Abstract
Background
Epicardial fat tissue refers to a type of visceral fat tissue that envelops the myocardial and visceral pericardium. It is recognized as a novel risk sign for cardiovascular issues, including coronary artery disease, our aim to assess the influence of body mass index on the amount of epicardial fat volume and its association with coronary artery disease in male patients.
Methods
Epicardial fat volume was quantified using cardiac Computed tomography scans in a cohort of 119 male patients who were believed to have coronary artery disease. Patients were categorized into three groups based on their body mass index values: Group I Body Mass Index (BMI ≥ 18.5–24.9 kg/m2), Group II (BMI ≥ 25–29.9 kg/m2), and Group III (BMI ≥ 30 kg/m2).
Results
In Group I, there were twenty-three patients (19.3%) with a mean epicardial fat volume of 40 ± .42.8 ml. In Group II, there were forty-three patients (36%) with a mean Epicardial Fat Volume (EFV) of 56 ± 21.5 ml. In Group III, there were 53 patients (44%) with a mean EFV of 56 ± 15.8 ml, when compared to the results of Group I, which were considerably higher (p = 0.0004). Regarding prevalence of obstructive coronary artery disease in our patient population, 86 individuals (72.3%) were diagnosed with obstructive coronary artery disease and exhibited a tendency to have a greater value of epicardial fat volume at 56.4 ± 18.9 ml, with a range of 19–121 ml, compared to those without coronary artery disease at 45.2 ± 6.7 ml, with a range of 33–55 ml (P < 0.02). The prevalence of coronary artery disease was 85% in group III and 87% in group II. Patients in group III with coronary artery disease exhibited a substantially higher EFV of 46 ± 6.63 vs 16 ± 3.2 ml (p = 0.001) compared to those without obstructive coronary artery disease.
Conclusions
Although epicardial fat volume was elevated in patients with coronary artery disease independent of their BMI, the predictive ability of epicardial fat volume for coronary artery disease was more pronounced in individuals with a body mass index more than 25 kg/m2.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Coronary artery disease (CAD) is responsible for the highest burden and mortality in 31% of global fatalities. Obesity, hypercholesterolemia, hypertension (HTN), smoking, and diabetes mellitus (DM) Contribute to the risk of developing CAD. Atherosclerosis is largely recognized as the primary etiology of CAD [1]. Epicardial adipose tissue (EAT) is now recognized as a novel clinical risk marker for CAD [2].
Epicardial fat (EF) buildup is a significant reservoir of free fatty acids (FFAs), inflammatory cytokines, and pro-atherogenic mediators. These substances contribute to the inflammation of blood vessel walls and promote the advancement of atherosclerosis, ultimately leading to the development of CAD. EAT refers to a specific type of visceral adipose tissue that surrounds the myocardium and the visceral layer of the pericardium [3]. Thus, it could contribute to the classification of risk for persons who experience chest pain [4]. The quantity of visceral fat found in the abdominal cavity was associated with the amount of EAT, which was assessed using several techniques such as CT, MRI, and echocardiography. The consumption of food is frequently associated with an increased likelihood of developing cardiovascular disease, beyond the influence of recognized biochemical and anthropometric risk factors. Non-invasive imaging techniques have demonstrated a strong and consistent relationship between the deposition of EAT and both BMI and CAD. This association has been established using measurements of EAT thickness in two dimensions and EAT volume in three dimensions [5]. The purpose of this research was to investigate the relationship between the BMI and the amount of epicardial fat in male patients, as well as the occurrence of CAD in these specific individuals.
Methods
Study design and populations
This study is a retrospective cross-sectional observational study conducted on 119 patients complaining of chest pain (The baseline probability of CAD was moderate) and sent to do multidetector CT and according to the results if positive proceeded to coronary angiography. Patients were seen et al. Zahraa Hospital and the Islamic Centre of Cardiology’s department between December 2021 and June 2023. Since the study was observational and all procedures followed standard care of our institution’s Ethics Committee of the Institutional Review Board gave their blessing to the study, and it was carried out in a manner that adhered to the ethical standards outlined in the Helsinki Declaration. The individuals who took part in the research provided their informed consent, and the processes were carried out in compliance with all applicable standards and rules. Patients with the following conditions were excluded from the study: significant arrhythmia, age below 18 years, history of previous revascularization (percutaneous intervention or coronary bypass graft), heart failure, cardiomyopathy, acute coronary syndrome, active inflammation and pregnancy.
