Abstract
Background
Several respiratory diseases are associated with specific respiratory sounds. In contrast to auscultation, computerized lung sound analysis is objective and can be performed continuously over an extended period. Moreover, audio recordings can be stored. Computerized lung sounds have rarely been assessed in neonates during the first year of life. This study was designed to determine and validate optimal cut-off values for computerized wheeze detection, based on the assessment by trained clinicians of stored records of lung sounds, in infants aged <1 year.
Methods
Lung sounds in 120 sleeping infants, of median (interquartile range) postmenstrual age of 51 (44.5–67.5) weeks, were recorded on 144 test occasions by an automatic wheeze detection device (PulmoTrack®). The records were retrospectively evaluated by three trained clinicians blinded to the results. Optimal cut-off values for the automatically determined relative durations of inspiratory and expiratory wheezing were determined by receiver operating curve analysis, and sensitivity and specificity were calculated.
Results
The optimal cut-off values for the automatically detected durations of inspiratory and expiratory wheezing were 2% and 3%, respectively. These cutoffs had a sensitivity and specificity of 85.7% and 80.7%, respectively, for inspiratory wheezing and 84.6% and 82.5%, respectively, for expiratory wheezing. Inter-observer reliability among the experts was moderate, with a Fleiss’ Kappa (95% confidence interval) of 0.59 (0.57-0.62) for inspiratory and 0.54 (0.52 - 0.57) for expiratory wheezing.
Conclusion
Computerized wheeze detection is feasible during the first year of life. This method is more objective and can be more readily standardized than subjective auscultation, providing quantitative and noninvasive information about the extent of wheezing.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Wheezes consisting of continuous musical sounds of one or more tonal components [1, 2] among the most common adventitious lung sounds in children [3]. Wheezes are usually louder than underlying breath sounds [4] and occur within a broad frequency range [1], with a mean dominant frequency in infants of 225.5 Hz [5]. Wheezing is the acoustic manifestation of lower airway obstruction limiting air-flow in a collapsible tube, thus inducing wall flutter [6]. This phenomenon is usually encountered in asthmatic children [7, 8], but can also occur in children with bronchiolitis [9], cystic fibrosis [10], foreign body aspiration [11], bronchomalacia [12] and primary ciliary dyskinesia [8]. Therefore, detection of wheezing can be useful in diagnosing respiratory disorders and in assessing the efficacy of treatments [9, 13].
Wheezing is most frequently diagnosed by auscultation using a stethoscope or is based on parental reports of wheezes. However, parents often differ in their understanding of wheeze [14, 15] and parentally reported wheezing often cannot be confirmed by auscultation [16]. Moreover, the inter-observer reliability between doctors has been questioned [17, 18] and the quality of auscultation has generally been described as insufficient [4, 7, 19]. This insufficiency is likely due to disparities in the nomenclature used to describe lung sounds [17, 20], in the varying quality of stethoscopes [4, 17] and high noise levels in clinical settings [3]. Computerized lung sound analysis, especially computerized wheeze detection, has been reported to be a more objective and standardizable method, which can overcome the limitations of subjective auscultation [3, 9, 21, 22]. In contrast to auscultation, computerized lung sound analysis can be performed continuously over an extended period of time, and audio recordings can be stored for later assessment and quality monitoring.
To date, few studies have used computerized methods to detect wheezes during the first year of life [9, 23]. The inspiratory and expiratory times are shorter in infants aged <1 year than in older infants, for which cut-off values for the duration of wheezing have been determined [24]. The authors hypothesized that, by determining optimal cut-off values for wheezing, computerized wheeze detection would be an objective, reliable and easy to use method of assessing wheezing also in infants aged <1 year. Therefore the aim of this feasibility study was to determine and validate optimal cut-off values for computerized wheeze detection, based on the assessment by trained clinicians of stored records of lung sounds in infants who recovered after a stay in the neonatal intensive care unit.
Methods
Subjects
Computerized wheeze detection and subjective lung sound assessment were performed in 120 infants, of median age 51 postmenstrual weeks, on 144 test occasions. Lung sounds were recorded during lung function testing (LFT) as part of our routine follow-up care of infants requiring intensive care [25]. Patient characteristics are shown in Table 1. Indications for LFT included bronchopulmonary dysplasia (BPD) in 51 infants, respiratory distress syndrome in 35, congenital diaphragmatic hernia in 13, respiratory maladaptation in 6, double aortic arch anomalies in 3, congenital cystic adenomatoid malformation in 2, tracheomalacia in 2 and others in 10.
All parents provided written informed consent before each LFT, and the study protocol was approved by our Institutional Data Safety Committee.
