Abstract
Background
Lung sound analysis parameters have been reported to be useful biomarkers for evaluating airway condition. We developed an automatic lung sound analysis software program for infants and children based on lung sound spectral curves of frequency and power by leveraging machine learning (ML) technology.
Methods
To put this software program into clinical practice, in Study 1, the reliability and reproducibility of the software program using data from younger children were examined. In Study 2, the relationship between lung sound parameters and respiratory flow (L/s) was evaluated using data from older children. In Study 3, we conducted a survey using the ATS-DLD questionnaire to evaluate the clinical usefulness. The survey focused on the history of wheezing and allergies, among healthy 3-year-old infants, and then measured lung sounds. The clinical usefulness was evaluated by comparing the questionnaire results with the results of the new lung sound parameters.
Results
In Studies 1 and 2, the parameters of the new software program demonstrated excellent reproducibility and reliability, and were not affected by airflow (L/s). In Study 3, infants with a history of wheezing showed lower FAP0 and RPF75p (p < 0.001 and p = 0.025, respectively) and higher PAP0 (p = 0.001) than healthy infants. Furthermore, infants with asthma/asthma-like bronchitis showed lower FAP0 (p = 0.002) and higher PAP0 (p = 0.001) than healthy infants.
Conclusions
Lung sound parameters obtained using the ML algorithm were able to accurately assess the respiratory condition of infants. These parameters are useful for the early detection and intervention of childhood asthma.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
An early diagnosis and intervention are important for of childhood asthma [1]. High-pitched continuous sound such as wheezing are considered an indicator of bronchial constriction, and repeated wheezing is an important factor in diagnosing asthma [1, 2]. Clinically, the evaluation of bronchial hyperresponsiveness using bronchoconstrictors has been used as an objective method that leads to a definitive asthma diagnosis [3]. However, the diagnosis of asthma in wheezy infants remains difficult for all physicians [1], because infants and preschool children are unable to spontaneously perform common lung function tests.
In recent years, studies on airway condition using lung sound analysis, which is expected to be a safe, simple and repeatable method [4], have been progressing [5, 6]. It has been reported that lung sounds are sensitive to changes in the airway condition [7], and that lung sound analyses can be applied to the clinical evaluation of airway changes even in infants and preschoolers [8]. In addition, recent advances in artificial intelligence (AI) technology have dramatically improved techniques for extracting information related to clinical diseases from lung sounds [9, 10].
However, in conventional lung sound analysis systems, operations related to the analysis have not been automated. To analyze the lung sound spectrum, two or more experts visually determined the highest frequency in each spectrum, or the zero point on the Y-axis of the spectrum [7, 11]. Although these previous methods also provide a certain degree of reproducibility from lung sound samples to the calculation of parameters, easy-to-handle and more stable methods are required for the evaluation of daily clinical practice or multicenter research [12].
Recently, we developed a new software program that uses machine learning (ML) algorithms to perform a lung sound analysis for infants and children that shows greater stability and is simpler and more accurate in comparison to the current methods. The purpose of this study was to evaluate the reliability and usefulness of our new method using a lung sound analysis software program to assess airway condition in infants and young children who are unable to undergo normal lung function tests.
Methods
Clinical evaluation of the new software program
Study 1: Evaluation of reproducibility and reliability of new parameters for analysis of lung sounds
The basic reliability and reproducibility of the new software program, which target younger children were retrospectively evaluated. That is, using inspiratory lung sound samples from younger children, intra-examiner reliability and inter-examiner reliability were determined and evaluated based on the intraclass correlation coefficients (ICCs) of Case 1 and Case 2 [13].
From February 1, 2022 to March 31, 2023, 24 young children (age, 1 month to 4 years, median age 15 months, male: female, 16: 8) visited the Department of Pediatrics, Tokai University Hachioji Hospital. In the Case 1 (1, 1) study to determine the intraclass correlation coefficient, doctors specializing in pediatric pulmonology measured the same sample twice. A doctor visually selected a 10-s interval that was considered to be a desirable part for a lung sound analysis at that time, and calculated it on different days using the new software program. The new parameters, FAP0 and PAP0, were calculated based on the analytical maximum point of the breath sound spectrum (0 points). New parameters based on a previous method [7], RPF50p, RPF75p, A3a/AT and B4a/AT were also calculated based on 0 points.
