Abstract
Background
Hypoglycemic episodes are infrequent in individuals without a history of diabetes mellitus or bariatric surgery. When hypoglycemia does occur in such individuals, an uncommon but important diagnosis to consider is non-islet cell tumor hypoglycemia (NICTH). We report a case of NICTH associated with paraneoplastic insulin-like growth factor-2 (IGF-2) production and review current relevant medical literature.
Case presentation
A 60 year old male with no relevant past medical history was referred to the endocrinology clinic with 18 month history of episodic hypoglycemic symptoms and, on one occasion was noted to have a fingerstick glucose of 36 mg/dL while having symptoms of hypoglycemia. Basic laboratory evaluation was unrevealing. Further evaluation however showed an elevated serum IGF-2 level at 2215 ng/mL (reference range 411–1248 ng/mL). Imaging demonstrated a large right suprarenal mass. A right nephrectomy with resection of the mass demonstrated a malignant solitary fibrous tumor. Post resection, the patient’s IGF-2 levels normalized and hypoglycemic symptoms resolved.
Conclusion
Due to the structural and biochemical homology between IGF-2 and insulin, elevated levels of IGF-2 can result in hypoglycemia. A posttranslational precursor to IGF-2 known as “big IGF” also possesses biologic activity. Review of recent reported cases of NICTH identified widespread anatomic locations and varied pathologic diagnoses of tumors associated with paraneoplastic production of IGF-2 causing hypoglycemia. Definitive management of hypoglycemia associated with paraneoplastic production of IGF-2 consists of resection of the tumor responsible for IGF-2 production. Accumulating literature provides a firm basis for routine IGF-2 laboratory evaluation in patients presenting with spontaneous hypoglycemia with no readily apparent cause.
Similar content being viewed by others
Background
Hypoglycemia is a common medical problem in patients with diabetes mellitus treated with insulin or insulin secretagogues. Hypoglycemia is also associated with gastric bypass weight loss surgery [1].
Although rare, hypoglycemia is occasionally encountered in non-diabetic, non-gastric bypass patients. In such patients, hypoglycemia is usually a manifestation of pancreatic islet cell tumors producing insulin, primary or secondary adrenal insufficiency, advanced liver disease, pheochromocytoma, IGF-1 secreting tumors, hypothyroidism, substances interfering with insulin and insulin receptor mediated metabolism [non-islet cell tumor hypoglycemia (NICTH)] or antibodies interfering with insulin receptors [2].
In subjects with recurrent hypoglycemia and no history of diabetes or weight loss surgery, NICTH is an important disorder to consider in the differential diagnosis. Tumors that have been reported to cause NICTH include malignancies associated with insulin receptor antibodies, tumor necrosis factor (TNF), interleukin (IL) -1 or -6; pheochromocytoma associated with excess catecholamine production; and paraneoplastic production of IGF-1 or IGF-2 [2].
In this article, we report a case of NICTH associated with paraneoplastic IGF-2 production. We have also reviewed the current literature on the subject and describe pathophysiology, diagnostic methods and treatment options.
Case presentation
A 60 year old male was referred to the Endocrinology clinic at the University of Minnesota for the evaluation of worsening symptoms that included diaphoresis, anxiety, inability to concentrate and episodic visual changes for the prior 18 months. Patient reported waking from sleep during the night with symptoms. He had discovered that carbohydrate rich snacks every 30 – 60 min prevented his symptoms. As a result of frequent snacking on carbohydrate containing foods, he had gained 30 pounds in the prior 12 months. He did not experience symptoms postprandially. During one of his episodes, he obtained a finger stick glucose value of 36 mg/dL. His past medical history included hypertension, dyslipidemia and obstructive sleep apnea. He did not have history of diabetes mellitus or bariatric surgery. His medications included metoprolol extended release, hydrochlorothiazide, irbesartan, amlodipine, aspirin, terazosin, simvastatin and omeprazole. He occasionally consumed alcohol and had a remote history of smoking. Physical examination was within normal limits except for body mass index (BMI) of 33.2 kg/m2.