We conducted measurements of our weight and height for the purpose of calculating our BMI. The traditional risk factors for CAD encompass age, HTN, dyslipidemia, DM, and active smoking. The epicardial fat volume (EFV) was consecutively measured in 119 male patients, with a mean age of 57.1 ± 9.3 years.
Measurement of EFV
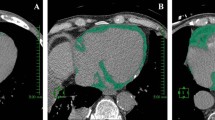
The 128-slice CT scanners; Toshiba Aquilion Prime was used to do CT scans on each patient. All participants in the study exhibited sinus rhythms consistently. Once the time delay is computed, there is an augmentation in the concentration of contrast (80–100 ml total contrast volume, 8 ml/sec) in the coronary arteries and ascending aorta. Using the workstation, axial images were rebuilt in three dimensions with optimal window settings. We analyzed the visual data by using photos that were scaled in many planes. To measure the openness of the blood vessels, several 2 D reconstructions of the coronary arteries were done. The acquisition of EFV took place. The manual tracing was performed on every fourth non-contrast cardiac CT slice, ranging from the level of the aortic root to the apex, using slices that were 5.0 mm thick. A range of 35 to 45 slices were obtained per heart, with attenuation ranging from − 190 to − 30 Hounsfield units (HU) defined as fat voxels, Fig. 1.
The EFV calculation involved multiplying the total cross-sectional area of fat by the thickness of the slice [6]. The software utilized automated tracing of the parietal pericardium in each manually drawn slice to calculate the EFV measured cm3.
Coronary angiography
Coronary angiography (CA) was conducted using traditional techniques, either by the radial or femoral approach. Each coronary angiography was assessed by an experienced cardiologist. They were not aware of the complete patient’s information. The most significant type of CAD found after a quantitative analysis of the CA was obstructive CAD. This was defined as having a ≥ 70% internal diameter narrowing in the left anterior descending coronary artery, left circumflex coronary artery, right coronary artery, or their major branches, or a ≥ 50% narrowing in the left main coronary artery [7].
The study’s participants were classified into three groups based on their BMI values: Group I (BMI ≥ 18.5–24.9 kg/m2), Group II (BMI ≥ 25–29.9 kg/m2), and Group III (BMI ≥ 30 kg/m2). Subsequently, afterwards, the participants were separated into two separate groups: group B exhibited no evidence of CAD, while group A displayed a significant presence of CAD as determined by the invasive coronary artery research.
Statistical analysis
Data management and statistical analysis were done using SPSS version 28 (IBM, Armonk, New York, United States). Quantitative data were assessed for normality using the Shapiro–Wilk test and direct data visualization methods. Quantitative data were summarized as means and standard deviations. Categorical data were summarized as numbers and percentages. Quantitative data were compared between the studied groups using independent t-test. Categorical data were compared using the Chi-square test. Agreement between 2 and 3D parameters was assessed using intraclass correlation coefficient. Correlation analyses were done using Pearson’s correlation. All statistical tests were two-sided. P values less than 0.05 were considered significant.
Results
The study found that the average age of the patients included was 57.1 ± 9.3 years, and the average EFV was 56.4 ± 19.3 ml. Additional essential patients features demonstrated in Table 1.
Clinical characteristics
The clinical features, categorized according to BMI, are presented in Table 2, 3. The normal BMI group had 23 individuals with a mean age of 59 ± 6.9 years. The high BMI group (group III) consisted of 53 individuals, with a mean age of 57 ± 9.6 years. When comparing the normal BMI group (group I) to the high BMI group (group III), it was shown that participants who had a high BMI had higher incidences of HTN and DM, as well as elevated levels of LDL and TG. However, they had decreased levels of HDL. However, those in group II exhibited elevated rates of HTN and DM, as well as decreased levels of total cholesterol, HDL, and LDL. However, their levels of triglyceride (TG) were only slightly higher, and this difference did not meet the criteria for statistical significance.
In the group of individuals with a normal BMI (I), the average EFV (end-diastolic volume) was 40 ± 9.42.8 ml. The average EFV in the high BMI group III was 56 ± 15.8 ml. In group II, individuals with a range of BMI (25–29.9) kg/m2 had a mean end-diastolic volume (EFV) of 56 ± 21.5 ml. When comparing group III with high BMI to group I with normal BMI, the average EFV was significantly greater in group III (56 ± 15.8 ml) compared to group I (40 ± 9.42.8 ml, p = 0.0004). In addition, it is worth noting that the average EFV was significantly larger in the BMI group II when compared to the normal BMI group I (56 ± 21.5 ml versus 40 ± 9.42.8 ml, p < 0.01), as demonstrated in Table 2.