In the Case 2 (2, 1) study, two doctors specializing in pediatric pulmonology visually selected a 10-s interval that was considered to be desirable for a lung sound analysis, and performed a lung sound analysis using the new software program.
The study protocol was approved by the institutional review board of Tokai University Hospital (No. 20R-324, approval date: March 17, 2021).
Study 2: Evaluation of characteristics of new parameters for the analysis of lung sounds
A retrospective study was conducted in older children for whom lung sounds and airflow (L/s) could be measured simultaneously [7]. That is, the airflow rate and each lung sound parameter were calculated at the maximum value of airflow rate of the sample during inspiration, and the relationship between the parameters and the airflow rate was confirmed.
From April 1, 2014 to March 31, 2015, 30 patients (age 5–15 years, median 9 years, male: female, 18:12), visited the pediatric department of Tokai University Hospital and lung sounds and airflow (L/s) were measured simultaneously. All patients had atopic asthma and the influence of airflow on new lung sound parameters was examined.
The study protocol was approved by the institutional review board of Tokai University Hospital (No. 11R-158, approval date: December 21, 2011 and No. 14R-133, approval date: October 23, 2014).
Study 3: Clinical evaluation of new parameters for the analysis of lung sounds
We prospectively investigated the relationship between the results of our lung sound analysis and the factors associated with the onset of asthma and allergic diseases in healthy infants who underwent a 3-year health checkup in the municipality from September 1 to December 28, 2023. During lung sound sampling, a pediatrician confirmed that all subjects had no fever or respiratory symptoms, such as cough, wheezing, or crackles, and no serious chronic diseases.
After administering the ATS-DLD questionnaire [12, 14, 15] to all subjects, lung sounds during resting breathing were collected in a quiet room, as previously reported [8]. Each lung sound sample was automatically calculated using the new software program. Thereafter, the relationship between the results of the lung sound analysis and the questionnaire responses was examined.
In these three studies, written informed consent of each subject was obtained from a parent and/or their legal guardian. The study protocol was approved by the institutional review board of Tokai University Hospital (No. 22R-136, approval date: October 20, 2022).
Basic details of the new lung sound analysis software program
The original lung sound analysis software program (Murata Manufacturing Co., Ltd, Version 1.6) can automatically: 1) select the subject's typical inspiratory sounds suitable for the analysis from breath sound samples on the lung sound spectrogram, and 2) analyze the lung sound parameters of the selected inspiratory sounds. That is, the first 10 s of the acquired spectrogram image (10 s or more) of each object were automatically selected, and each parameter was automatically calculated, and then saved as individual data. In addition, to avoid external noises and crying sounds, the examiner can select the optimal 10 s to start the automatic analysis. The selection of inspiratory sound samples mentioned above and the creation of an appropriate power spectrum for inspiratory sounds were automatically analyzed using the ML algorithm (Fig. 1).
The lung sound data were transformed into a spectrogram in an automatic procedure. From the spectrogram, multiple sound source components are extracted using non-negative matrix factorization (NMF) [16, 17], and the exact time when inspiration is relatively strong is determined from each component. The power spectrum at time was smoothed using Bayesian estimation [18, 19]. Simultaneously, new lung sound parameters were calculated (Fig. 2).
Flowchart of the newly developed procedure for automatic lung sound analysis. In the ML algorithm, multiple basis components are extracted from the inspiratory sound sample using non-negative matrix factorization (NMF), and the exact time when inspiration is relatively strong is determined from each basis component. The analytical basis point, 0 point, is estimated after smoothing the power spectrum of the inspiratory sound sample at the determined time using Bayesian estimation. STFT; short-time Fourier transform, NMF, nonnegative matrix factorization; GMM, Gaussian mixture model
As sound parameters, the frequency at the basic point, 0 point, calculated from the spectrum of the subject's inspiratory sound, was FAP0 (kHz), and the power was PAP0 (dBm) based on previous studies [7, 11] (Fig. 3). Furthermore, RPF50p is the index obtained by dividing the power at 1/2 of the frequency (dBm) by the frequency at the same point (F50p), which is obtained by subtracting 100 Hz from the frequency at the 0 point. RPF75p was calculated by dividing the power at 3/4 of the frequency (dBm) by the frequency at the same point (F75p). Furthermore, 2/3 of the frequency obtained by subtracting 100 Hz from the frequency of 0 point was defined as A3p, the area from A3p in the high-pitched range to 0 point was defined as A3a, and the value divided by the total area (AT) was defined as A3a/AT. B4p and B4a/AT were calculated by using the same procedure [7, 11].