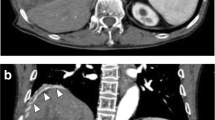
Serial finger stick blood glucose monitoring during symptomatic episodes demonstrated recurrent hypoglycemia with blood glucose values of 41, 35 and 41 mg/dL. Diagnoses considered included medication induced hypoglycemia, insulinoma, chronic liver disease, pheochromocytoma and adrenal insufficiency. These diagnoses were excluded based on laboratory evaluation (Table 1).Subsequently, IGF-2 was measured and found to be 2215 ng/mL (reference range: 414 – 1248 ng/mL). Computed Tomography (CT) without contrast of chest, abdomen and pelvis showed a large right suprarenal mass measuring 14 × 17 × 16 cm, which was lobular in shape with central necrosis and calcifications (Figure 1). Technetium radionuclide bone scan did not show any metastatic disease.The patient underwent right nephrectomy and resection of the mass. Surgical pathology showed a malignant solitary fibrous tumor with spindled to epithelioid cells and focal high-grade nuclear atypia (Figure 2). The tumor cell proliferation marker Ki67 index was elevated. The tumor was positive for CD34, CD99 and Bcl-2 consistent with a diagnosis of malignant solitary fibrous tumor.
The patient’s follow-up IGF-2 levels were within the normal range (Table 2). He did not experience further episodes of hypoglycemia and was able to lose 10 lbs over the next six months. A three year follow-up CT scan of the abdomen did not demonstrate any evidence of recurrence.
Discussion
The IGF-2 gene is located on short arm of chromosome 11 (11p15) adjacent to the insulin (INS) gene [3]. IGF-2 is a 67 amino acid polypeptide with 47% sequence homology with insulin. Post translational prepro IGF-2 contains 180 amino acids with a carboxy terminal peptide of 89 amino acids and a signal peptide of 24 amino acids. Prepro IGF-2 is cleaved to form mature IGF-2 [4]. Prepro IGF-2 molecules (known as “big IGF-2”) have also been shown to have biologic activity and can produce hypoglycemia in the setting of a normal IGF-2 level [5]. It is due to the structural and biochemical homology between IGF-2 and insulin that an unregulated and elevated level of IGF-2 stimulates glucose metabolism pathways thus leading to hypoglycemia [6].
Under physiologic conditions, IGF-2 is mainly produced by the liver. However, other tissues may also produce IGF-2, allowing it to exert effects through endocrine, autocrine and paracrine pathways [7]. Physiologically, IGF-2 plays an important role in human fetal and post-natal development but whether it has significant physiological function in adults remain unknown [8]. As opposed to IGF-1, IGF-2 regulation is independent of growth hormone [9]. IGF-1 and IGF-2 both have glucose lowering potency that is approximately 5% of insulin’s potency. However, plasma concentrations of IGF-1 and IGF-2 can be 1000 times greater than insulin in NICTH allowing them to cause hypoglycemia [10]. More than 95% of IGF-2 in the circulation is bound to insulin like growth factor binding proteins (IGFBP) that have high affinity for both IGF-1 and IGF-2 [11]. In spite of structural homology with insulin, normal levels of IGF-2 do not cause hypoglycemia. At the cellular level, IGF-2 binds with IGF2R which is responsible for endocytosis, intracellular hormone transport and degradation of circulating IGF-2 [12].
Tumors producing elevated levels of circulating IGF-2 with resulting hypoglycemia are categorized as NICTH. NICTH has also been reported in conjunction with paraneoplastic production of insulin receptor antibodies, tumor necrosis factor (TNF), interleukin (IL) -1 and -6, catecholamine (pheochromocytoma) and IGF-1 [2]. For reasons not clear, there are several neoplasms noted to have high levels of IGF-2 mRNA without exhibiting elevated hormone activity [13].
The IGF-2 gene is an imprinted gene; normally only one parental allele is expressed [14]. Loss of imprinting leads to over expression of IGF-2 and has been demonstrated in multiple tumors including solitary fibrous tumors (independent of anatomical location), Wilm’s tumors, metastatic hemangiopericytomas, mesotheliomas, hepatocellular carcinomas, gastrointestinal stromal tumors (GIST), colorectal adenomas, osteosarcomas, rhabdomyosarcomas, leiomyosarcomas, paragangliomas, prostate cancers, breast cancers and bladder cancers [2, 15]. Although these neoplasms often have elevated levels of IGF-2 mRNA, they do not always produce elevated circulating levels of IGF-2 or big IGF-2. Genetic and epigenetic mechanisms that determine isolated transcription of mRNA and the production of IGF-2 and big IGF-2 are not well understood; however, these mechanisms are thought to play an important role in carcinogenesis and tumor growth [13, 16].