For the inter-and intraobserver variability of the EFV measurement, the magnitudes of the average paired differences were 0.0201 ± 0.39526, p = 0.873 and 0.0032 ± 0.5586, p = 0.978, respectively. These results demonstrate that the EFV measurement is highly reproducible. 27.7% of patients with inconclusive results on MSCT did not show any significant coronary angiography. Consequently, these patients were classified as group B and underwent ICA.
Group A comprised the remaining participants, accounting for 72.2% of the total, and exhibited significant CAD. Specifically, out of the total number of patients, 33 individuals (27.7%) did not exhibit obstructive CAD, while the remaining 86 patients (72.3%) did have obstructive CAD. persons diagnosed with obstructive CAD are likely to have significantly larger EFV values (56.5 ± 19.4 ml) compared to persons without obstructive CAD (44.9 ± 7.7 ml) with a statistically significant difference (P < 0.02) as shown in Table 4 There were no notable disparities in terms of BMI, HTN, DM, smoking, and dyslipidemia between the two groups. The ROC curve analysis of the diagnostic accuracy of EFV, as measured by MDCT, in predicting CAD revealed a threshold value of 49 ml or greater. The sensitivity was 61.6% and the specificity was 64.3%. The statistical significance was P < 0.003, as shown in Table 5.
EFV serves as a prognostic indicator for CAD based on BMI categorization
EFV was elevated significantly in CAD patients in comparable to healthy ones (50.5 ml vs. 45.5 ml, p = 0.02). In group III, where the BMI was greater than 30 kg/m2, the prevalence of CAD was 85%, while in group II, where the BMI was from 25 to 30 kg/m2, the prevalence was 87%. CAD Patients in both the normal group I and high BMI group III had substantially higher mean end-diastolic volume (EFV) compared to those without CAD (36 vs 41 ± 9.9 ml, p = 0.19 and 46 ± 6.63 vs. 16.2 ml, p = 0.001, respectively).
A comparison was made between patients with coronary artery disease (CAD) in group II, who had a BMI greater than 25 kg/m2, and subjects without CAD. The results showed that the average end-diastolic volume (EFV) was significantly higher in the CAD group (57 ± 22.7 ml) compared to the non-CAD group (48 ± 6.8 ml, p = 0.08), as illustrated in Fig. 2.
In addition to established risk factors for CAD such as age, smoking, HTN, and DM, EFV has been identified as a separate factor that is linked to CAD. Additionally, it was discovered that there was a noteworthy association between the variable and age and weight (p = 0.01). Nevertheless, no substantial association was detected between the variable and to Cholesterol, TG, HDL, LDL, and BMI. The information is presented in Table 6.
Discussion
BMI is a numerical quantity that define an individual’s percentage of body fats by taking into account their height and weight. To calculate the BMI, divide the weight in kilograms by the square of the height in meters (weight (kg)/height (m2). NIH has substituted the utilization of outdated height vs. weight charts with the BMI for classifying persons into categories of underweight, normal weight, overweight, or obese. The WHO and the NIH utilize the BMI classifications for individuals belonging to White, Hispanic, and Black ethnicity [1].
The study included 23 patients, which accounted for 19% of the total patients recruited. These patients were classified in group I, which consisted of patients (BMI ≥ to 18.5 to 24.9 kg/m2). Group II, consisting of individuals who were overweight or had pre-obesity, accounted for 36 percent of the patients (BMI = 25 to 29.9 kg/m2), while Group III, consisting of obese patients, accounted for 44 percent of the patients (with a BMI of 30 kg/m2 or above). The study’s findings suggest that in order to accurately forecast CAD, it is necessary to include certain EFV values dependent on BMI.
BMI is commonly employed in extensive epidemiological and observational studies as a reliable and replicable tool for classifying obesity and assessing overall body fatness. Increasing clinical data shows that obesity significantly contributes to cardiovascular disorders, both directly and indirectly [8].
