Abstract
Background
What currently appears to be irreversible axonal loss in normal appearing white matter, measured by proton magnetic resonance spectroscopy is of great interest in the study of Multiple Sclerosis. Our aim is to determine the axonal damage in normal appearing white matter measured by magnetic resonance spectroscopy and to correlate this with the functional disability measured by Multiple Sclerosis Functional Composite scale, Neurological Rating Scale, Ambulation Index scale, and Expanded Disability Scale Score.
Methods
Thirty one patients (9 male and 22 female) with relapsing remitting Multiple Sclerosis and a Kurtzke Expanded Disability Scale Score of 0–5.5 were recruited from four hospitals in Andalusia, Spain and included in the study. Magnetic resonance spectroscopy scans and neurological disability assessments were performed the same day.
Results
A statistically significant correlation was found (r = -0.38 p < 0.05) between disability (measured by Expanded Disability Scale Score) and N-Acetyl Aspartate (NAA/Cr ratio) levels in normal appearing white matter in these patients. No correlation was found between the NAA/Cr ratio and disability measured by any of the other disability assessment scales.
Conclusions
There is correlation between disability (measured by Expanded Disability Scale Score) and the NAA/Cr ratio in normal appearing white matter. The lack of correlation between the NAA/Cr ratio and the Multiple Sclerosis Functional Composite score indicates that the Multiple Sclerosis Functional Composite is not able to measure irreversible disability and would be more useful as a marker in stages where axonal damage is not a predominant factor.
Similar content being viewed by others
Background
Multiple sclerosis (MS) is a chronic disease of the central nervous system characterised by multicentral inflammation and myelin destruction. Although axonal pathology in Multiple Sclerosis (MS) has been recognized since the initial description of Charcot [1], emphasis generally has been placed on the relative sparing of axons in traversing lesions. Recent pathology studies [2–5] have stressed the importance of axonal pathology in MS, in addition to demyelination, in lesions and in Normal Appearing White Matter (NAWM).
Magnetic resonance imaging (MRI) is now widely used for diagnosing MS and detecting clinically silent lesions in clinical trials on MS treatment. However, correlations found between conventional techniques, such as T2-weighted lesion load, and disability, are weak or absent [6–8] and therefore, neither the magnetic resonance techniques used so far nor clinical measurements are gold standard to assess disease or disability, in vivo. This reflects the inability of conventional MRI to accurately depict the pathology of MS as regards to histopathologic heterogeneity of MS lesions and the pathological processes occurring in the NAWM [9]. These limitations may account for the weak correlation between disability and MRI findings in patients with MS.
Clinical measurements used so far lack the accuracy to demonstrate small changes, show only moderate reproducibility and therefore do not fulfil requirements for being reliable outcome assessment measures [10–12] Assessment of clinical disability began to improve with use of the Multiple Sclerosis Functional Composite (MSFC). This scale has some advantages over the Expanded Disability Status Scale (EDSS) [13] as it is a more sensitive and reproducible outcome measure, and covers more independent neurological domains [14–16]. In addition MSFC correlates better than EDSS with T1 and T2 weighted lesion volume (LV) [16].
Alteration of NAWM is of great importance because its true patho-physiological significance is not completely understood. Decrease in N-Acetyl Aspartate (NAA) has been used as an marker of axonal damage or loss that presumably appears secondary to inflammation or demyelination [5, 17], although primary axonal damage is not excluded [3]. The limitation of T2-weighted MRI, in delineating tissue damage occurring in CNS, can be partially overcome by the use of other MR modalities as hypointense lesions load of T1-weighted images [18, 19], Magnetization Transfer Imaging (MTI), Diffusion Tensor Imaging (DTI) [20–24], and Proton Magnetic Resonance Spectroscopy (H-MRS).
Proton magnetic resonance spectroscopy (H-MRS) is a technique that has the potential to detect axonal loss non-invasively. The normal proton spectrum is dominated by N-acetyl derived groups (NA). The predominant component of the NA peak is N-acetyl aspartate (NAA) [25], an amino acid metabolite which has been shown to be contained almost exclusively within neurons [26]. A loss of neurons would thus predict a persistent reduction in the levels of NAA. Such a reduction has been reproducibly demonstrated in several diseases characterised by neuronal loss [27–29]. There are now many studies in the literature that report a reduction of NAA from acute lesions [30–33], in chronic multiple sclerosis lesions [30–34], in areas of normal appearing white matter [32, 35, 36], and form grey matter [37–40]. Furthermore, the degree of NAA reduction seems to be most marked in those patient groups with greatest neurological disability [41–43].
In this study we focus on the axonal loss in NAWM measured by H-MRS and attempt to determine a correlation between this damage and the functional disability measured by Multiple Sclerosis Functional Composite scale (MSFC) [44], Neurological Rating Scale (NRS) [45], Ambulation Index scale (AI), and Expanded Disability Scale Score (EDSS) [13].