In a previous extensive review of this topic by de Groot and coauthors in 2007, authors noted that 41% of the IGF-2 producing tumors causing hypoglycemia were of mesenchymal origin, 43% of epithelial origin, 1% of neuroendocrine and hematopoietic origin and 14% of unknown origin [2]. Although symptoms of hypoglycemia were predominant, other symptoms included skin tags, acne and rhinophyma.
We reviewed case reports of NICTH associated with paraneoplastic production of IGF-2 from 2008 to 2012 (Table 3) [17–38]. On our review of literature, we identified 22 published reports of NICTH with elevated IGF-2 levels causing hypoglycemia. Age range of the patients was 27 – 83 years (mean age 58 years), with 14 males and 8 females. Predominant symptoms included those of hypoglycemia (diaphoresis, tremor, anxiety, loss of consciousness) and mechanical symptoms depending on site of the tumor. Some tumors were associated with acromegaly. The anatomic location of tumors in these case reports included liver (n = 9), pleural cavities (n = 5), lungs (n = 3), retroperitoneum (n = 3), bones (n = 2), pelvis (n = 2), breasts (n = 2), cranium (n = 1), kidney (n = 1), uterus (n = 1), spleen (n = 1) and adrenal gland (n = 1). The tumor involved more than one organ system in 6 patients. Fifteen patients were diagnosed with NICTH for the first time, whereas 7 patients presented with recurrent disease. Pathologic diagnosis included solitary fibrous tumor (n = 10), hemangiopericytoma (n = 3), phyllodes tumor and sarcoma (n = 2), hepatocellular carcinoma (n = 2), uterine leiomyoma (n = 1), gastric adenocarcinoma (n = 1), ovarian germ cell tumor (n = 1), pheochromocytoma (n = 1) and desmoplastic small round cell tumor (n = 1). Hypoglycemia associated with IGF-2 production has also been reported in pancreatic islet cell tumor [39]. Biochemical profiles of the patients varied and included elevated IGF-2, elevated big IGF-2 with normal IGF-2, and elevated IGF-2 to IGF-1 ratio.
Currently, different assays are being used in clinical and research settings for measurement of IGF-2. These assays utilize liquid chromatography–mass spectrometry (LC-MS) [40] or immunoassay (ELISA and RIA). Western blot is used for measurement big IGF-2 [41]. The possibility of NICTH secondary to IGF-2 is suggested by low blood glucose along with suppressed insulin, C-peptide and IGF-1 levels. Concurrent normal to high morning cortisol and normal response on cosyntropin stimulation can rule out adrenal insufficiency and suggest NICTH secondary to IGF-2. IGF-2 levels are frequently within the normal range in NICTH, and assays for big IGF-2 are not commercially available. Thus, the IGF-2: IGF-1 ratio is used as a surrogate marker for big IGF-2 concentration. A ratio of >10 is considered to be clinically significant [42].
Management of NICTH can be divided into issues related to 1) hypoglycemia and 2) the underlying tumor. Hypoglycemia can be managed in a typical manner by administration of oral glucose, intravenous dextrose or glucagon depending upon the severity. Glucocorticoid therapy has been shown to suppress big IGF-2 in a dose dependent manner using doses of 30–60 mg/d of prednisolone or 4 mg/d of dexamethasone [43]. Surgical resection of the tumor, whenever possible, is the treatment of choice and, if successful, usually results in the resolution of hypoglycemia and other metabolic abnormalities. In cases where complete resection is not feasible due to tumor infiltration and/or distant metastasis, surgical debulking along with chemoradiation can be considered for treatment of hypoglycemia. The choice of chemotherapy depends upon the primary pathology of the tumor. Metastatic disease and tumor recurrence are associated with a poor prognosis.
Conclusion
NICTH is a rare cause of hypoglycemia in the general population. It is associated with elevated levels of IGF-2, high molecular weight IGF-2 (big-IGF-2) or an increased IGF-2 to IGF-1 ratio. Diagnosis of paraneoplastic IGF-2 induced hypoglycemia is an important and time sensitive consideration since earlier detection of tumors provides a better opportunity for complete resection and subsequent resolution of hypoglycemia. Whether IGF-2 level should be routinely measured in the evaluation of hypoglycemia depends on the prevalence of the condition. Accumulating literature supports screening for IGF-2 in non-diabetic individuals who present with hypoglycemia along with suppressed insulin and c-peptide levels.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review.
