Abstract
Background
Fluoxetine was the first molecule of a new generation of antidepressants, the Selective Serotonin Re-uptake Inhibitors (SSRIs). It is recurrently the paradigm for the development of any new therapy in the treatment of depression. Many controlled studies and meta-analyses were performed on Fluoxetine, to improve the understanding of its real impact in the psychiatric area. The main objective of this review is to assess the quality and the results reported in the meta-analyses published on Fluoxetine.
Methods
Published articles on Medline, Embase and Cochrane databases reporting meta-analyses were used as data sources for this review.
Articles found in the searches were reviewed by 2 independent authors, to assess if these were original meta-analyses. Only data belonging to the most recent and comprehensive meta-analytic studies were included in this review.
Results
Data, based on a group of 9087 patients, who were included in 87 different randomized clinical trials, confirms that fluoxetine is safe and effective in the treatment of depression from the first week of therapy. Fluoxetine's main advantage over previously available antidepressants (TCAs) was its favorable safety profile, that reduced the incidence of early drop-outs and improved patient's compliance, associated with a comparable efficacy on depressive symptoms. In these patients, Fluoxetine has proven to be more effective than placebo from the first week of therapy.
Fluoxetine has shown to be safe and effective in the elderly population, as well as during pregnancy. Furthermore, it was not associated with an increased risk of suicide in the overall evaluation of controlled clinical trials.
The meta-analysis available on the use of Fluoxetine in the treatment of bulimia nervosa shows that the drug is as effective as other agents with fewer patients dropping out of treatment.
Fluoxetine has demonstrated to be as effective as chlomipramine in the treatment of Obsessive-Compulsive-Disorder (OCD).
Conclusion
Fluoxetine can be considered a drug successfully used in several diseases for its favorable safety/efficacy ratio. As the response rate of mentally ill patients is strictly related to each patient's personal characteristics, any new drug in this area, will have to be developed under these considerations.
Similar content being viewed by others
Background
The release of fluoxetine was the beginning of a new era of safe and effective treatment for patients with depression[1]. Fluoxetine was introduced into clinical use for the treatment of patients with depression in 1988. Since then, fluoxetine has become the most widely prescribed antidepressant drug in the world. In the following years, it was approved for use in the treatment of patients with OCD and bulimia nervosa. Other indications for its use, outside of Italy, are Premenstrual Dysphoric Disorder (PMDD) and major depression in children and adolescents.
Fluoxetine is a selective inhibitor of serotonin re-uptake; it has little effect on other neurotransmitters [2]. It is well absorbed after oral administration, with peak plasma concentrations observed after 6 to 8 hours. The parent compound, fluoxetine, has an elimination half-life of 1 to 4 days, whereas the active metabolite, norfluoxetine, has an half-life of 7 to 10 days[3]. This extended half-life appears to protect against sporadic noncompliance [2] and against the occurrence of withdrawal phenomena.
Objectives
Fluoxetine has been widely studied and described in the scientific literature; its use has been reported in over 8,500 articles present in the most important literature databases (Medline, Embase).
The objectives of this review were the following:
1. to evaluate the strength of the information available in reviewed meta-analyses
2. to understand if the use of fluoxetine is clinically effective and safe compared with previously available drugs
3. to point out the drug's current role in the treatment of diseases where fluoxetine is indicated.
This original review approach based on scientific evidence seems the most appropriate to appreciate and understand the main clinical characteristics of fluoxetine.
Methods
Searching and selection of studies
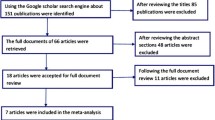
We attempted to identify all relevant meta-analyses on fluoxetine as published and reported on Medline or Embase databases.