Sound spectrum parameters. FAP0: analysis parameter of frequency at the 0 point, PAP0: analysis parameter of power at the 0 point, AT: Total area of sound spectrum, A3a/AT: Ratio of high-pitched third area to total area, B4a/AT: Ratio of high-pitched fourth area to total area, dBF50p: dB at F50p, dBF75p: dB at F75p, RPF50p: Ratio of power to (F99p-F50p)[= dBF50p/(F99p-F50p)], RPF75p: Ratio of power to (F99p-F75p)[= dBF75p/(F99p-F75p)]
To optimize the estimation process in the ML analysis software program, we used encrypted lung sound research data collected by our group from March 2012 to March 2023. Three hundred seventeen children of 0–15 years of age participated in this study, including 169 children diagnosed with asthma, 49 asthma-suspected wheezy infants, 54 non-asthmatic children of regular outpatient visits, and 45 infants of health checkups in Isehara City.
Each subject was asked if they had a history of wheezing or allergic disease, asthma or asthmatic bronchitis by a doctor, and if they had an RSV infection through a detailed interview at the outpatient clinic or the ATS-DLD questionnaire at the infant health checkup. We also checked the patient's history of hospitalization due to respiratory tract diseases, family history of allergies, smoking, pets, and air pollution at home.
Correction of lung sounds
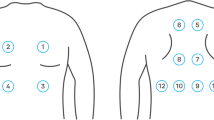
Lung sounds were collected in accordance with previous reports [7, 8]. Breath sounds were recorded using a handheld microphone for at least 10 s in a quiet room. A microphone was placed in the second intercostal space along the midclavicular line. The inhalation sound analysis was performed using an LSA-2000/2008/2012 acoustic spectrometer (Kenz Medico Co., Saitama, Japan). Sound amplification units have been found to be effective for analyzing sounds in the 100–2500 Hz range.
The recorded lung sounds were analyzed using at fast Fourier transform. The sampling frequency was 10,240 Hz, and the spectra were acquired using a Hanning window. The lung sounds were displayed as a spectrogram. The dBm values are plotted on the Y-axis and the Hz values are plotted on the X-axis [7]. Through an automatic analysis using our new software program, a sample of inspiratory sound with an ideal shape on the lung sound spectrogram was selected, and data around the maximum frequency of that sample were used as a lung sound spectrum for the lung sound analysis.
Statistical analyses
All statistical analyses were conducted using SPSS (IBM SPSS Statistics, Version 22 for Windows; IBM Corp., Armonk, N.Y., USA). Paired parameters were compared using the Mann–Whitney U-test. P values of < 0.05 were considered to indicate statistical significance. Correlations between individual breath sound parameters and other measurements were determined using Pearson’s correlation coefficient. To examine the reliability and reproducibility of analyses performed by the new software program, intra-observer and inter-observer reliability were determined using the ICCs of Case 1 and Case 2.
Results
Study 1, repeatability of new lung sound analysis parameters
A significant correlation was observed among the new parameters, FAP0, PAP0, RPF50p, RPF75p, A3a/AT and B4a/AT (Table 1a). In the study of Case 2 (2, 1), two doctors specializing in pediatric pulmonology measured the same sample, and significant correlations were observed for each parameter (Table 1b). The inhalation sounds were extracted from the selected 10-s frame of continuous breathing data on the spectrogram image. The median total sampling time for the lung sounds was 23.1 s.
Study 2, effect of respiratory flow on new lung sound analysis parameters
Although F99 was highly correlated with the airflow rate, there is no correlation with the airflow rate for the new parameters, FAP0, PAP0, RPF50p, RPF75p, A3a/AT and B4a/AT (Table 2).
Study 3, relationship between questionnaire items and new lung sound analysis parameters
We examined the relationship between lung sound data from 48 healthy children (age 3 years 0 months, boys: girls, 27: 21) who underwent infant health checkups at the age of 3 in Isehara City from September 1 to December 28, 2023, and the results of the ATS-DLD version of the questionnaire on asthma and allergic diseases. It was possible to perform sound analysis of 47 samples (97.9%). The sample of one girl was omitted due to persistent crying. The results are shown in Table 3.