Abbreviations
- IGF-1:
-
Insulin like growth factor–1
- IGF-2:
-
Insulin like growth factor–2
- NICTH:
-
Non-islet cell tumor hypoglycemia
- TNF:
-
Tumor necrosis factor
- IL:
-
Interleukin
- BMI:
-
Body mass index
- CT:
-
Computed tomography
- LC-MS:
-
Liquid chromatography–mass spectrometry.
References
Vella A, Service FJ: Incretin hypersecretion in post-gastric bypass hypoglycemia: primary problem or red herring?. J Clin Endocrinol Metab. 2007, 92: 4563-4565. 10.1210/jc.2007-2260.
de Groot JW, Rikhof B, van Doorn J, Bilo HJ, Alleman MA, Honkoop AH, van der Graaf WT: Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007, 14: 979-993. 10.1677/ERC-07-0161.
Bell GI, Gerhard DS, Fong NM, Sanchez-Pescador R, Rall LB: Isolation of the human insulin-like growth factor genes: insulin-like growth factor II and insulin genes are contiguous. Proc Natl Acad Sci U S A. 1985, 82: 6450-6454. 10.1073/pnas.82.19.6450.
O’Dell SD, Day IN: Insulin-like growth factor II (IGF-II). Int J Biochem Cell Biol. 1998, 30: 767-771. 10.1016/S1357-2725(98)00048-X.
Alkemade GM, Bakker M, Rikhof B, Ijpma FF, van Ginkel RJ, Kluin PM, van Doorn J, Dullaart RP: Hypoglycemia in a Patient With a Big “Big”-IGF-II-Producing Tumor. J Clin Endocrinol Metab. 2013, 98: 3113-3114. 10.1210/jc.2013-2170.
Hoekman K, van Doorn J, Gloudemans T, Maassen JA, Schuller AG, Pinedo HM: Hypoglycaemia associated with the production of insulin-like growth factor II and insulin-like growth factor binding protein 6 by a haemangiopericytoma. Clin Endocrinol (Oxf). 1999, 51: 247-253. 10.1046/j.1365-2265.1999.00833.x.
Butler AA, Le Roith D: Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu Rev Physiol. 2001, 63: 141-164. 10.1146/annurev.physiol.63.1.141.
Han VK, D’Ercole AJ, Lund PK: Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science. 1987, 236: 193-197. 10.1126/science.3563497.
Jones JI, Clemmons DR: Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995, 16: 3-34.
Baxter RC: The insulin-like growth factors and their binding proteins. Comp Biochem Physiol B. 1988, 91: 229-235.
Humbel RE: Insulin-like growth factors I and II. Eur J Biochem. 1990, 190: 445-462. 10.1111/j.1432-1033.1990.tb15595.x.
Malaguarnera R, Belfiore A: The Emerging Role of Insulin and Insulin-Like Growth Factor Signaling in Cancer Stem Cells. Front Endocrinol (Lausanne). 2014, 5: 10-eCollection 2014
Tennant MK, Thrasher JB, Twomey PA, Drivdahl RH, Birnbaum RS, Plymate SR: Protein and messenger ribonucleic acid (mRNA) for the type 1 insulin-like growth factor (IGF) receptor is decreased and IGF-II mRNA is increased in human prostate carcinoma compared to benign prostate epithelium. J Clin Endocrinol Metab. 1996, 81: 3774-3782.
Lewis A, Reik W: How imprinting centres work. Cytogenet Genome Res. 2006, 113: 81-89. 10.1159/000090818.
Gallagher EJ, LeRoith D: The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010, 21: 610-618. 10.1016/j.tem.2010.06.007.
Tricoli J, Rall LB, Karakousis CP, Herrera L, Petrelli NJ, Bell GI, Shows TB: Enhanced levels of insulin-like growth factor messenger RNA in human colon carcinomas and liposarcomas. Cancer Res. 1986, 46: 6169-6173.