Relevant meta-analytic trials, identified according to the Gass definition of studies having characteristics summarized in Table 1[4], were identified by searching the following electronic databases, accessed by Datastarweb interface, using the following search strategy:
(i)MEDLINE (January 1966 to May 2003). The following specific search for this review: [fluoxetin$ AND (metanal$ OR meta-anal$ OR meta ADJ analis$ OR meta ADJ analys$)] was performed
(ii) EMBASE (January 1988 to May 2003). The following specific search for this review: [(fluoxetin$ AND (metanal$ OR meta-anal$ OR meta ADJ analis$ OR meta ADJ analys$))] was performed.
Documents reported in more than one database were removed using the Datastarweb "remove duplicates" function. A total of 438 unique records were identified.
The reference lists of all papers selected were inspected for relevant studies where an original meta-analytic evaluation was performed.
Major reviews published on the use of fluoxetine were also inspected to assess the presence of relevant studies in their references[5–9] as the Cochrane database.
Validity assessment
The abstract of each reference identified by the search was independently evaluated by two of the authors (AR, PD) to assess it's relevancy. All meta-analyses, where fluoxetine was directly compared with placebo or with other drugs, were eligible for this paper. A total of 25 articles were identified as suitable.
Data abstraction
In order to ensure that variation in results was not caused by systematic errors in the design of selected studies, two independent reviewers (AR, PD) assessed the methodological quality of each trial. Only the articles that met these criteria were included. Reviewers were not blind to the names of the authors, institutions and journal of publication. Any disagreement was discussed and decisions were documented.
16 articles were not included in this paper:
9 because fluoxetine was not analyzed separately from other drugs[10–18]
3 because the same analysis was done in another more recent, complete and updated article [19–21]
3 because the articles were reviews or randomized studies, even if titles suggest them as a meta-analysis[22–24]
1 because of the inconsistency between the methodology reported in the scope and methodology of the study[25]
Study characteristics
The 9 remaining articles, included in this paper, are summarized in the following table (Table 2). These meta-analyses evaluate the efficacy and/or safety of fluoxetine as a treatment for major depression (MDD), obsessive-compulsive disorder (OCD), bulimia nervosa, including pregnant and elderly populations
All outcomes (clinical improvement, remission, drop-outs, adverse events) will be summarized using descriptive statistics according to the method used in each study.
Results
Depression
Meta-analyses were based on original data from the US IND database, on a virtual total of 87 studies of 9,084 potential patients using the drug.
Main results and study characteristics are summarized in table 3.
The paper evaluating efficacy and safety of fluoxetine for the short-term treatment of major depression[31], calculated the odds ratio analysis and the percentage of responders (based on HDRS-17 improvement and CGI outcome) compared with placebo and TCAs. All performed analysis showed a statistically significant benefit compared with placebo. No statistically significant differences were observed in the comparison with TCAs in terms of efficacy.
In terms of discontinuations, significantly more dropouts because of lack of efficacy were observed in the placebo treated group. No significant difference was observed in the comparison with TCAs.
Significantly more TCA treated patients discontinued the studies than fluoxetine treated patients (on an average of about 2 times more); more fluoxetine treated patients discontinued for the same reason.
Overall, significantly fewer patients on fluoxetine discontinued treatment due to any adverse event compared (as compared with TCAs), while a not-significant difference in discontinuation rate for any reason was found vs placebo.
Minor differences in the Fluoxetine group, regarding the discontinuation rate for any reason, were not found to be statistically significant, as compared to the placebo group.
The Beasley meta-analysis is on the safety of fluoxetine compared with TCAs or placebo[28], substantially confirmed these results in terms of safety. It also adds some interesting information about types of ADE and better points out the role of fluoxetine's dosage. Considering only events with an incidence above 5%, it was observed in the TCA higher group, an incidence of cholinergic ADEs (dry mouth, constipation, abnormal vision), sedation (somnolence), dizziness and peresthesia, than in patients using fluoxetine at dosages from 20 to 80 mg/die. Fluoxetine treated patients showed a higher incidence of nausea, insomnia, diarrhea, anorexia and rhinitis.