In this study, we employed the WHO classification of BMI using the unit kg/m2. Therefore, any value equal to or exceeding 30 kg/m2 was regarded as the BMI indicating obesity [9]. Previous study indicates that individuals with a BMI of 30 kg/m2 or more have an increased likelihood of mortality due to cardiovascular illnesses. Recent research on Asian individuals has established a correlation between low BMI and cardiovascular diseases. According to a 2007 Kangwha Cohort Study [10], the Korean population with a BMI category more than 27 kg/m2 experienced a substantially elevated susceptibility to cardiovascular diseases.
A growing number of people are beginning to acknowledge the idea that myocardial obesity is becoming an emergent risk factor for CAD. There is a suggestion that EAT can serve as a reliable signal for both an unfavorable metabolic profile and visceral obesity. The study found that the EFV was 56 ± 15.8 ml in the high BMI (obesity) group III, and 56 ± 21.5 ml in the non-high (overweight) group II. These values were substantially higher than the EFV of 40 ± 9.42.8 ml in the normal BMI group I [11].
Lately, there has been a growing interest in understanding the impact of epicardial adipose tissue on the development of coronary atherosclerosis. There is a proposal suggesting that the fat in the pericardial space tissue has complex and contradictory functional characteristics that can be both advantageous and harmful, particularly in its interaction with the coronary veins. The mechanisms regulating pericardial fat expansion and the temporal relationship between pericardial fat changes and the development of coronary atherosclerosis remain uncertain. In addition, the current investigation discovered that individuals with obstructive CAD had significantly larger values of EFV compared to the non-obstructive group (56.5 ± 19.4 ml vs. 44.9 ± 7.7 ml; P < 0.02). Nevertheless, there were no notable disparities between the two groups regarding BMI, HTN, DM, smoking, or dyslipidemia. The diagnostic accuracy of EFV, as determined by MDCT, in predicting CAD was found to have a threshold of ≥ 49 ml. The sensitivity was measured at 61.6% and the specificity at 64.3%; the p-value was less than 0.003, which is statistically significant [12].
A recent study has shown that evaluating pericardial fat volume (PFV) using MDCT, which is a measure of fat around the heart, is a more accurate method than BMI for measuring the overall cardiometabolic problems linked to increasing fat accumulation [13].
The evidence from this study indicates that PFV, rather than BMI, is a additional relevant indication of cardiometabolic problems associated with various obesity phenotypes. CAD patients in both the high BMI group (group III) and the normal group I showed significantly larger average end-diastolic volume (EFV) values (57 ± 16.2 vs 46 ± 6.63; p = 0.001 and 41 ± 9.9 ml vs 36 ml, respectively) compared to participants without CAD. Patients in group II with CAD demonstrated a notably larger average EFV (57 ± 22.7 ml vs 48 ± 6.8 ml, p = 0.08). Conversely, though, there was a distinguishable variation in the distribution of PFV between patients who were obese and those who were of normal weight [2]. Obese individuals exhibited a markedly increased PFV compared to individuals with a normal weight.
Research conducted on individuals has shown that EAT is a notable producer of pro-inflammatory adipokines and is metabolically active [14]. Based on a study, individuals with CAD exhibited significantly reduced amounts of the anti-inflammatory protein adiponectin in their epicardial adipose tissue (EAT) compared to those without CAD [15].
EFV was identified as a major independent factor linked to CAD, exhibiting a substantial correlation with age and weight (p = 0.01). However, it showed no significant correlation with total cholesterol, TG, LDL, HDL, and BMI. EFV, along with other established risk factors for CAD such as age, smoking, HTN, and DM, has been found to be associated with CAD. This association was reported in studies conducted by Alina Cristina Silaghi et al. in 2011 [16] and Bénédicte Gaborit et al. in 2015 [10], with a p-value of 0.2. Additionally, it was observed that there was no significant link between the EAT and BMI in this study, as indicated by the p-values of 0.82 and 0.315.
The ongoing analysis revealed a notable correlation between EFV and CAD specifically in those with a BMI exceeding 30 kg/m2. The study conducted by Gorter et al. found a correlation between CAD and increased CT measures of pericoronary fat volume and EAT volume in individuals with a BMI below 27 kg/m2 [4]. While other studies utilized a different approach to assess EAT, by evaluating its thickness using echocardiography, the findings agreed with the results documented by Gorter et al.