Methods
Population studied
A total of 31 patients (9 male and 22 female) diagnosed with MS according to Poser [46] criteria and who had had at least two episodes in the previous two years, were included in the study. Patients were from four hospitals in Andalusia, Spain (Hospital Universitario Virgen Macarena, Seville; Hospital Puerta del Mar, Cádiz; Hospital San Cecilio, Granada; and Hospital Ciudad de Jaén, Jaén). These patients had been previously diagnosed with RRMS [47] and had an EDSS of 0 to 5.5 as an inclusion criteria. None of the patients had been previously treated with an immunosuppressive agent or an immunomodulating agent or corticosteroids within the previous month.
Prior to H-MRS examination each patient's disability was measured using MSFC scale [44], NRS [45], AI scale and EDSS [13]. The H-MRS was performed the same day as the neurological examination by the same neuroradiologist at the same center using the same MRI scanner and following the same protocol. The neuroradiologist was blinded to the neurological examination results and all the neurologists were blinded to the MRS results.
This was a multicentre, cross-sectional study within an ongoing prospective, longitudinal study. The study was approved by the Ethics Committees of the participating hospitals and informed consent was obtained from all the patients.
At the beginning of the study MRI and H-MRS were also performed on 10 healthy, age matched control subjects with not known systemic or neurological disease by the same neuroradiologist using the same MRI scanner and following the same protocol as well.
Magnetic resonance measurements
H-MRS and conventional MRI examinations of the brain were obtained in a single session for each patient using the same 1.5 T scanner (Signa Horizon, GE, Mass).
In the conventional MRI examinations a sagittal T2 weighted sequence (TR = 4000; TE = 109; slice thickness = 4 mm; gap = 1 mm; 13 slices) was acquired. These slices were used to obtain transverse and coronal proton density weighted and T2 weighted images (TR = 3420; TE = 13.5/121; slice thickness = 5 mm; gap = 2 mm; 30 slices) and a coronal T1 weighted sequence (TR = 400; TE = 15; slice thickness = 5 mm.; gap = 2 mm; 15 slices).
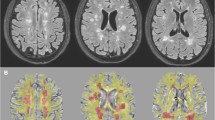
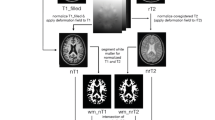
These conventional MR images were used to position a spectroscopic volume of interest (VOI) in one or two areas with demyelinating lesions and one area of normal appearing white matter in MS patients and in one area of normal white matter in the healthy control group. Spectroscopy volume selection was performed using a PROBE-P sequence (PROBE/SV: PROton Brain Examination/Single Volume), a version of the PRESS sequence (Point Resolved Spectroscopy), which is a double spin echo that uses a 90°-180°-180° pulse sequence to acquire the proton spectra (TR = 2000; TE = 37; field of vision = 22 × 16 cm). Each VOI measured 20 mm anteroposterior × 20 mm craneocaudal × 20 mm left-right, obtaining a brain tissue volume of 8 cc. (see Figure 1) VOIs were selected manually by the only neuroradiologist of the study (P.P.) and they were kept constant in size and position in the follow up examinations. Magnetic field homogeneity was optimized to a line width of about 5 Hz over the VOI using the proton signal from water. Water suppression was achieved by a selective inversion of the water resonance prior to volume selection using adiabatic inversion pulse and adjustment of the waiting time so that the spectrum was acquired when the water signal passed through zero.
Clinical assessments
MS patients were examined to determine, the same day, their EDSS [13], Neurological Rating Scale [45], Ambulation Index, 25 foot timed walk test (TWT) [14], nine hole peg test (9 HPT) [48], and paced auditory serial addition test (PASAT 3 s interval) [49] scores. The average of two trials for the TWT and average of four trials of the 9 HPT [44] were calculated and, together with PASAT and Multiple Sclerosis Functional Composite (MSFC) [44] scores were used in further analyses. All neurologists are trained to use Kurtzke's scale, AI scale, NRS, and MSFC scale, but they were retrained to use these scales by the same person and on the same day.
Statistical analysis
Data was entered into the SAS programme and a descriptive and analytical statistical analysis was carried out.
Absolute frequencies and percentages were used for the description of the categorical variables using the SAS/BASE PROC TABULATE programme (SAS Software release 6.12; Cary, NC, 1989 to 1996).
The mean, standard deviation (SD), median, interquartile range and the upper and lower quartiles were used for the description of the numerical variables, using the SAS/BASE PROC TABULATE programme.
Correlation among the different variables was determined by Spearman's rank coefficient (considering the variables as ordinal) and Pearson's coefficient (considering the variables as quantitative). A comparison between NAWM area in MS patients and controls and between White Matter Lesion (WML) and NAWM within the patient group was performed.