Wheezing (total) is the sum of the questions Q2: wheezing history, and Q3: cold-induced wheezing history. Using these new parameters, a clear difference was observed between the presence or absence of wheezing (total) and the presence or absence of asthma/asthma-like bronchitis diagnosed by a physician in Q7. No clear difference was observed in the presence or absence of a history of RSV and atopy, which combined Q10, history of allergies and Q11, and history of atopic dermatitis [12, 14].
The new parameters FAP0 and PAP0 were both considered to strongly suggest a history of wheezing. An ROC curve analysis revealed that FAP0 had an AUC of 0.887, asymptotic significance probability of < 0.001, and a 95% confidence interval of 0.795 to 0.979), while the PAP0 had AUC of 0.798, asymptotic significance probability of 0.001, and a 95% confidence interval of 0.667 to 0.930. In FAP0, the cutoff value for wheezing (total) determined using Youden's index was 1.79 (kHz). This showed 81% sensitivity and 87% specificity.
Discussion
In this report, we demonstrated the reliability of a new software program that utilizes ML algorithms to perform lung sound analysis in infants and children. Compared to previous methods [7, 8], the new software program is able to more accurately classify 3-year infants with a history of wheezing and/or asthma, and is superior in that it is fully automated. It is preferable that a diagnosis can be made even during a healthy period with resting breathing.
In recent years, lung sound analysis techniques have been proposed as a safe and simple approach that can be used to evaluate airway changes in children [7, 8]. Previous reports have shown that lung sound parameters change during histamine [20] and methacholine inhalation tests [21] and that there is a strong relationship between changes in lung sound parameters and airway narrowing in asthmatic patients [22].
One major problem with these analysis methods is that lung sounds are affected by airflow [23]; however, we previously developed reliable methods that are not affected by airflow by utilizing lung sound spectrum curves [7]. Using this method, it was possible to investigate airway hyperresponsiveness and reversibility using lung sounds in children with asthma and to study the factors that cause asthma in healthy infants [12, 14, 15].
As described above, it is clear that the lung sound analysis parameters are useful biomarkers of lung function in children. However, another problem with conventional methods is that the operations of lung sound analysis are not fully automated. Before starting the lung sound analysis, two or more pediatric pulmonology experts were required to visually determine the highest frequency (Hz) at 0 dB of inspiratory sound in the lung sound spectrum of interest [7]. Although this method is sufficiently reliable to be used in clinical research, a simpler, more reliable, and unbiased method is desired.
In recent years, lung sound analysis has made remarkable progress in diagnosing respiratory diseases in children [24] and adults [25], and the use of deep learning-based AI [26, 27] or ML algorithm [28, 29] has made it even more accurate. Although these techniques have great power to support the screening and diagnosis of respiratory diseases, they are still considered inferior to the evaluations performed by specialists for the differential diagnosis of individual patients. Therefore, we developed a new system using the ML algorithm, which allows clinicians to select a desirable region in the collected lung sound samples. The system automatically selects one ideal inspiratory sound from the lung sound spectra within that region, and automatically calculates the 0 point and other lung sound parameters for each patient.
The 0 point is not a simple maximum frequency, which is a lung sound parameter up to now [7, 30], but is on a curve that is integrally calculated from the lung sound spectrum as an analysis parameter. In Study 1, intra- and inter-examiner reproducibility was satisfactory, confirming the reliability of the new analysis software program. As Study 2 demonstrated that the new parameters are not so affected by airflow, we believe that it is possible to measure them accurately and repeatedly in large-scale, prospective studies at multiple institutions, as well as in daily medical care and during infant health checkups.
Furthermore, in Study 3, which is a clinical study, it was found that a history of wheezing and diagnosis of asthma/asthma-like bronchitis can be estimated with high probability based on lung sound parameters in 3-year-old children. However, it was also shown that there is still no clear relationship between atopy and airway conditions at the age of 3 years. This appears to be a peculiarity of the airways prior to the diagnosis of asthma at three years of age.