Computerized wheeze detection
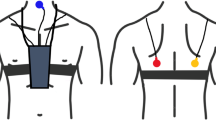
Wheezes were detected using the PulmoTrack® Model 2020 (Karmel Sonix Ltd., Israel), an instrument developed for the continuous tracking and recording of breathing sounds and the detection of wheezing. Lung sounds were analyzed using a fast Fourier transform (FFT)-based algorithm for lung sound analysis and two phonopneumographic contact sensors, one applied to the region of the manubrium and the other over the left axillary line (Figure 1). The sensors are coin-shaped piezoelectric elements with linear ±3 dB frequency responses from 75 to 2000 Hz, a resonance at 2.7 kHz and an useable range that extends beyond 4 kHz [9]. The sensors were attached to the skin via adhesive foam pads to reduce ambient noise. Another air-coupled microphone was placed next to each infant to record ambient noises and improve the signal-to-noise ratio, and a respiration belt fitted with tension sensors was strapped around each infant’s chest to detect breathing activity (times of inspiration and expiration). Sound artifacts due to movements of the infant or occasional crying could not totally be eliminated.
The PulmoTrack® calculates the relative inspiratory and expiratory wheeze rates as.
where Tw in/ex is the breathing time with wheeze during inspiration/expiration and Tin/ex is the total inspiratory/expiratory breathing time.
Subjective wheeze detection
Recorded sounds coded for each infant were retrospectively evaluated by three medical doctors working in the neonatal intensive care unit and trained before the study using a computer aided instruction on respiratory sounds (R.A.L.E.® Lung Sounds 3.2). Using headphones that minimized surrounding noise, each observer listened to the sound of each infant in a blinded fashion and assessed if wheezing was present or absent, independent of the strength and duration of sounds.
Measurement protocol
Lung sounds were recorded in clinically stable and sleeping infants who had no respiratory infections during the 3 weeks preceding the tests. Sleep was induced 15–30 min before LFT by oral administration of chloral hydrate (50 mg∙kg−1), since sedation was necessary for subsequent more complex LFT [25, 26].
To prevent any interactions lung sound recordings were performed before LFT and before a face mask was applied. Sounds were measured while the infants were supine, with the neck in a neutral position and supported by a neck roll. After attachment of the microphones and breathing belt, an adaptation time of 10–15 min was allowed before lung sounds were recorded. The duration of each recording was 10 minutes. No other lung function tests were performed simultaneously.
Statistical methods
Patient characteristics and lung sound data are reported as rates (%) or as medians and interquartile ranges (IQR). Incidences of wheezing were compared using Fisher’s exact test. The Kruskal-Wallis rank test was used to investigate the influence of birth weight, mechanical ventilation and BPD on wheeze rates. Inter-observer reliability of lung sound assessment was assessed using Fleiss’ kappa, which is a generalization of Cohen’s kappa to multiple raters that provides a conservative measure of agreement. The 95% confidence interval of Fleiss’ kappa was calculated as described [27], with Fleiss’ kappa scores of 1.0, 0.81–0.99, 0.61–0.80, 0.41–0.60, 0.2–0.40 and <0.2 indicating perfect, almost perfect, substantial, moderate and poor agreement, respectively. Receiver operating characteristic (ROC) curves were calculated to determine the optimal cut-off values for inspiratory and expiratory wheezing times, as measured by the PulmoTrack® and compared with subjective evaluations. All statistical analyses were performed using Statgraphics Centurion® software (Version 16.0, Statpoint Inc., Herndon, VA, USA) and MEDCALC (Version 9.1.0.1, MedCalc Software, Mariakerke Belgium), with p < 0.05 defined as statistically significant.
Results
Study population
Lung sounds were recorded in 120 infants on 144 test occasions, with 98 infants (82%) tested on one occasion, 20 (17%) on two occasions and 2 (1.7%) on three occasions. Patient characteristics are shown in Table 1. Most patients (95%) were premature infants with less than 37 gestational weeks and almost half (49%) of all patients were former extremely low birth weight (ELBW) infants, with a birth weight <1000 g. On the day of measurement, their median postmenstrual age (PMA) was 51 weeks, with none requiring any respiratory support.
Computerized wheeze detection
The distributions of inspiratory and expiratory wheeze rates are shown in Figure 2. Both distributions showed a distinct skewness, with maxima at wheeze rates of 1%. The PulmoTrack® detected wheezing in the majority of measurements. Only 27 (19%) of the measurements and 18 (13%) of the expiratory measurements were without wheezing, a difference that was not statistically significant. Wheeze rates >10% were significantly more frequent during expiration than during inspiration (18.8% versus 9.7%, p = 0.042).