To calculate the degree of association and agreement amongst the main indexes of functional capacity and magnetic resonance, the variables were divided and the median was taken as a reference. The Chi-squared test was then applied.
Consistency of the internal measurements was determined by dividing the total patient sample into two sub-samples, each with half of the patients.
Results
Subject demographics and clinical parameters
Table 1 reports the demographics and clinical characteristics of the MS Patients. The mean number of relapses in the previous two years was 2.6 ± 0.6 (range 2–5).
The healthy control group was made up of 10 patients (5 male and 5 female) with a mean age of 42.5 years ± 24 years (median 42, range 14 – 74).
Tissue metabolite ratio
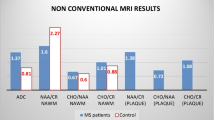
Table 2 shows the metabolite value of control subjects and MS patients (in NAWM and in WML). MS patients showed lower values of NAA compared with the controls. No significant differences were found between the NAA, Cr, Cho peak signal intensities of patients and control subjects. Table 2 shows the metabolite ratio of control subjects and MS patients (in NAWM and in WML). The reduction of NAA in MS patients without significant differences in Cr induced lower NAA/Cr in patients than in controls (p > 0.05). We found significant differences between the Cho/Cr metabolite ratio in WML and NAWM.
Correlation between tissue metabolite concentration and clinical parameters
The objective of the study was to determine the correlation among the different disability indexes and the NAA/Cr ratio, in order to ascertain if this technique is a good disability marker.
There is a clear correlation between the EDSS and NRS disability scores, the correlation coefficient (r) being -0.79 (p < 0.0001). A clear correlation between the EDSS and AI scores (r = 0.55; p < 0.001) and between the NRS and the AI scores (r = 0.63; p < 0.0001) was also found. Evaluation of other correlations amongst the EDSS, NRS and AI disability tests with the MSFC are given in Table 3.
There is a correlation among the two H-MRS values, NAWM and White Matter Lesion (the correlation coefficient (r) is 0.47, p < 0.01). However the correlation between the EDSS and the H-MRS in NAWM value was low (r = -0.3; p = 0.056). This latter correlation was calculated using Pearson's test which is more sensitive than the former one. The coefficient was -0.38 (p < 0.03) which is statistically significant. We did not find correlation among the two NAA/Cr values, in NAWM and in WML, with the others disability scales.
Correlations between metabolite ratio and measures of disability were significant too for NAWM mI/Cr with NRS (r = -0.468; p = 0.007), NAWM mI/Cr with AI (r = 0.439; p = 0.01), WML Cho/Cr with EDSS (r = -0.4, p = 0.02), WML Cho/Cr with NRS (r = 0.37, p = 0.04) and WML Cho/Cr with AI (r = -0.42, p = 0.01). We have found as well a non linear correlation between metabolite ratio and MSFC score: NAWM mI/Cr with TWT (r = -0.72, p = 0.0001, cubic correlation), NAWM mI/Cr with 9 HPT (r = -0.51, p = 0.01, cubic correlation), NAWM Cho/Cr with TWT (r = 0.42, p = 0.02, inverse correlation).
Internal consistency of measurements
To ensure the internal consistency of the measurements the patient samples were sub-divided and the stability of the parameters that determine the measurements was checked. It was found that on dividing the samples both the mean and the median EDSSs varied greatly, and therefore vary with respect to the overall mean.
The mean and median H-MRS values were very similar, which leads us to believe that this is a stable measurement.
Discussion
This study demonstrates that patients' disability correlates with levels of NAA, a marker of axonal loss. However, this correlation is only seen when the EDSS but not the MSFC is used. We have found significant correlations between myoinositol (a gliosis marker) with NRS, AI and MSFC score (TWT, 9 HPT). Our data also shows that there is a weak correlation among the different disability scales studied, as also occurs among the values measured with H-MRS.
Markers of axonal loss and gliosis
In our study we found that, from the early stages of the disease, NAA:Cr ratios determined by H-MRS, in NAWM (1.67 ± 0.18) and White Matter Lesions (1.60 ± 0.22) were lower in MS subjects than in healthy controls. These results coincide with others published earlier, using similar techniques [50–54].