Limitation
The present investigation exhibits certain limitations. Originally, the study was conducted using a cross-sectional design instead of a cohort one. Pericardial fat has been demonstrated to independently forecast the occurrence of coronary heart disease in a community-based prospective cohort study, regardless of traditional risk factors. The researchers assessed the association between pericardial fat and CAD without stratifying the study participants based on their level of obesity [17].
Furthermore, the EFV by CT does not accurately reflect the overall amount of EAT. While echocardiography may not be the most optimal method for measuring EAT, previous studies have utilized echocardiography to examine epicardial fat thickness and have discovered a significant correlation between the overall amount of EAT and the measured thickness [13]. EAT refers to the accumulation of visceral fat surrounding the heart. The splanchnopleuritic mesoderm, which is attached to the gut, serves as the shared precursor for epicardial, mesenteric, and omental fat [9]. Furthermore, further investigation is necessary to highlight the numerous roles of EFV in relation to BMI taking into consideration gender and racial inequalities.
Conclusions
The ability of EFV to predict CAD was greater in persons with a BMI > 25 kg/m2, despite the fact that EFV was significantly higher in CAD patients regardless of BMI.
Availability of data and materials
All data are available.
Abbreviations
- EFT:
-
Epicardial fat tissue
- CAD:
-
Coronary artery disease
- EFV:
-
Epicardial fat volume
- BMI:
-
Body mass index
- FFA:
-
Free fatty acids
- EF:
-
Ejection fraction
- HU:
-
Hounsfield units
- EAT:
-
Epicardial adipose tissue
- HDL:
-
High density lipoprotien
- LDL:
-
Low density lipoprotien
- TG:
-
Triglyceride
References
Gruzdeva OV, Dyleva YA, Belik EV et al (2022) Relationship between epicardial and coronary adipose tissue and the expression of adiponectin, leptin, and interleukin 6 in patients with coronary artery disease. J Pers Med 12:129
Mahabadi AA, Berg MH, Lehmann N et al (2013) Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf recall study. J Am Coll Cardiol 61:1388–1395
Monti CB, Codari M, de Cecco CN et al (2020) Novel imaging biomarkers: Epicardial adipose tissue evaluation. Br J Radiol 93:20190770
Ridker PM, Everett BM, Thuren T et al (2017) Antiinfammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377(12):1119–1131
Gorter PM, de Vos AM, van der Graaf Y et al (2008) Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol 102:380–385
Levelt E, Pavlides M, Banerjee R et al (2016) Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol 68(1):53–63
Baker AR, Silva NF, Quinn DW et al (2006) Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 5:1–7
Koliaki C, Liatis S, Kokkinos A (2019) Obesity and cardiovascular disease: revisiting an old relationship. Metabolism 92:98–107
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363(9403):157–163
Cui R, Iso H, Toyoshima H et al (2005) JACC study group: body mass index and mortality from cardiovascular disease among Japanese men and women: the JACC study. Stroke 36(7):1377–1382
Rabkin SW (2014) The relationship between epicardial fat and indices of obesity and the metabolic syndrome:a systematic review and meta-analysis. Metab Syndr Relat Disord 12:31–42
Pierdomenico SD, Pierdomenico AM, Cuccurullo F et al (2013) Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol 111:73–78
Antonopoulos AS, Tousoulis D (2017) The molecular mechanisms of obesity paradox. Cardiovasc Res 113(9):1074–1086
Ahn SG, Lim HS, Joe DY et al (2008) Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart 94:e7
Juan V, Julio O, Angela G et al (2011) National cardiology and cardiovascular surgery institute1: institute of tropical medicine pedro kourí´ 2-havana-cuba. Arq Bras Cardiol 97(2):122–129
Shaper AG, Wannamethee SG, Walker M (1997) Body weight: implications for the prevention of coronary heart disease, stroke, and diabetes mellitus in a cohort study of middle aged men. BMJ 314:1311–1317
AnsL AM, Montecucco F, Sahebkar A et al (2019) Epicardial adipose tissue and cardiovascular diseases. Int J Cardiol 278:254–260
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All the authors have contributed equally to the current study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was done by the local University authorities.
Consent for publication
A written consent was provided by all the study participants after explanation of the study steps.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elkareem, T.s.A., Elhady, F. & Ali, A.A. Impact of body mass index on the relationship of Epicardial fat volume to coronary artery disease in males. Egypt J Radiol Nucl Med 55, 155 (2024). https://doi.org/10.1186/s43055-024-01303-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01303-1