Subjective wheeze detection and inter-observer reliability
Evaluation of wheezing in the 144 recordings by’ the three observers is shown in Table 2. The incidence of expiratory wheezing (36%-50%) was higher than the incidence of inspiratory wheezing (24%-29%). The agreement of the three observers in detection of wheezing was moderate, with Fleiss' kappas (95% confidence interval) of 0.54 (0.52–0.57) for expiratory wheezing and 0.59 (0.57–0.62) for inspiratory wheezing.
Cut-off values for computerized wheeze detection
Because the PulmoTrack® detected wheezing in almost all infants, cut-off values for the duration of wheezing were needed to compare computerized and subjective assessments of wheezing. For this purpose, the results of the three observers were classified into three groups: no wheezing detected by all three (group 1), lack of agreement on the presence of wheezing (group 2) and wheezing detected by all three (group 3). Classifications of inspiratory and expiratory wheezing into these three groups are shown in Figure 3. Using ROC analysis, optimal cut-offs for the computer-measured wheezing rates were calculated. The cutoff value for the inspiratory wheezing rate was >2%, which had a sensitivity of 85.7% and a specificity of 80.7%; whereas the cut-off value for the expiratory wheezing rate was >3%, which had a sensitivity of 84.6% and a specificity of 82.5%. For ROC analysis only files in groups 1 and 3 were used. Of the total study population sensitivity and specificity were 85.7% and 71.4% for the inspiratory wheeze detection and 84.6% and 74.3% for the expiratory wheeze detection.
Discussion
The study showed that the PulmoTrack® can reliably detect wheezing in neonates, with sensitivities of 85.7% for inspiratory and 84.6% for expiratory wheezes and specificities of 80.7% and 82.5%, respectively, using appropriate cut-off values. Computerized wheeze detection reliably detects even short periods of wheezing, as reflected by the low cut-off values of 2% for inspiratory and 3% for expiratory wheezing.
The equipment used in this study differs from that used in previous studies in older children, in which sound was recorded by five sensors [13, 23]. Due to the smaller thoraxes in infants aged <1 year, we used only two chest microphones, as suggested by the developers of the PulmoTrack®. Using five sensor positions simultaneously, the PulmoTrack® has been validated in children aged 6–14 years, with a slightly higher sensitivity (91%) and specificity (89%) in wheeze detection than the consensus by a panel of pulmonary experts who performed auscultation of the same respiratory sounds [13].
The inter-observer reliability for wheeze detection, expressed as the Fleiss Kappa coefficient, was moderate in our study, reflecting a higher inter-observer reliability than reported in most previous studies [17, 28–30]. ROC analysis showed cut-off values of >2% for inspiratory and >3% for expiratory wheeze. In contrast, wheeze rates <5% in older children were not considered clinically significant [24], as healthy children have wheeze rates <5%, with a wheeze rate >10% proposed as a cutoff value [13].
The disparity in lung sound nomenclature has been cited as contributing to disagreements among observers [4, 5, 31]. To prevent this disparity we followed the standardized nomenclature proposed by the American Thoracic Society (ATS) [32] and the International Symposium on Lung Sounds (ILSA) [33]. Although the frequency and duration of wheezes in adults have been defined [32, 34], these definitions are lacking for neonates. Cutoff values in neonates <1 year may differ from those in older children and adults.
This study has several strengths and limitations. The main strengths include the use of a larger sample size than in previous studies on wheeze detection in infants [3, 20] and the use of the same investigators, equipment, and protocol for all patients. Moreover, all the assessed lung sounds were recorded, allowing the three observers to listen to exactly the same sounds. To our knowledge, this study is one of the largest single-center comprehensive studies to compare computerized wheeze detection with the assessment of an expert panel and to analyze inter-observer reliability regarding the detection of wheezing.
One study limitation was that all sound recordings were performed in a quiet lung function testing unit. Thus, we cannot determine the quality of computerized wheeze detection in noisier clinical settings. Moreover, all infants included in our study were sedated for LFT, preventing a determination of the quality of computerized wheeze detection in awake and possibly restless infants.
Conclusion
Computerized wheeze detection using PulmoTrack® is feasible and reliable in neonates <1 year when using appropriate cut-off values for inspiratory and expiratory wheeze rate. This method provided quantitative and noninvasive information about the extent of wheezing, in contrast to the assessment by trained clinicians, which was subjective and only moderate in the inter-observer agreement. Since this included only infants indicated for LFT due to pulmonary impairment, further studies are needed to evaluate lung sounds in healthy infants.