Since it was first described, MS has been considered a demyelinating disease of the central nervous system, and until recently axonal loss had not been studied in depth. The N-Acetyl Aspartate levels detected by H-MRS could provide a precise marker of axonal loss. [30–32, 55–58]
Although the function of myoinositol (mI) is uncertain, we know that it is involved in the polyphosphoinositol second messenger cascade [71] and that it is relatively concentrated in glial cells when compared with neurones as was shown by multinuclear spectroscopy techniques performed in rats [40, 52]. So an increase in mI may reflect neuroglial homeostasis and gliosis. [52]
Clinical outcome and H-MRS parameters
It has been suggested by various authors who have used H-MRS [33, 35, 65–67] that the weakness of correlations between MRI lesions and disability was due to abnormalities in the NAWM. In a longitudinal study in 11 RRMS patients, De Stefano and colleagues [9, 68] demonstrated a significant correlation between changes in NAA/Cr ratio in NAWM and changes in disability score (measured by EDSS), concluding that axonal damage in NAWM is important in the understanding of chronic disability in MS. Twenty nine patients with MS, 18 with secondary progressive MS and 11 with RRMS (EDSS: median of 5, range 3–6.5) were included in this study. Unlike the De Stefano et al. study we did not evaluate the change in EDSS but the baseline EDSS in patients whose disease was less developed and in a more uniform patient group. Our study included 31 patients with RRMS with a lower EDSS (median = 2, range of 1–5.5) and a short disease duration (median 4 years). This leads us to believe that perhaps an NAA decrease already exists in normal appearing white matter since early stages of the disease.
In our study the correlation between NAA/Cr in NAWM and EDSS was found to be statistically significant (r = -0.38 (p < 0.03)). Tourbah et al [52] found a negative and significant correlation between NAA/Cr in NAWM and EDSS (r = -0.39). Davie [43] found a correlation of r = 0.364 in lesions in MS patients. De Stefano et al [59], and Mainero et al [69] found similar correlations in recent studies (r = -0.5), and this latter author point out that it is the best correlation found in multiparametric MRI. They agree with us therefore in observing that the NAA/Cr value is which best correlates with the Kurtzke EDSS.
Bonneville et al [70] did not report this correlation between NAA levels and EDSS. However, they used an overall NAA measurement that does not distinguish between NAWM and T1 and T2-weighted lesion volumes. This supports the theory that NAA behaves differently in damaged areas in T2 and T1 probably because they include areas with demyelination and remyelination inflammation in which NAA recovery is possible (Table 4).
Discrepancy among the results of H-MRS studies in MS could be due to the different patient selection and inclusion criteria, the different techniques used, the different area selected and the difference in the size of selected volume of interest [71].
What it is new in our study is that it was designed to measure axonal loss in relation to a multidimensional measure of disability (MSFC), and it showed no correlations between axonal damage (measured by NAA/Cr ratio) and the MSFC in patients in early stages of the disease. This suggests that the MSFC, which measures disability, is more closely associated with MRI markers that measure global disease burden, lesion burden and gliosis than with axonal loss. Thus, we agree with Kalkers et al [72] that a major part of the variability in the MSFC still remains unidentified. In this article, Kalkers et al found no significant correlation between ventricular fraction (VF) of MS patients with short disease duration (≤5 years) and MSFC.
The relationship between clinical disability and NAWM mI implies that glial proliferation in NAWM is associated with a negative effect on clinical function (either NRS or AI or MSFC (TWT, 9 HPT)) something we have not found between clinical disability and WML mI. All these findings support the idea that glial proliferation, or another pathological process leading to it, has a significant role in disability [40].
Study limitations
In this study H-MRS results are expressed as NAA/Cr ratio. The resonance intensity of intravoxel Cr has been widely used as an internal standard in H-MRS studies in vivo, as it is relatively equally present in all brain cells and tends to be stable in non-acute pathology [33]. We agree with Arnold et al [59] that though changes in apparent brain Cr levels have been reported in MS in recent H-MRS studies attempting absolute quantitation, all current quantitative approaches have considerable limitations when applied to clinical studies. Discrepancies are found in the results of studies in MS patients which report increases, decreases and absence of Cr changes in MRI lesions and NAWM [35, 43, 60–63]. A study using high resolution H-MRS in vitro, which does not suffer from the limitations of in vivo quantification, has demonstrated that Cr does not change in normal appearing tissue of the brains of MS patients [64]
Because of the short duration of the disease (median 4 years) and the low lesional load in T2 of our patients it was impossible to choose large T2 weighted lesions without NAWM included in the VOI to minimise the partial volume effects. This is the reason why the mean NAA/Cr ratio of T2 Weighted Lesions is close to the mean NAA/Cr ratio in NAWM.
Conclusions
In conclusion, Magnetic Resonance Spectroscopy is a tool that may play an important part in studies on natural history of the disease and the assessment of immunosuppressive or immunomodulatory treatment in clinical trials [42, 73]. It provides important data on axonal degeneration which is the subject of this study. We have clearly demonstrated a correlation between an MRS measure and EDSS. EDSS seems to reflect the existence of irreversible disability probably related to axonal degeneration, whereas the MSFC does not appear to do so. MSFC is probably a more sensitive measure of both global disease burden and lesion burden than axonal loss. This will have to be determined in longitudinal studies.
References
Charcot M: Histologie de la sclerose en plaques. Gaz Hosp. 1868, 141: 554-8.