The same type of effects were substantially observed in the comparison with placebo, but some of these (nervousness, tremor, dizziness, dyspepsia) were not found to be statistically higher than in placebo treated patients, when only 20 mg/die dose was used.
The results of the analysis of discontinuations was consistent with the one reported in the Bech study[31]. Furthermore, the drop out ratio due to adverse events of patients using 20 mg/die of fluoxetine, was similar to the ratio observed in the placebo-treated group.
These results, in terms of safety, are substantially confirmed by the Beasley study[29] where only a 20 mg/die dosage was compared with placebo. Furthermore, these fluoxetine treated patients demonstrated significantly greater remission and response rates, mean changes on HAMD-17 total score, anxiety/somatization, retardation and cognitive disturbance factor score, than placebo treated patients (p < 0,01).
All these results confirm the hypothesis that fluoxetine at 20 mg/die, the most commonly used effective dose in the treatment of major depression, has an improved safety and tolerability profile compared with higher doses of fluoxetine.
The results of the Tollefson[34] study assess that the probability of achieving a clinical response, defined as HAMD-21 score reduction from baseline of at least 50%, was similar for both fluoxetine and placebo at the end of week 1. However, by week 2 and after, the probability of response was greater for fluoxetine than placebo. These results challenged the current belief that a 3 to 4 week delay in the onset of antidepressant action is to be expected.
Bulimia
A recent Cochrane group overview on the use of antidepressants in the treatment of bulimia nervosa, including randomized placebo-controlled studies published until 2000, found that the use of drugs decreased the relative risk of binge episodes. The only SSRI included in the analysis was fluoxetine (60 mg/die).
No statistically significant differential effect could be demonstrated regarding efficacy among TCAs, SSRIs, MAOIs and other classes of antidepressants.
The results of this meta-analysis show that patients treated with antidepressants were more likely to prematurely interrupt the treatment due to an adverse event. Patients treated with TCAs dropped out for any reason more frequently than patients treated with placebo. The opposite was found with fluoxetine.
The authors conclude, "fluoxetine is the most systematically studied antidepressant agent. Even if it is not superior to other drugs in terms of efficacy, its better tolerability may justify its use as a first line antidepressant in bulimia nervosa. A daily dose of 60 mg is more effective that the antidepressant doses of 20 mg. Eight weeks seems to be an appropriate period to obtain a relevant clinical improvement. If only a partial response is noted, an alternative therapeutic approach is indicated"[27].
OCD
In an analysis of the results from one fluoxetine and two clomipramine studies, Jenije et al. found both treatments to be effective with fluoxetine having fewer side effects. In this study all three treatments (clomipramine, fluoxetine and behavior therapy) were significantly effective for OCD symptoms, anxiety and depression. Only behavior therapy was not significantly effective for depressed mood.
The authors conclude: "There are still not enough appropriate treatment studies available to determine statistically the superiority of any treatment"[26].
Elderly depression
Up to 4% of the elderly experience major depression and as many as 44% experience depressive symptoms[27–31]. At least three published, population-based studies associate depression in the elderly patient with greater than expected mortality[32–34].
The mean improvement in baseline-to-endpoint HAMD-17 scores was significantly greater in fluoxetine (-7.9 ± 7.5) vs placebo-treated patients (-6.3 ± 7.1) (p < 0.01).
In the global population the anxious and nonanxious subgroups, the analysis of psychomotor agitation, psychotic anxiety or somatic anxiety, shows a consistent, but not statistically significant, trend in the improvement rate in fluoxetine treated patients as compared to the placebo group.
The only adverse event most frequently reported by fluoxetine treated patients wisthin the anxious subgroup was nervousness (p=0.03). No statistically significant differences were reported between fluoxetine and placebo-treated patients within the nonanxious subgroup.