References
Pasterkamp H, Kraman SS, Wodicka GR: Respiratory sounds. Advances beyond the stethoscope. Am J Respir Crit Care Med. 1997, 156: 974-987. 10.1164/ajrccm.156.3.9701115.
Ellington LE, Gilman RH, Tielsch JM, Steinhoff M, Figueroa D, Rodriguez S, Caffo B, Tracey B, Elhilali M, West J, Checkley W: Computerised lung sound analysis to improve the specificity of paediatric pneumonia diagnosis in resource-poor settings: protocol and methods for an observational study. BMJ Open. 2012, 2: e000506-
Prodhan P, Dela Rosa RS, Shubina M, Haver KE, Matthews BD, Buck S, Kacmarek RM, Noviski NN: Wheeze detection in the pediatric intensive care unit: comparison among physician, nurses, respiratory therapists, and a computerized respiratory sound monitor. Respir Care. 2008, 53: 1304-1309.
Loudon R, Murphy RL: Lung sounds. Am Rev Respir Dis. 1984, 130: 663-673.
Elphick HE, Ritson S, Rodgers H, Everard ML: When a “wheeze” is not a wheeze: acoustic analysis of breath sounds in infants. Eur Respir J. 2000, 16: 593-597. 10.1034/j.1399-3003.2000.16d04.x.
Gavriely N, Shee TR, Cugell DW, Grotberg JB: Flutter in flow-limited collapsible tubes: a mechanism for generation of wheezes. J Appl Physiol. 1989, 66: 2251-2261.
Baughman RP, Loudon RG: Quantitation of wheezing in acute asthma. Chest. 1984, 86: 718-722. 10.1378/chest.86.5.718.
Tenero L, Tezza G, Cattazzo E, Piacentini G: Wheezing in preschool children. Early Hum Dev. 2013, 89 (Suppl 3): S13-S17.
Beck R, Elias N, Shoval S, Tov N, Talmon G, Godfrey S, Bentur L: Computerized acoustic assessment of treatment efficacy of nebulized epinephrine and albuterol in RSV bronchiolitis. BMC Pediatr. 2007, 7: 22-10.1186/1471-2431-7-22.
Ren CL, Konstan MW, Rosenfeld M, Pasta DJ, Millar SJ, Morgan WJ, Fibrosis IaCotESoC: Early childhood wheezing is associated with lower lung function in cystic fibrosis. Pediatr Pulmonol. 2014, 49: 745-750. 10.1002/ppul.22894.
Kiyan G, Gocmen B, Tugtepe H, Karakoc F, Dagli E, Dagli TE: Foreign body aspiration in children: the value of diagnostic criteria. Int J Pediatr Otorhinolaryngol. 2009, 73: 963-967. 10.1016/j.ijporl.2009.03.021.
Saikia B, Sharma PK, Sharma R, Gagneja V, Khilnani P: Isolated severe bilateral bronchomalacia. Indian J Pediatr. 2014, 81: 707-708. 10.1007/s12098-013-1079-7.
Bentur L, Beck R, Shinawi M, Naveh T, Gavriely N: Wheeze monitoring in children for assessment of nocturnal asthma and response to therapy. Eur Respir J. 2003, 21: 621-626. 10.1183/09031936.03.00036302.
Cane RS, Ranganathan SC, McKenzie SA: What do parents of wheezy children understand by “wheeze”?. Arch Dis Child. 2000, 82: 327-332. 10.1136/adc.82.4.327.
Brand PL, Baraldi E, Bisgaard H, Boner AL, Castro-Rodriguez JA, Custovic A, de Blic J, de Jongste JC, Eber E, Everard ML, Frey U, Gappa M, Garcia-Marcos L, Grigg J, Lenney W, Le Souëf P, McKenzie S, Merkus PJ, Midulla F, Paton JY, Piacentini G, Pohunek P, Rossi GA, Seddon P, Silverman M, Sly PD, Stick S, Valiulis A, van Aalderen WM, Wildhaber JH, et al: Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J. 2008, 32: 1096-1110. 10.1183/09031936.00002108.
Peterson-Carmichael SL, Rosenfeld M, Ascher SB, Hornik CP, Arets HG, Davis SD, Hall GL: Survey of clinical infant lung function testing practices. Pediatr Pulmonol. 2014, 49: 126-131. 10.1002/ppul.22807.
Brooks D, Thomas J: Interrater reliability of auscultation of breath sounds among physical therapists. Phys Ther. 1995, 75: 1082-1088.