Ferguson B, Matyszak MK, Esiri MM, Perry VH: Axonal damage in acute multiple sclerosis lesions. Brain. 1997, 120 (pt 3): 393-399. 10.1093/brain/120.3.393.
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bo L: Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998, 338: 278-285. 10.1056/NEJM199801293380502.
Waxman SG: Demyelinating diseases – new pathological insights, new therapeutic targets. N Engl J Med. 1998, 338 (5): 323-325. 10.1056/NEJM199801293380510.
McDonald WI: Rachelle Fishman – Matthew Moore Lecture. The pathological and clinical dynamics of multiple sclerosis. J Neuropathol Exp Neurol. 1994, 53: 338-343.
Miller DH, Albert PS, Barkhof F, Francis G, Frank JA, Hodgkinson S, Lublin FD, Paty DW, Reingold SC, Simon J: Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. US National MS Society Task Force. Ann Neurol. 1996, 39: 6-16.
Filippi M, Paty DW, Kappos L, Barkhof F, Compston DA, Thompson AJ, Zhao GJ, Wiles CM, McDonald WI, Miller DH: Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: a follow-up study. Neurology. 1995, 45: 255-260.
Thorpe JW, Barker GJ, Jones SJ, Moseley I, Losseff N, MacManus DG, Webb S, Mortimer C, Plummer DL, Tofts PS, et al: Magnetisation transfer ratios and transverse magnetisation decay curves in optic neuritis: correlation with clinical findings and electrophysiology. J Neurol Neurosurg Psychiatry. 1995, 59: 487-492.
Fu L, Matthews PM, De Stefano N, Worsley KJ, Narayanan S, Francis GS, Antel JP, Wolfson C, Arnold DL: Imaging axonal damage of normal-appearing white matter in multiple sclerosis. Brain. 1998, 121: 103-13. 10.1093/brain/121.1.103.
Rudick R, Antel J, Confavreux C, Cutter G, Ellison G, Fischer J, Lublin F, Miller A, Petkau J, Rao S, Reingold S, Syndulko K, Thompson A, Wallenberg J, Weinshenker B, Willoughby E: Clinical Outcomes assessment in multiple sclerosis. Ann Neurol. 1996, 40: 469-479.
Whitaker JN, McFarland HF, Rudge P, Reingold SC: Outcomes assessments in multiple sclerosis clinical trials: a critical analysis. Mult Scler. 1995, 1: 37-47.
Fischer JS, Rudick RA, Cutter GR, Reingold SC: The Multiple Sclerosis Functional Composite measure (MSFC): an integrated approach to MS clinical outcome assessment. NMSS Clinical Outcomes Assessments Task Force. Mult Scler. 1999, 5: 244-250. 10.1191/135245899678846168.
Kurtzke JF: Rating neurologic impairment in Multiple Sclerosis (EDSS). Neurology. 1983, 33: 1444-52.
Cutter GR, Baier ML, Rudick RA, Cookfair DL, Fischer JS, Petkau J, Syndulko K, Weinshenker BG, Antel JP, Confavreux C, Ellison GW, Lublin F, Miller AE, Rao SM, Reingold S, Thompson A, Willoughby E: Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999, 122: 871-882. 10.1093/brain/122.5.871.
Kalkers NF, De Groot V, Lazeron RHC, Killestein J, Ader HJ, Barkhof F, Lankhorst GJ, Polman CH: MS functional composite: relation to disease phenotype and disability strata. Neurology. 2000, 54: 1233-1239.
Kalkers NF, Bergers L, De Groot V, Lazeron RH, van Walderveen MA, Uitdehaag BM, Polman CH, Barkhof F: Concurrent validity of the MS functional composite using MRI as a biological disease marker. Neurology. 2001, 56: 215-219.
Barnes D, Munro PM, Youl BD, Prineas JW, McDonald WI: The longstanding MS lesion. A quantitative MRI and electron microscopic study. Brain. 1991, 114: 1271-80.
Truyen L, van Waesberghe JHTM, van Walderveen MAA, van Oosten BW, Polman CH, Hommes OR, Ader HJ, Barkhof F: Accumulation of hypointense lesions (Black Holes) on T1 spin echo MRI correlates with disease progressión in multiple sclerosis. Neurology. 1996, 47: 1469-1476.
van Walderveen MAA, Kamphorst W, Scheltens PH, van Waesberghe JH, Ravid R, Valk J, Polman CH, Barkhof F: Histopathologic correlate of hypointense lesions on T1 weighted spin echoMRI in multiple sclerosis. Neurology. 1998, 50: 1282-1288.
