, Berman DS, Lahiri A: Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012, 220: 223-230. 10.1016/j.atherosclerosis.2011.09.041.
Ding J, Kritchevsky SB, Harris TB, Burke GL, Detrano RC, Szklo M, Jeffrey Carr J: Multi-Ethnic Study of Atherosclerosis. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring). 2008, 16: 1914-1919. 10.1038/oby.2008.278.
Gensini GG: A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983, 51: 606-10.1016/S0002-9149(83)80105-2.
Hoffmann U, Massaro JM, Fox CS, Manders E, O'Donnell CJ: Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008, 102: 1136-1141. 10.1016/j.amjcard.2008.06.038.
Reqitz-Zaqrosek V, Lehmkuhl E, Weickert MO: Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006, 95: 136-147. 10.1007/s00392-006-0351-5.
Evangelista O, McLaughlin MA: Review of cardiovascular risk factors in women. Gend Med. 2009, 6: 17-36.
Shimabukuro M: Cardiac adiposity and global cardio-metabolic risk: New concept and clinical implication. Circ J. 2009, 73: 27-34. 10.1253/circj.CJ-08-1012.
Lim S, Despres JP, Hoh KK: Prevention of atherosclerosis in overweight/obese patients. In need of novel multi-targeted approaches. Circ J. 2011, 75: 1019-1027. 10.1253/circj.CJ-10-1240.
Park JS, Ahn SG, Hwang JW, Lim HS, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH: Impact of body mass index on the relationship of epicardial adipose tissue to metabolic syndrome and coronary artery disease in an Asian population. Cardiovasc Diabetol. 2010, 9: 29-10.1186/1475-2840-9-29.
Eroglu S, Sade LE, Yildirir A, Bal U, Ozbicer S, Ozgul AS, Bozbas H, Aydinalp A, Muderrisoglu H: Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovasc Dis. 2009, 19: 211-217. 10.1016/j.numecd.2008.05.002.
Kim HM, Kim KJ, Lee HJ, Yu HT, Moon JH, Kang ES, Cha BS, Lee HC, Lee BW, Kim YJ: Epicardial adipose tissue thickness is an indicator for coronary artery stenosis in asymptomatic type 2 diabetic patients: its assessment by cardiac magnetic resonance. Cardiovasc Diabetol. 2012, 11: 83-10.1186/1475-2840-11-83.
Tanaka K, Nagata D, Hirata Y, Tabata Y, Nagai R, Sata M: Augmented angiogenesis in adventitia promotes growth of atherosclerotic plaque in apolipoprotein E-deficient mice. Atherosclerosis. 2011, 215: 366-373. 10.1016/j.atherosclerosis.2011.01.016.
Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, Dong N, Chen X: Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol. 2011, 10: 2-10.1186/1475-2840-10-2.
Gao X, Mi S, Zhang F, Gong F, Lai Y, Gao F, Zhang X, Wang L, Tao H: Association of chemerin mRNA expression in human epicardial adipose tissue with coronary atherosclerosis. Cardiovasc Diabetol. 2011, 10: 87-10.1186/1475-2840-10-87.
Shaw LJ, Bairey MCN, Pepine CJ, et al: Insights from the NHLBI-sponsored WISE study, part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006, 47 (3 Suppl): S4-S20.
Nasir K, Gopal A, Blankstein R, et al: Noninvasive assessment of gender differences in coronary plaque composition with multidetector computed tomographic angiography. Am J Cardiol. 2010, 105: 453-458. 10.1016/j.amjcard.2009.09.053.
Diamond GA, Staniloff HM, Forrester JS, Pollock BH, Swan HJ: Computer-assisted diagnosis in the noninvasive evaluation of patients with suspected coronary artery disease. J Am Coll Cardiol. 1983, 1: 444-455. 10.1016/S0735-1097(83)80072-2.
Reis SE, Holubkov R, Conrad Smith AJ, et al: Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001, 141: 735-741. 10.1067/mhj.2001.114198.
Sade LE, Eroglu S, Bozbaş H, Ozbiçer S, Hayran M, Haberal A, Müderrisoğlu H: Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009, 204: 580-585. 10.1016/j.atherosclerosis.2008.09.038.
Acknowledgements
This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare (MHLW).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DM and M Shimabukuro designed and conducted this study and drafted the manuscript; JU, ST, and MH assembled the application for multidetector computed tomography; TN contributed to patient management; DM, DF, YH, HK, TS, TI, KK, TN, KY, YT, SY, NT, HY, and TW measured EATV and participated in the analysis; TK and M Sata supervised the study. All authors read and approved the final manuscript.
Electronic supplementary material
12933_2012_531_MOESM2_ESM.tiff
Additional file 2: Comparison of BMI (upper panel) and EAT/BSA (lower panel) in Non-CAD (○) and CAD (●)subjects with less or greater than 65 years of age. BMI, body mass index; EATV, epicardial adipose tissue volume; BSA, body surface area. Coronary artery disease (CAD) was defined if one has plaque lesion(s) causing greater than 50% luminal narrowing. Unpaired t test was made between Non-CAD and CAD subjects. p: P values. (TIFF 7 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Dagvasumberel, M., Shimabukuro, M., Nishiuchi, T. et al. Gender disparities in the association between epicardial adipose tissue volume and coronary atherosclerosis: A 3-dimensional cardiac computed tomography imaging study in Japanese subjects. Cardiovasc Diabetol 11, 106 (2012). https://doi.org/10.1186/1475-2840-11-106
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-11-106