Elphick HE, Lancaster GA, Solis A, Majumdar A, Gupta R, Smyth RL: Validity and reliability of acoustic analysis of respiratory sounds in infants. Arch Dis Child. 2004, 89: 1059-1063. 10.1136/adc.2003.046458.
Guntupalli KK, Alapat PM, Bandi VD, Kushnir I: Validation of automatic wheeze detection in patients with obstructed airways and in healthy subjects. J Asthma. 2008, 45: 903-907. 10.1080/02770900802386008.
Levy ML, Godfrey S, Irving CS, Sheikh A, Hanekom W, Bush A, Lachman P: Wheeze detection: recordings vs. assessment of physician and parent. J Asthma. 2004, 41: 845-853. 10.1081/JAS-200038451.
Oliveira A, Marques A: Respiratory sounds in healthy people: a systematic review. Respir Med. 2014, 108: 550-570. 10.1016/j.rmed.2014.01.004.
Marques A, Oliveira A, Jácome C: Computerized adventitious respiratory sounds as outcome measures for respiratory therapy: a systematic review. Respir Care. 2014, 59: 765-776. 10.4187/respcare.02765.
Bentur L, Beck R, Berkowitz D, Hasanin J, Berger I, Elias N, Gavriely N: Adenosine bronchial provocation with computerized wheeze detection in young infants with prolonged cough: correlation with long-term follow-up. Chest. 2004, 126: 1060-1065. 10.1378/chest.126.4.1060.
Eising JB, Uiterwaal CS, van der Ent CK: Nocturnal wheeze measurement in preschool children. Pediatr Pulmonol. 2014, 49: 257-262. 10.1002/ppul.22803.
Schmalisch G, Wilitzki S, Roehr CC, Proquitté H, Bührer C: Differential effects of immaturity and neonatal lung disease on the lung function of very low birth weight infants at 48–52 postconceptional weeks. Pediatr Pulmonol. 2013, 48: 1214-1223. 10.1002/ppul.22770.
Schmalisch G, Wilitzki S, Roehr CC, Proquitté H, Bührer C: Development of lung function in very low birth weight infants with or without bronchopulmonary dysplasia: longitudinal assessment during the first 15 months of corrected age. BMC Pediatr. 2012, 12: 37-10.1186/1471-2431-12-37.
Reichenheim ME: Confidence intervals for the kappa statistics. Stata J. 2004, 4: 421-428.
Lati J, Pellow V, Sproule J, Brooks D, Ellerton C: Examining interrater reliability and validity of a paediatric cardiopulmonary physiotherapy discharge tool. Physiother Can. 2014, 66: 153-159. 10.3138/ptc.2013-23.
Wipf JE, Lipsky BA, Hirschmann JV, Boyko EJ, Takasugi J, Peugeot RL, Davis CL: Diagnosing pneumonia by physical examination: relevant or relic?. Arch Intern Med. 1999, 159: 1082-1087. 10.1001/archinte.159.10.1082.
Brooks D, Wilson L, Kelsey C: Accuracy and reliability of ‘specialized’ physical therapists in auscultating tape-recorded lung sounds. Physiother Can. 1993, 45: 21-24.
Wilkins RL, Dexter JR, Murphy RL, DelBono EA: Lung sound nomenclature survey. Chest. 1990, 98: 886-889. 10.1378/chest.98.4.886.
Anonymous: American Thoracic Society Ad Hoc Commitee on Pulmonary Nomenclature - Updated nomenclature for membership reaction. Am Thoracic Soc News. 1977, 3: 5-6.
Mikami R, Murao M, Cugell DW, Chretien J, Cole P, Meier-Sydow J, Murphy RL, Loudon RG: International Symposium on Lung Sounds. Synopsis of proceedings Chest. 1987, 92 (2): 342-345. 10.1378/chest.92.2.342.
Sovijärvi AR, Dalmasso F, Vanderschoot J, Malmberg LP, Righini G, Stoneman S: Definition of terms for applications of respiratory sounds. Eur Respir Rev. 2000, 10: 597-610.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2431/14/257/prepub
Acknowledgments
The authors thank Dr. Scott Butler of English Manager Science Editing, Sydney, Australia, for linguistic revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
LP, HF and GS had primary responsibility for study design, data analysis and writing of the manuscript. SW measured all lung sounds and GS performed statistical analysis. LP, HF and JU assessed all sound records to determine if wheezing was present or absent. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Puder, L.C., Fischer, H.S., Wilitzki, S. et al. Validation of computerized wheeze detection in young infants during the first months of life. BMC Pediatr 14, 257 (2014). https://doi.org/10.1186/1471-2431-14-257
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2431-14-257