Filippi M, Iannucci G, Tortorella C, Minicucci L, Horsfield MA, Colombo B, Sormani MP, Comi G: Comparision of MS clinical phenotypesusing conventional and magnetization transfer MRI. Neurology. 1999, 52: 588-594.
van Waesberghe JHTM, Kamphorst W, De Groot CJ, van Walderveen MA, Castelijns JA, Ravid R, Lycklama a Nijeholt GJ, van der Valk P, Polman CH, Thompson AJ, Barkhof F: Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999, 46: 747-754. 10.1002/1531-8249(199911)46:5<747::AID-ANA10>3.3.CO;2-W.
Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH: Diffusion tensor imaging of lesions and normal appearing white matter in multiple sclerosis. Neurology. 1999, 52: 1626-1632.
Cercignani M, Iannucci G, Rocca MA, Comi G, Horsfield MA, Filippi M: Pathologic damage in MS assessed by Diffusión weighted and magnetization transfer MRI. Neurology. 2000, 54: 1139-1144.
Filippi M, Iannucci G, Cercignani M, Assunta Rocca M, Pratesi A, Comi G: A quantitative study of water diffusion in MS lesions and NAWM using echo planar imaging. Neurology. 2000, 54: 1139-1144.
Birken DL, Oldendorf WH: N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev. 1989, 13: 23-31.
Urenjak J, Williams SR, Gadian DG, Noble M: Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993, 13: 981-9.
Gideon P, Henriksen O, Sperling B, Christiansen P, Olsen TS, Jorgensen HS, Arlien-Soborg P: Early time course of N acetylaspartate, creatine and phosphocreatine, and compounds containing choline in the brain after acute stroke. A proton magnetic resonance spectroscopy study. Stroke. 1992, 23: 1566-72.
Van der knaap MS, Van der Grond J, Luyten PR, den Hollander JA, Nauta JJ, Valk J: 1H and 31P magnetic resonance spectroscopy of brain degenerative cerebral disorders. Ann Neurol. 1992, 31: 202-11.
Chong WK, Sweeney B, Wilkinson ID, Paley M, Hall-Craggs MA, Kendall BE, Shepard JK, Beecham M, Miller RF, Weller IV, et al: Proton spectroscopy of the brain in HIV infection: correlation withclinical, immunologic and MR imaging findings. Radiology. 1993, 188: 119-24.
Matthews PM, Francis G, Antel J, Arnold DL: Proton magnetic resonance spectroscopy for metabolic characterisation of plaques in multiple sclerosis. Neurology. 1991, 41: 1251-6.
Miller DH, Austin SJ, Connelly A, Youl BD, Gadian DG, McDonald WI: Proton magnetic resonance spectroscopy of an acute and chronic lesion in multiple sclerosis [letter]. Lancet. 1991, 337: 58-9. 10.1016/0140-6736(91)93383-K.
Davie CA, Hawkins CP, Barker GJ, Brennan A, Tofts PS, Miller DH, McDonald WI: Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain. 1994, 117: 49-58.
De Stefano N, Matthews PM, Antel JP, Preul M, Francis G, Arnold D: Chemical pathology of acute demyelinating lesions and its correlation with disability. Ann Neurol. 1995, 38: 901-9.
Arnold DL, Matthews PM, Francis G, Antel J: Proton magnetic resonance spectroscopy of human brain in vivo in the evaluation of multiple sclerosis:assessment of the load of disease. Magn Reson Med. 1990, 14: 154-9.
Husted CA, Goodin S, Hugg JW, Maudsley AA, Tsuruda JS, de Bie SH, Fein G, Matson GB, Weiner MW: Biochemical alterations in multiple sclerosis lesions and normal appearing white matter detected by in vivo 31P and 1H spectroscopic imaging. Ann Neurol. 1994, 36: 157-65.
Narayanan S, Fu L, Pioro E, De Stefano N, Collins DL, Francis GS, Antel JP, Matthews PM, Arnold DL: Imaging of axonal damage in multiple sclerosis: spatial distribution of magnetic resonance imaging lesions. Ann Neurol. 1997, 41: 385-91.
Kapeller P, McLean MA, Griffin CM, Chard D, Parker GJ, Barker GJ, Thompson AJ, Miller DH: Preliminary evidence for neuronal damage in cortical grey matter and normal appearing white matter in short duration relapsing remitting multiple sclerosis: a quantitative MR spectroscopic imaging study. J Neurol. 2001, 248: 131-138. 10.1007/s004150170248.
Kapeller P, Brex PA, Chard D, Dalton C, Griffin CM, McLean MA, Parker GJ, Thompson AJ, Miller DH: Quantitative H-MRS imaging 14 year after presenting with a clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler. 2002, 8 (3): 207-210. 10.1191/1352458502ms822oa.
Sarchielli P, Presciutti O, Tarducci R, Gobbi G, Alberti A, Pelliccioli GP, Chiarini P, Gallai V: Localized magnetic resonance spectroscopy in mainly cortical grey matter of patients with multiple sclerosis. J Neurol. 2002, 249 (7): 902-910. 10.1007/s00415-002-0758-5.