Even in our previous lung sound analysis method, it was clear that the previous parameters, RPF75, RPF50, A3/AT, and B4/AT, reflected airway condition [7, 11, 20]. In subjects with airway constriction, such as patients with asthma, the high-pitched region of the lung sound spectrum is thought to increase even in patients who do not complain of respiratory symptoms [31, 32]. In addition, the new lung sound parameters are thought to accurately indicate airway constriction during the asymptomatic period. Being able to clearly identify the presence of an airway condition in early childhood is considered to have important implications for the infant's future [2].
For the examiner, it is not very difficult to identify the 0 point of each lung sound spectrum by visual observation of samples collected without distortion [7, 8]. However, the power of lung sounds near the 0 point of the lung sound spectrum is low, and noises tends to exist over a wide range, making accurate analysis difficult in some cases. In infants and young children, lung sounds are of low power and often contain wide-range noises from the upper respiratory tract [33]. For these reasons, a clear analysis is difficult in many cases. For many years, we have considered the use of ML and have been researching the automatic analysis of all parameters based on lung sound spectrum curves. Through this research, we determined the ideal 0 point using an ML algorithm. It was confirmed that it is possible to analyze the breath sound spectra of infants and young children more accurately.
Unlike the previous maximum frequency or F99, the new parameter is desirable based on a spectral curve that is not affected by airflow, and is an indicator with excellent sensitivity and reproducibility. Above all, it is considered groundbreaking that, by using new parameters, we were able to obtain better results than previous reports [12, 14] regarding the relationship between the results of lung sound measurements in healthy children and a history of wheezing and asthma.
Based on these results, physicians will be able to take the examination one step further and include questions about not only the history of wheezing, but also the history of allergic diseases, family history, smoking, and the indoor environment. There are many confounding factors for asthma development that have been reported. To confirm their influence, we are currently planning to use our method to conduct a large-scale, multi-center prospective study. If abnormalities in the parameters can be confirmed, it is possible to consider more advanced blood tests and image diagnosis. It is assumed that the new criteria, which consider the results of lung sound analysis in addition to existing evaluation methods [34, 35], will lead to an early diagnosis of childhood asthma.
Conclusions
Although spirometry is the gold standard for lung function testing due to its accuracy, it cannot be performed in small children [36]. However, this new method, which can be safely and easily repeated during resting breathing and can be automatically analyzed, is the preferred lung function test for infants and young children. However, based on previous studies, we understand that lung sound analyses are still experimental, and it may be difficult to implement such an analysis as a standard lung function test. The present findings suggest that it is still too early to use the results of lung sound analyses alone to evaluate asthma in children, but we believe that a lung sound analysis is a useful element to add to the medical history at this stage.
Childhood asthma is a long-term chronic disease and an important risk factor for adult-onset COPD [37, 38]. The performance of a lung sound analysis during infant period when infants visit outpatient clinics or undergo health checkups may enable the early detection and diagnosis of childhood asthma. If it were possible to prevent the onset of asthma or provide protection to prevent it from becoming more severe before 3 years of age, this would have greater significance.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available as they are pending patent application, but are available from the corresponding author on reasonable request.
Abbreviations
- ATS-DLD:
-
American Thoracic Society-Division of Lung Diseases
- AI:
-
Artificial intelligence, ML: machine learning, NMF: Non-negative matrix factorization
- AUC:
-
Area under the curve
- F99 :
-
99% Of maximum point in breath sound spectrum
- 0 point:
-
The analytical maximum point of breath sound spectrum
- RPF50p :
-
Ratio of power and frequency at 50% of the 0 point
- RPF75p :
-
Ratio of power and frequency at 75% of the 0 point
- FAP0 :
-
Analysis parameter of frequency at the 0 point
- PAP0 :
-
Analysis parameter of power at the 0 point
- AT :
-
Total area under the curve of 100 Hz to the 0 point
- A3p :
-
2/3 Of the frequency obtained by subtracting 100 Hz from the frequency of the 0 point
- B4p :
-
3/4 Of the frequency obtained by subtracting 100 Hz from the frequency of the 0 point
- A3a :
-
High-pitched third area under the curve
- B4a :
-
High-pitched forth area under the curve
- ICC:
-
Intraclass correlation coefficient
References
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. https://ginasthma.org/wp-content/uploads/2023/07/GINA-2023-Full-report-23_07_06-WMS.pdf (ginasthma.org), Accessed 5 Jan 2024.