Wagner S, Greco F, Hamza A, Hoda RM, Holzhausen HJ, Fornara P: Retroperitoneal malignant solitary fibrous tumor of the small pelvis causing recurrent hypoglycemia by secretion of insulin-like growth factor 2. Eur Urol. 2009, 55: 739-742. 10.1016/j.eururo.2008.09.050.
Fama F, Le Bouc Y, Barrande G, Villeneuve A, Berry MG, Pidoto RR, Saint Marc O: Solitary fibrous tumour of the liver with IGF-II-related hypoglycaemia. A case report. Langenbecks Arch Surg. 2008, 393: 611-616. 10.1007/s00423-008-0329-z.
Anaforoglu I, Simsek A, Turan T, Algun E: Hemangiopericytoma-associated hypoglycemia improved by glucocorticoid therapy: a case report. Endocrine. 2009, 36: 151-154. 10.1007/s12020-009-9197-8.
Tani Y, Tateno T, Izumiyama H, Doi M, Yoshimoto T, Hirata Y: Defective expression of prohormoneconvertase 4 and enhanced expression of insulin-like growth factor II by pleural solitary fibrous tumor causing hypoglycemia. Endocr J. 2008, 55: 905-911. 10.1507/endocrj.K08E-062.
Yamakawa-Yokota F, Ozaki N, Okajima A, Nishio H, Nagasaka T, Oiso Y: Retroperitoneal solitary fibrous tumor-induced hypoglycemia associated with high molecular weight insulin-like growth factor II. Clin Med Res. 2010, 8: 159-162. 10.3121/cmr.2010.888.
Okabe R, Sonobe M, Bando T, Date H: Large solitary fibrous tumor with overexpression of insulin-like growth factor-2. Interact Cardiovasc Thorac Surg. 2010, 11: 688-690. 10.1510/icvts.2010.240770.
Lawson EA, Zhang X, Crocker JT, Wang WL, Klibanski A: Hypoglycemia from IGF2 overexpression associated with activation of fetal promoters and loss of imprinting in a metastatic hemangiopericytoma. J Clin Endocrinol Metab. 2009, 94: 2226-2231. 10.1210/jc.2009-0153.
Hu Y, Mahar TJ, Hicks DG, Raymond D, Jones C, Wandtke JC, Powers JM, Xu H: Malignant solitary fibrous tumor: report of 3 cases with unusual features. Appl Immunohistochem Mol Morphol. 2009, 17: 451-457. 10.1097/PAI.0b013e318198f23e.
Hata T, Tsuruta Y, Takamori S, Shishikura Y: Non-islet cell tumor hypoglycemia at the second recurrence of malignant solitary fibrous tumor in the retroperitoneum and pelvis: a case report. Case Rep Oncol. 2012, 5: 420-427. 10.1159/000340012.
Chan JK, Cheuk W, Ho LC, Wen JM: Recurrent meningeal hemangiopericytoma with multiple metastasis and hypoglycemia: a case report. Case Rep Med. 2012, 2012: 628756-
Thabit H, Healy ML, Royston D, Broe P, Scarramuzzi N, Walsh TN, Sreenan S: A case of spontaneous hypoglycaemia and impaired glucose tolerance in the same patient. Ann Clin Biochem. 2011, 48: 183-185. 10.1258/acb.2010.010064.
Krishnan L, Clark J: Non-islet cell tumour hypoglycaemia. BMJ Case Rep. 2011, doi:10.1136/bcr.02.2011.3914
Hino N, Nakagawa Y, Ikushima Y, Yoshida M, Tsuyuguchi M: A case of a giant phyllodes tumor of the breast with hypoglycemia caused by high-molecular-weight insulin-like growth factor II. Breast Cancer. 2010, 17: 142-145. 10.1007/s12282-009-0094-z.
Renard E, Langbour-Remy C, Klein M, Le Bouc Y, Weryha G, Cuny T: Severe hypoglycemia with “Big”-IGF-2 oversecretion by a giant phyllode tumor of the breast: a rare case of non-islet cell tumor-induced hypoglycemia (NICTH). Ann Endocrinol. 2012, 73: 488-491. 10.1016/j.ando.2012.04.011.
Ndzengue A, Deribe Z, Rafal RB, Mora M, Desgrottes S, Schmidt F, Becher R, Wright AM, Guillaume J, Jaffe EA: Non-islet cell tumor hypoglycemia associated with uterine leiomyomata. Endocr Pract. 2011, 17: e109-e112. 10.4158/EP10381.CR.