The percentage of fluoxetine treated patients that discontinued studies due to an adverse event (11.5%) was not statistically different from placebo treated patients (9.6%) (p=0.39)[33].
Suicide
Suicidal ideation, assessed using the item 3 of HAMD scale which systematically rates suicidality, was evaluated using data as belonging to clinical studies comparing fluoxetine with TCAs and placebo. These were considered as emergencies (any change from 0 or 1 to 3 or 4 in the item during the double blind period) and as "worsening" any increase from baseline. The pooled incidence of suicidal acts was 0.3% for fluoxetine, 0.2% for placebo and 0.4% for TCAs; fluoxetine did not differ statistically from any comparator group. Suicidal ideation emerged slightly below the significance rate, less often than with placebo (0.9% vs 2.6%: p=0.094) and numerically less often than TCAs (1.7% vs 3.4%; p=0.102). The pooled incidence of substantial suicidal ideation emergencies was 1.2% for fluoxetine, 2.6% for placebo and 3.6% for TCAs; the incidence was significantly lower with fluoxetine than with placebo (p=0.042) and TCAs (p=0.001). The pooled incidence of "worsening", as the pooled incidence of improvement of suicidal ideation, did not differ between groups except with the incidence of improvement with fluoxetine (72.2%); which was statistically superior than with placebo (54.8%; p < 0.001) [30].
Pregnancy
All meta-analyses previously reviewed did not include or did not evaluate the safety of fluoxetine in pregnant women. An ad-hoc meta-analysis on data belonging to different sources, examined the increased risk for major malformations following the use of fluoxetine during the first trimester of pregnancy. The pooled relative risk and 95% confidence interval for major malformations does not suggest an association between the use of fluoxetine during the first trimester of pregnancy and the increased risk of major malformations. The authors conclude that the use of fluoxetine during the first trimester of pregnancy is not associated with measurable teratogenic effects in humans[26].
Discussion
Fluoxetine is a widely and well-known drug successfully used in treating several diseases. Its unique combination of efficacy and its safety profile explains its key role in the history of the pharmacotherapy of several diseases. Fluoxetine could be considered the standard comparator for the development of new drugs to be used in the treatment of serious and socially invasive pathologies as Major Depression, Bulimia Nervosa, Obsessive Compulsive Disorder, Premenstrual Dysphoric Disorder, as it confirms its effectiveness in elderly, children, adolescents and pregnant women suffering from depression.
All the above studies on depressed patients tend to underestimate the efficacy pattern of fluoxetine, mainly because of the type of studies considered. In fact the drop-out rate is always higher in the placebo arm than in the fluoxetine one, so that those patients with a placebo response completed the study and increased the revealed effectiveness of placebo. An even higher increase in the response rate or in the efficacy score in the fluoxetine arm has to be added to the greatest number of patients that, consistently between different studies, completed the trial period.
The dose showing the better effectiveness was 20 mg/die.
Fluoxetine results also effective and safe for the treatment of Bulimia Nervosa and OCD.
Furthermore, the use of fluoxetine has shown a general improvement in the suicidal actions and ideations in depressed patients.
The most commonly reported side effects of fluoxetine include sexual dysfunction, headache and nausea, but fortunately, even in the small minority of patients who have them, such effects generally disappear after about 2 weeks, although, as with other antidepressants, sexual dysfunction can persist[35].
The incidence of spontaneous adverse events resulted quite impressive, but the vast majority of depressed patients were proud to report their symptoms to physician in a controlled study environment. If we compare the true incidence of adverse events, only dry mouth appeared in over 50% of TCAs treated patients; as depressive patients often have hypochondriacal attitudes, when they know they are in a clinical trial, their answers to specific questions regarding their physical conditions overestimate their feelings compared with non-trial environment. A confirmation about this belongs to the fact that physical symptoms were often reported by the placebo treated patients, in some cases statistically more often than in the fluoxetine arm (back pain arthralgia)[29].