Japanese Society of Pediatric Allergy and Clinical Immunology. Guidelines for the treatment and management of pediatric bronchial asthma 2023 (Japanese). Tokyo: Kyowa Kikaku; 2023.
McFadden ER Jr, Kiser R, DeGroot WJ. Acute bronchial asthma. Relations between clinical and physiologic manifestations. N Engl J Med. 1973;288(5):221–5.
Baughman RP, Loudon RG. Lung sound analysis for continuous evaluation of airflow obstruction in asthma. Chest. 1985;88(3):364–8.
Anderson K, Aitken S, Carter R, MacLeod JE, Moran F. Variation of breath sound and airway caliber induced by histamine challenge. Am Rev Respir Dis. 1990;141(5 pt 1):1147–50.
Oweis RJ, Abdulhay EW, Khayal A, Awad A. An alternative respiratory sounds classification system utilizing artificial neural networks. Biomed J. 2015;38(2):153–61.
Tabata H, Hirayama M, Enseki M, Nukaga M, Hirai K, Furuya H, et al. A novel method for detecting airway narrowing using breath sound spectrum analysis in children. Respir Invest. 2016;54(1):20–8.
Enseki M, Nukaga M, Tabata H, Hirai K, Matsuda S, Mochizuki H. A clinical method for detecting bronchial reversibility using a breath sound spectrum analysis in infants. Respir Invest. 2017;55(3):219–28.
Demir F, Sengur A, Bajaj V. Convolutional neural networks based efficient approach for classification of lung diseases. Health Inf Sci Syst. 2019;8(1):4.
Hafke-Dys H, Kuźnar-Kamińska B, Grzywalski T, Maciaszek A, Szarzyński K, Kociński J. Artificial intelligence approach to the monitoring of respiratory sounds in asthmatic patients. Front Physiol. 2021;12: 745635.
Tabata H, Enseki M, Nukaga M, Hirai K, Matsuda S, Furuya H, et al. Changes in the breath sound spectrum during methacholine inhalation in children with asthma. Respirology. 2018;23(2):168–75.
Shioya H, Tadaki H, Yamazaki F, Miyamoto M, Yoshihara S, Enseki M, et al. Characteristics of breath sound in infants with risk factors for asthma development. Allergol Int. 2019;68(1):90–5.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8.
Ishizu H, Shioya H, Tadaki H, Yamazaki F, Miyamoto M, Enseki M, et al. A lung sound analysis in infants with risk factors for asthma during acute respiratory infection. Pediatr Allergy Immunol Pulmonol. 2020;33(3):147–54.
Miyamoto M, Yoshihara S, Shioya H, Tadaki H, Imamura T, Enseki M, et al. Lung sound analysis in infants with risk factors for asthma development. Health Sci Rep. 2021;4(3): e379.
Smaragdis P, Brown JC. Non-negative matrix factorization for polyphonic music transcription. IEEE Workshop on Applications of Signal Processing to Audio and Acoustics, 2003 pp.177–180. https://ieeexplore.ieee.org/document/1285860.
Virtanen T. Monaural sound source separation by nonnegative matrix factorization with temporal continuity and sparseness criteria. IEEE Transactions on Audio, Speech and Language Processing 2007 15(3), pp.1066–1074. https://ieeexplore.ieee.org/document/4100700.
Brochu E, Cora VM, Freitas ND. A tutorial on Bayesian optimization of expensive cost functions, with application to active user modeling and hierarchical reinforcement learning. arXiv preprint arXiv:1012.2599, 2010. https://arxiv.org/abs/1012.2599.
Blei DM, Kucukelbir A, McAuliffe JD. Variational inference: A review for statisticians. Journal of the American statistical Association, 2017, 112.518: 859–877. https://arxiv.org/abs/1601.00670.
Malmberg LP, Sorva R, Sovijärvi AR. Frequency distribution of breath sounds as an indicator of bronchoconstriction during histamine challenge test in asthmatic children. Pediatr Pulmonol. 1994;18(3):170–7.
Imamura T, Enseki M, Murayama Y, Furuya H, Mochizuki H. Characteristics of breath sounds during methacholine-induced bronchoconstriction in children with asthma. Tokai J Exp Clin Med. 2022;47(3):125–30.