Matsuyama M, Sugiura S, Kakita A, Sato Y, Kuroda M: Hepatocellular carcinoma arising from ectopic liver tissue in the spleen producing insulin-like growth factor II. Pathol Res Pract. 2011, 207: 124-126. 10.1016/j.prp.2010.09.003.
Maruyama H, Tatsumi M, Kitayama H, Enomoto Y, Kuniyasu H, Uematsu K, Fukuda I, Kameya T, Konishi Y: A case of gastric cancer with non-islet cell tumor hypoglycemia detected by insulin-like growth factor II. Pathol Int. 2010, 60: 595-597. 10.1111/j.1440-1827.2010.02563.x.
Powter L, Phillips S, Husbands E: A case report of non-islet cell tumourhypoglycaemia associated with ovarian germ-cell tumour. Palliat Med. 2013, 27: 281-283. 10.1177/0269216312462273.
Macfarlane DP, Leese GP: Hypoglycaemia, phaeochromocytoma and features of acromegaly: a unifying diagnosis?. QJM. 2011, 104: 983-986. 10.1093/qjmed/hcq219.
Barra WF, Castro G, Hoff AO, Siqueira SA, Hoff PM: Symptomatic hypoglycemia related to inappropriately high igf-ii serum levels in a patient with desmoplastic small round cell tumor. Case Rep Med. 2010, 2010: 684045-
Okushin K, Asaoka Y, Fukuda I, Fujiwara N, Minami T, Sato M, Mikami S, Uchino K, Enooku K, Kondo Y, Tateishi R, Goto T, Shiina S, Yoshida H, Koike K: IGF-II Producing Hepatocellular Carcinoma Treated with Sorafenib: Metabolic Complications and a Foresight to Molecular Targeting Therapy to the IGF Signal. Case Rep Gastroenterol. 2012, 6: 784-789. 10.1159/000346462.
Tominaga N, Kawarasaki C, Kanemoto K, Yokochi A, Sugino K, Hatanaka K, Uekusa T, Fukuda I, Aiba M, Hizuka N, Uda S: Recurrent solitary fibrous tumor of the pleura with malignant transformation and non-islet cell tumor-induced hypoglycemia due to paraneoplastic overexpression and secretion of high-molecular-weight insulin-like growth Factor II. Intern Med. 2012, 51: 3267-3272. 10.2169/internalmedicine.51.7906.
Chung JO, Hong SI, Cho DH, Lee JH, Chung DJ, Chung MY: Hypoglycemia associated with the production of insulin-like growth factor II in a pancreatic islet cell tumor: a case report. Endocr J. 2008, 55: 607-612. 10.1507/endocrj.K07E-153.
Bystrom C, Sheng S, Zhang K, Caulfield M, Clarke NJ, Reitz R: Clinical utility of insulin-like growth factor 1 and 2; determination by high resolution mass spectrometry. PLoS One. 2012, 7: e43457-10.1371/journal.pone.0043457.
Qiu Q, Yan X, Bell M, Di J, Tsang BK, Gruslin A: Mature IGF-II prevents the formation of “big” IGF-II/IGFBP-2 complex in the human circulation. Growth Horm IGF Res. 2010, 20: 110-117. 10.1016/j.ghir.2009.11.001.
Marks V, Teale JD: Tumours producing hypoglycaemia. Endocr Relat Cancer. 1998, 5: 111-129. 10.1677/erc.0.0050111.
Teale JD, Marks V: Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol. 1998, 49: 491-498. 10.1046/j.1365-2265.1998.00564.x.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6823/14/49/prepub
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ contribution
AK reviewed the literature and wrote the manuscript. BJR edited the manuscript, wrote the abstract and participated in the literature review. JB was involved in clinical management and reviewed and edited the manuscript. AM was involved in clinical management and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Khowaja, A., Johnson-Rabbett, B., Bantle, J. et al. Hypoglycemia mediated by paraneoplastic production of Insulin like growth factor–2 from a malignant renal solitary fibrous tumor – clinical case and literature review. BMC Endocr Disord 14, 49 (2014). https://doi.org/10.1186/1472-6823-14-49
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1472-6823-14-49