Chard D, Griffin CM, McLean MA, Kapeller P, Kapoor R, Thompson AJ, Miller DH: Brain Metabolites changes in cortical grey and normal appearing white matter in clinically early relapsing remitting multiple sclerosis. Brain. 2002, 125: 2342-2352. 10.1093/brain/awf240.
Davie CA, Barker GJ, Webb S, Tofts PS, Thompson AJ, Harding AE, McDonald WI, Miller DH: Persistent functional deficit in multiple sclerosis and autosomal dominant cerebellar ataxia is associated with axon loss. Brain. 1995, 118: 1583-92.
Matthews PM, Pioro E, Narayanan S, De Stefano N, Fu L, Francis G, Antel J, Wolfson C, Arnold DL: Assessment of lesion pathology in multiple sclerosis using quantitative MRI morphometry and magnetic resonance spectroscopy. Brain. 1996, 119: 715-22.
Davie CA, Barker GJ, Thompson AJ, Tofts PS, McDonald WI, Miller DH: 1H Magnetic resonance spectroscopy of chronic cerebral white matter lesions and normal appearing white matter in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1997, 63: 736-42.
Fischer JS, Jak AJ, Kniker JE, Rudick RA, Cutter G: Administration and scoring manual for the multiple sclerosis functional composite measure. (MSFC). 1999, New York: Demos
Sipe JC, Knobler RL, Braheny SL, Rice GP, Panitch HS, Oldstone MB: A neurological rating scale (NRS) for use in multiple sclerosis. Neurology. 1984, 34: 1368-1372.
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW: New diagnostic criteria for MS: guideline for research protocols. Ann Neurol. 1983, 13: 227-31.
Lublin FD, Reingold SC: Defining the clinical course of Multiple Sclerosis: results of an international survey. National Muntiple Sclerosis Society (USA) Advisory Committee on Clinical trials of New Agents in Multiple Sclerosis. Neurology. 1996, 46: 907-911.
Goodkin DE, Hertsgaard D, Seminary J: Upper extremity function in multiple sclerosis: improving assessment sensitivity with box and block and nine hole peg test. Arch Phys Med Rehabil. 1988, 69: 850-4.
Gronwell DMA: Paced auditory serial addition task: a measure of recovery from concussion. Percept Mot Skills. 1977, 44: 367-73.
Rooney WD, Goodkin DE, Schuff N, Meyerhoff DJ, Norman D, Weiner MW: H-MRS of normal appearing white matter in multiple sclerosis. Mult Scler. 1997, 3 (4): 231-237.
Leary SM, Davie CA, Parker GJ, Stevenson VL, Wang L, Barker GJ, Miller DH, Thompson AJ: H magnetic resonance spectroscopy of normal appearing white matter in primary progresive multiple sclerosis. J Neurol. 1999, 246 (11): 1023-1026. 10.1007/s004150050507.
Tourbah A, Stievenart JL, Gout O, Fontaine B, Liblau R, Lubetzki C: Localized proton magnetic resonance spectroscopy in relapsing remitting versus secondary progressive multiple sclerosis. Neurology. 1999, 53 (5): 1091-1097.
Tartaglia MC, Narayanan S, De Stefano N, Arnaoutelis R, Antel SB, Francis SJ, Santos AC, Lapierre Y, Arnold DL: Choline is increased in pre lesional normal appearing white matter in multiple. J Neurol. 2002, 249 (10): 1382-1390. 10.1007/s00415-002-0846-6.
Enzinger C, Ropele S, Strasser-Fuchs S, Kapeller P, Schmidt H, Poltrum B, Schmidt R, Hartung HP, Fazekas F: Lower levels of N-Acetilaspartate in Multiple Slerosis Patients with the Apolipoprotein E 4 Allele. Arch Neurol. 2003, 60: 65-70.
Wolinsky JS, Narayana PA, Fenstermacher MJ: Proton magnetic resonance spectroscopy in multiple sclerosis. Neurology. 1990, 40: 1764-9.
Arnold DL, Matthews PM, Francis GS, O Connor J, Antel JP: Proton Magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol. 1992, 31: 35-41.
Van Hecke P, Marcal G, Johannik K, Demaerel P, Wilms G, Carton H, Baert AL: Human brain proton localized NMR spectroscopy in multiple sclerosis. Magn Reson Med. 1991, 18: 199-206.
Grossman RI, Lenkinski RE, Ramer KN, Gonzalez-Scarano F, Cohen JA: MR Proton spectroscopy in multiple sclerosis. Am J Neuroradiol. 1992, 13: 1535-43.
De Stefano N, Narayanan S, Francis GS, Arnaoutelis R, Tartaglia MC, Antel JP: Evidence of axonal damege in the early stages of Múltiple sclerosis and its relevance to disability. Arch Neurol. 2001, 58: 65-70. 10.1001/archneur.58.1.65.