Nukaga M, Tabata H, Enseki M, Hirai K, Furuya H, Kato M, et al. Changes in the breath sound spectrum with bronchodilation in children with asthma. Respir Investig. 2018;56(5):392–8.
Sanchez I, Pasterkamp H. Tracheal sound spectra depend on body height. Am Rev Respir Dis. 1993;148(4 pt 1):1083–7.
Grzywalski T, Piecuch M, Szajek M, Bręborowicz A, Hafke-Dys H, Kociński J, et al. Practical implementation of artificial intelligence algorithms in pulmonary auscultation examination. Eur J Pediatr. 2019;178(6):883–90.
Islam MA, Bandyopadhyaya I, Bhattacharyya P, Saha G. Multichannel lung sound analysis for asthma detection. Comput Methods Progr Biomed. 2018;159:111–23.
Ntalianis V, Fakotakis ND, Nousias S, Lalos AS, Birbas M, Zacharaki EI, et al. Deep CNN sparse coding for real time inhaler sounds classification. Sensors (Basel). 2020;20(8):2363.
Zulfiqar R, Majeed F, Irfan R, Rauf HT, Benkhelifa E, Belkacem AN. Abnormal respiratory sounds classification using deep CNN through artificial noise addition. Front Med (Lausanne). 2021;8: 714811.
Park JS, Kim K, Kim JH, Choi YJ, Kim K, Suh DI. A machine learning approach to the development and prospective evaluation of a pediatric lung sound classification model. Sci Rep. 2023 13(1);1289. https://doi.org/10.1038/s41598-023-27399-5. http://www.nature.com/articles/s41598-023-27399-5.
Palaniappan R, Sundaraj K, Ahamed NU. Machine learning in lung sound analysis: A systematic review. Biocybern Biomed Eng. 2013 33(3);129–135. https://doi.org/10.1016/j.bbe.2023.07.001. http://www.sciencedirect.com/science/article/pii/S0208521613000168.
Hidalgo HA, Wegmann MJ, Waring WW. Frequency spectra of normal breath sounds in childhood. Chest. 1991;100(4):999–1002.
Imamura T, Enseki M, Furuya H, Niimura F, Mochizuki H. Changes in the breath sound spectrum with bronchodilator inhalation in asthmatic children with long-term management. Tokai J Exp Clin Med. 2020;45(1):24–30.
Sakama T, Ichinose M, Obara T, Shibata M, Kagawa T, Takakura H, et al. Effect of wheeze and lung function on lung sound parameters in children with asthma. Allergol Int. 2023;72(4):545–50.
Kagawa T, Imamura T, Enseki M, Tabata H, Furuya H, Niimura F, et al. Effect of inspiratory flow on breath sound analysis in children with asthma. Arerugi (Japanese). 2020;69(3):184–91.
Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4 pt 1):1403–6.
Guilbert TW, Morgan WJ, Krawiec M, Lemanske RF Jr, Sorkness C, Szefler SJ, et al. The prevention of early asthma in kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004;25(3):286–310.
Mochizuki H, Hirai K, Tabata H. Forced oscillation technique and childhood asthma. Allergol Int. 2012;61(3):373–83.
McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374(19):1842–52.
Berry CE, Billheimer D, Jenkins IC, Lu ZJ, Stern DA, Gerald LB, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 2016;194(5):607–12.
Acknowledgements
We would like to thank the city officials of Isehara City and Hachioji City for their cooperation in the implementation of the questionnaires for infant health checkups.
Funding
This study was supported by the Environmental Restoration and Conservation Agency of Japan in 2009–2019, No. 1–1.
Author information
Authors and Affiliations
Contributions
HM, MI and HN conceptualized the study, drafted the initial manuscript, and performed all analyses. KH, TO and KS contributed to data collection. HF and FN performed the statistical analysis and critically reviewed and revised the manuscript. All authors read and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In these three studies, written informed consent of each subject was obtained from a parent and/or their legal guardian and approval from the institutional review board of Tokai University Hospital was obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mochizuki, H., Hirai, K., Furuya, H. et al. The analysis of lung sounds in infants and children with a history of wheezing/asthma using an automatic procedure. BMC Pulm Med 24, 394 (2024). https://doi.org/10.1186/s12890-024-03210-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03210-7