Conclusion
All data from the above meta-analyses confirm that in the treatment of patients with major depression, fluoxetine is equally effective as, and has a distinctly more benign side-effect profile and lower rates of discontinuation than the TCAs, is safer in overdose and easier and simpler for patients to use and physicians to prescribe.
Fluoxetine was found to be similar in side-effect profile to the other SSRIs, including paroxetine, sertraline, fluvoxamine and citalopram. Fluoxetine has demonstrated the least need for dose titration of any available antidepressant. Most of the studies comparing SSRIs were 6 to 8 weeks in duration, but one study comparing fluoxetine and sertraline followed up 57 patients for 8 months and found the efficacy was maintained with a low incidence of adverse events[8].
In summary, fluoxetine is effective in treating all degrees of depression and is clearly better tolerated (ie, has a more benign adverse-events profile) and safer in cases of overdose than the older antidepressant drugs[8]. Response to pharmacotherapy is likely incremental, and the rate of response highly individualized, so more detailed attention to patient heterogeneity and early response patterns has to be studied in the development of any new treatment for depressive pathologies.
Conflict of interests
The authors are employed at Eli Lilly Italia S.p.A.
Eli Lilly Italia paid only publication fees.
References
Wong DT, Bymaster FP, Engleman EA: Minireview: Prozac (Fluoxetine, Lilly 110140), the first selective selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995, 57: 411-441. 10.1016/0024-3205(95)00209-O.
Guze BH, Gitlin M: New antidepressants and the treatment of depression. J Fam Pract. 1994, 38: 49-57.
American Medical Association: Psychopharmacologic drugs. In: Drugs used in mood disorders. Drug evaluation subscription. 1992, Chicago Ill, 1: 32-
Glass GV: Primary, secondary and meta-analysis of research. Educ Res. 1976, 5: 3-9.
Cheer SM, Goa KL: Fluoxetine: A review of its therapeutic potential in the treatment of depression associated with physical illness. Drugs. 2001, 61 (1): 81-110.
Simpson K, Noble S: Fluoxetine: A review of its use in women's health. CNS Drugs. 2000, 14 (4): 301-328.
Wilde MI, Benfield P: Fluoxetine: A pharmacoeconomic review of its use in depression. PharmacoEconomics. 1998, 13 (5): 543-561.
Stokes PE, Holtz A: Fluoxetine tenth anniversary update: The progress continues. Clinical Therapeutics. 1997, 19 (5): 1135-1150. 10.1016/S0149-2918(97)80066-5.
Scott LV: Fluoxetine: A review of its pharmacology and clinical applications. Journal of Serotonin Research. 1997, 3 (4): 173-191.
Bakker A, Van Balkom AJLM, Spinhoven P: SSRIs vs. TCAs in the treatment of panic disorder: A meta-analysis. Acta Psychiatrica Scandinavica. 2002, 106 (3): 163-167. 10.1034/j.1600-0447.2002.02255.x.
Khan A, Leventhal RM, Khan S, Brown WA: Suicide risk in patients with anxiety disorders: A meta-analysis of the FDA database. Journal of Affective Disorders. 2002, 68 (2-3): 183-190. 10.1016/S0165-0327(01)00354-8.
Furukawa TA, Streiner DL, Young LT: Is antidepressant–benzodiazepine combination therapy clinically more useful? A meta-analytic study. Journal of Affective Disorders. 2001, 65 (2): 173-177. 10.1016/S0165-0327(00)00254-8.
Van der Linden GJH, Stein DJ, Van Balkom AJLM: The efficacy of the selective serotonin reuptake inhibitors for social anxiety disorder (social phobia): A meta-analysis of randomized controlled trials. International Clinical Psychopharmacology. 2000, 15 (SUPPL. 2): S15-S23.
De Lima MS, Hotoph M, Wessely S: The efficacy of drug treatments for dysthymia: A systematic review and meta-analysis. Psychological Medicine. 1999, 29 (6): 1273-1289. 10.1017/S0033291799001324.