Narayana PA, Doyle TJ, Lai D, Wolinsky JS: Serial proton magnetic resonance spectroscopic imaging, contrast-enhanced magnetic resonance imaging, and quantitative lesion volumetry in multiple sclerosis. Ann Neurol. 1998, 43: 56-71.
Pan JW, Hetherington HP, Vaughan JT, Mitchell G, Pohost GM, Whitaker JN: Evaluation of multiple sclerosis by spectroscopic imaging at 4.1 T. Magn Reson Med. 1996, 36: 72-77.
Sarchielli P, Presciutti O, Pelliccioli GP, Tarducci R, Gobbi G, Chiarini P, Alberti A, Vicinanza F, Gallai V: Absolute quantification of brain metabolites by Proton Magnetic resonance spectroscopy in normal appearing white matter of multiple sclerosis patients. Brain. 1999, 122 (pt 3): 513-521. 10.1093/brain/122.3.513.
van Walderveen MA, Barkhof F, Pouwels PJ, van SR, Polman CH, Castelijns JA: Neuronal damage in T1 hypointense multiple sclerosis lesions demostrated in vivo using Proton Magnetic resonance spectroscopy. Ann Neurol. 1999, 46: 79-87. 10.1002/1531-8249(199907)46:1<79::AID-ANA12>3.3.CO;2-0.
Davies SE, Newcombe J, Williams SR, McDonald WI, ClarK JB: Hihg resolution proton MR spectroscopy of multiple sclerosis lesions. J Neurochem. 1995, 64: 742-748.
Barbosa S, Blumhardt LD, Roberts N, Lock T, Edwards RHT: Magnetic resonance relaxation time mapping in multiple sclerosis: normal appearing white matter and the "invisible" lesion load. Magn Reson Imaging. 1994, 12: 33-42. 10.1016/0730-725X(94)92350-7.
Filippi M, Campi A, Dousset V, Baratti C, Martinelli V, Canal N, Scotti G, Comi G: A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology. 1995, 45: 478-482.
Tourbah A, Stievenart JL, Iba-Zizen MT, Zannoli G, Lyon-Caen O, Cabanis EA: In vivo localized NMR proton spectroscopy of normal appearing white matter in patients with multiple sclerosis. J Neuroradiol. 1996, 23: 49-55.
De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS, Antel JP, Arnold DL: Axonal damage correlates with disability in patients with relapsing remitting multiple sclerosis. Brain. 1998, 121: 1469-77. 10.1093/brain/121.8.1469.
Mainero C, De Stefano N, Iannucci G, Sormani MP, Guidi L, Federico A, Bartolozzi ML, Comi G, Filippi M: Correlates of MS disability assessed in vivo using aggregates of MR quantities. Neurology. 2001, 56: 1331-1334.
Bonneville F, Moriarty DM, Belina SY, Babb JS, Grossman RI, Gonen O: Whole-Brain N-Acetylaspartate concentration: correlation with T2-weighted lesion volume and Expanded Disability Satatus Scale Score in case of Relapsing-Remiting Multiple Sclerosis. Am J Neuroradiol. 2002, 23: 371-375.
Sarchielli P, Presciutti O, Tarducci R, Gobbi G, Alberti A, Pelliccioli GP, Orlacchio A, Gallai V: H-MRS in patients with multiple sclerosis undergoing treatment with Interferon B 1a; results of a preliminary study. J Neurol Neurosurg Psychiatry. 1998, 64: 204-212.
Kalkers NF, Bergers E, Castelijns JA, van Walderveen MA, Bot JC, Ader HJ, Polman CH, Barkhof F: Optimizing the association between disability and biological markers in MS. Neurology. 2001, 57: 1253-1258.
Arnold DL, Riess GT, Matthews PM, Francis GS, Collins DL, Wolfson C, Antel JP: Use of proton magnetic resonance spectroscopy for monitoring disease progression in multiple sclerosis. Ann Neurol. 1994, 36: 76-82.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2377/4/8/prepub
Acknowledgements
We are grateful to Yolanda Gata for the administrative tasks performed. This study has been partially sponsored by Neuroinvest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
This study has been partially sponsored by Schering – Plough.
Authors' contributions
GI conceived of the study, and participated in its design and coordination. PP carried out the Magnetic Resonance studies. JLRP drafted the manuscript. GS performed the statistical analysis. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ruiz-Peña, J.L., Piñero, P., Sellers, G. et al. Magnetic resonance spectroscopy of normal appearing white matter in early relapsing-remitting multiple sclerosis: correlations between disability and spectroscopy. BMC Neurol 4, 8 (2004). https://doi.org/10.1186/1471-2377-4-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2377-4-8