Rossy LA, Buckelew SP, Dorr N, Hagglund KJ, Thayer JF, McIntosh MJ, Hewett JE, Johnson JC: A meta-analysis of fibromyalgia treatment interventions. Annals of Behavioral Medicine. 1999, 21 (2): 180-191.
Whittal MC, Agras WS, Gould RA: Bulimia nervosa: A meta-analysis of psychosocial and pharmacological treatments. Behavior Therapy. 1999, 30 (1): 117-135.
Anderson IM: SSRIS versus tricyclic antidepressants in depressed inpatients: A meta-analysis of efficacy and tolerability. Depression and Anxiety. 1998, 7 (SUPPL 1): 11-17. 10.1002/(SICI)1520-6394(1998)7:1+<11::AID-DA4>3.3.CO;2-O.
Michael KD, Crowley SL: How effective are treatments for child and adolescent depression? A meta-analytic review. Clinical Psychology Review. 2002, 22 (2): 247-269. 10.1016/S0272-7358(01)00089-7.
Greenberg RP, Bornstein RF, Zborowski MJ, Fisher S, Greenberg MD: A meta-analysis of fluoxetine outcome in the treatment of depression. Journal of Nervous and Mental Disease. 1994, 182 (10): 547-551.
Burrows GD, Norman TR: Suicide, violent behaviour and fluoxetine: Meta-analysis of clinical trials shows that fluoxetine does not cause or increase suicidal behaviour or violent acts. Medical Journal of Australia. 1994, 161 (7): 404-405.
Beasley CM, Dornseif BE, Bosomworth JC, Sayler ME, Rampey AH, Heiligenstein JH, Thompson VL, Murphy DJ, Masica DN: Fluoxetine and suicide: a meta-analysis of controlled trials of treatment for depression. BMJ. 1991, 303 (6804): 685-92.
Falsetti AE: Fluoxetine–induced suicidal ideation: An examination of the medical literature, case law, and the legal liability of drug manufacturers. Food and Drug Law J. 2002, 57: 247-267.
Jarema M: The use of meta–analysis in the evaluation of antidepressive effects of fluoxetine. Psychiatria Polska. 1996, 30 (4): 569-581.
Carr Roxane R, Ensom Mary HH: Fluoxetine in the treatment of premenstrual dysphoric disorder. The annals of pharmacotherapy. 2002, 36 (4): 713-7.
Anderson IM: Meta–analytical studies on new antidepressants. British Medical Bulletin. 2001, 57: 161-178. 10.1093/bmb/57.1.161.
Jenike MA, Bear L, Greist JH: Clomipramine versus fluoxetine in obsessive-compulsive disorder: A retrospective comparison of side effects and efficacy. Journal of Clinical Psychopharmacology. 1990, 10: 122-124.
Myers JK, Weissman MM, Tischler GL, Holzer CE, Leaf PJ, Orvaschel H, Anthony JC, Boyd JH, Burke JD, Kramer M, et al: Six-month prevalence of psychiatric disorders in three communities 1980 to 1982. Archives of General Psychiatry. 1984, 41 (10): 959-67.
Borson S, Barnes RA, Kukull WA, Okimoto JT, Veith RC, Inui TS, Carter W, Raskind MA: Symptomatic depression in elderly medical outpatients. I. Prevalence, demography, and health service utilization. Journal of the American Geriatrics Society. 1986, 34 (5): 341-7.
Blazer D, Hughes DC, George LK: The epidemiology of depression in an elderly community population. Gerontologist. 1987, 27 (3): 281-7.
Alexopoulos GS, Young RC, Meyers BS, Abrams RC, Shamoian CA: Late-onset depression. Psychiatric Clinics of North America. 1988, 11 (1): 101-15.
Girling DM, Barkley C, Paykel ES, Gehlhaar E, Brayne C, Gill C, Mathewson D, Huppert FA: The prevalence of depression in a cohort of the very elderly. Journal of Affective Disorders. 1995, 34 (4): 319-29. 10.1016/0165-0327(95)00030-Q.
Markush RE, Schwab JJ, Farris MA, et al: Mortality and community mental health. Arch Gen Psychiatry. 1977, 34: 1393-1401.
Enzell K: Mortality among persons with depressive symptoms and among responders and non-responders in a health check-up. An investigation of persons born in 1905 and followed up from age 66 to 75. Acta Psychiatrica Scandinavica. 1984, 69 (2): 89-102.
Bruce ML, Seeman TE, Merrill SS, Blazer DG: The impact of depressive symptomatology on physical disability: MacArthur Studies of Successful Aging. American Journal of Public Health. 1994, 84 (11): 1796-9.
xx x: Prozac (fluoxetine hydrocloride) Product Information. Dista Products Company, a division of the Eli Lilly industries, Inc., Carolina, Puerto Rico, a subdivision of Eli Lilly and Co., Indianapolis, Indiana. May 17, 1995
Addis A, Koren G: Safety of fluoxetine during the first trimester of pregnancy: A meta–analytical review of epidemiological studies. Psychological Medicine. 2000, 30 (1): 89-94. 10.1017/S0033291799001270.
Bacaltchuk J, Hay P: Antidepressants versus placebo for people with bulimia nervosa. The Cochrane Library. 2003, 1
Beasley CM, Koke SC, Nilsson ME, Gonzales JS: Adverse events and treatment discontinuations in clinical trials of fluoxetine in major depressive disorder: An updated meta-analysis. Clinical Therapeutics. 2000, 22 (11): 1319-1330. 10.1016/S0149-2918(00)83028-3.
Beasley CM, Nilsson ME, Koke SC, Gonzales JS: Efficacy, adverse events, and treatment discontinuations in fluoxetine clinical studies of major depression: A meta-analysis of the 20-mg/day dose. Journal of Clinical Psychiatry. 2000, 61 (10): 722-728.
Beasley CM, Dornseif BE, Bosomworth JC, Sayler ME, Rampey AH, Heiligenstein JH, Thompson VL, Murphy DJ, Masica DN: Fluoxetine and suicide: a meta–analysis of controlled trials of treatment for depression. International Clinical Sychopharmacology. 1992, 6 (SUPPL. 6): 35-57.
Bech P, Cialdella P, Haugh MC, Birkett MA, Hours A, Boissel JP, Tollefson GD: Meta-analysis of randomised controlled trials of fluoxetine v. placebo and tricyclic antidepressants in the short-term treatment of major depression. British Journal of Psychiatry. 2000, 176: 421-428. 10.1192/bjp.176.5.421.
Cox BJ, Swinson RP, Morrison B, Lee PS: Clomipramine, fluoxetine, and behavior therapy in the treatment of obsessive–compulsive disorder: A meta–analysis. Journal of Behavior Therapy and Experimental Psychiatry. 1993, 24 (2): 149-153. 10.1016/0005-7916(93)90043-V.
Hoog S, Tepner R, Nilsson ME, Romano S, Kennedy JS: Changes in anxiety, agitation, and insomnia during treatment of depression for patients age 55 years and older: Analysis from fluoxetine double-blind, placebo-controlled trials. International Journal of Geriatric Psychopharmacology. 1999, 2 (1): 33-39.
Tollefson GD, Holman SL: How long to onset of antidepressant action: a meta-analysis of patients treated with fluoxetine or placebo. International Clinical Psychopharmacology. 1994, Winter 9 (4): 245-50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Rossi, A., Barraco, A. & Donda, P. Fluoxetine: a review on evidence based medicine. Ann Gen Hosp Psychiatry 3, 2 (2004). https://doi.org/10.1186/1475-2832-3-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2832-3-2