Abstract
Background
Catheter-related bacteraemias (CRBs) contribute significantly to morbidity, mortality and health care costs in dialysis populations. Despite international guidelines recommending avoidance of catheters for haemodialysis access, hospital admissions for CRBs have doubled in the last decade. The primary aim of the study is to determine whether weekly instillation of 70% ethanol prevents CRBs compared with standard heparin saline.
Methods/design
The study will follow a prospective, open-label, randomized controlled design. Inclusion criteria are adult patients with incident or prevalent tunneled intravenous dialysis catheters on three times weekly haemodialysis, with no current evidence of catheter infection and no personal, cultural or religious objection to ethanol use, who are on adequate contraception and are able to give informed consent. Patients will be randomized 1:1 to receive 3 mL of intravenous-grade 70% ethanol into each lumen of the catheter once a week and standard heparin locks for other dialysis days, or to receive heparin locks only. The primary outcome measure will be time to the first episode of CRB, which will be defined using standard objective criteria. Secondary outcomes will include adverse reactions, incidence of CRB caused by different pathogens, time to infection-related catheter removal, time to exit site infections and costs. Prospective power calculations indicate that the study will have 80% statistical power to detect a clinically significant increase in median infection-free survival from 200 days to 400 days if 56 patients are recruited into each arm.
Discussion
This investigator-initiated study has been designed to provide evidence to help nephrologists reduce the incidence of CRBs in haemodialysis patients with tunnelled intravenous catheters.
Trial Registration
Australian New Zealand Clinical Trials Registry Number: ACTRN12609000493246
Similar content being viewed by others
Background
Central venous catheterization is an increasingly common method of providing rapid, temporary access for the provision of haemodialysis (HD) to patients with serious acute or chronic kidney failure. Unfortunately, the clinical usefulness of this method is severely limited by the frequent occurrence of bloodstream infections in up to 40% of cases [1–4]. A number of registry [5, 6] and observational cohort studies [7] have indicated that there has been an increasing reliance on haemodialysis catheters in incident haemodialysis patients ranging from 30% of patients in Europe and Australia to 60% in the United States of America [8–14]. Hospital admissions for catheter-related bacteraemias (CRBs) have doubled in the last decade [15] and a recent analysis of the Dialysis Morbidity and Mortality Wave 2 study [16] identified initial dialysis access as the main antecedent of bacteraemia in dialysis patients. Recent studies have further suggested that the use of haemodialysis catheters is associated with a 1.5- to 3-fold increase in both all-cause and infectious mortality [17–19].
Blood stream infections can result from extraluminal (exit site or tunnel infections) or intraluminal infection of the catheter [20]. Strategies for preventing CRBs have generally focused on cutaneous antisepsis, topical antibiotic application, antibiotic lock solutions and the use of devices coated or impregnated with antimicrobial agents [21, 22]. However, these prophylactic strategies have been limited by concerns about antibiotic toxicity and the appearance of antibiotic-resistant microbial isolates [23, 24]. Sodium citrate locks have also been investigated for prophylaxis against CRBs [25], although increased rates of catheter thrombosis have been reported [26].
Locking of catheters with ethanol is a promising technique in this respect as the agent is bactericidal, has low toxicity, is unlikely to produce resistant organisms, is able to disinfect organisms in biofilms, and is cheap. Ethanol is an antiseptic agent which is bacteriocidal by protein denaturation and is active against a wide variety of organisms including Gram-positive bacteria, Gram-negative bacteria, and fungi. In vitro studies have been published demonstrating the bactericidal effect of 70% ethanol against plastic-adherent organisms that commonly cause line infections [27]. Complete eradication of Gram-negative bacilli, Gram-positive cocci, and Candida albicans biofilms occurred with 30 minutes treatment with 60% ethanol compared to no eradication with trisodium citrate 46.7%.
The effects of catheter exposure to ethanol have been assessed and published. Silicone dialysis catheter integrity is maintained as assessed by scanning electron microscopy, even after 15 days exposure to 95% ethanol solutions [28]. The amount of silicone released was not significantly different when the catheter was submerged in ethanol compared to 0.9% sodium chloride. Mechanical testing of polyurethane and silicone catheters exposed to ten weeks of 70% ethanol proved ongoing structural integrity of both catheter types after prolonged ethanol exposure [29].
Published data has also demonstrated the effectiveness of ethanol lock therapy in the prevention of CRBs in haematology inpatients [16]. A short daily lock in haematology inpatients was used, which is not practical for long term use in renal dialysis outpatients. The current study is designed to ask whether a weekly lock could reduce the rate of CRBs in haemodialysis outpatients.
Methods/design
Ethics approval for the trial has been obtained from the Princess Alexandra Hospital Human Research Ethics Committee prior to study initiation and patient enrolment. The study will be performed in accordance with the 2000 Edinburgh, Scotland Revision of the Declaration of Helsinki, the National Health and Medical Research Committee (NHMRC) Statement on Human Experimentation, Joint NHMRC/AVCC Statement and Guidelines on Research Practice, applicable ICH guidelines and the Therapeutic Goods Administration (TGA) – Note for guidance on good clinical practice (CPMP/ICH/135/95) annotated with TGA.
Participants
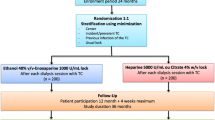
Participants will be selected from patients undergoing haemodialysis with incident and prevalent tunnelled intravenous catheters (Fig. 1). Inclusion criteria will include the presence of a tunnelled intravenous catheter and the ability to give informed consent. Exclusion criteria will include:
-
1.
Pregnancy or breast-feeding
-
2.
Religious or personal objection to the use of ethanol
-
3.
Intolerance to ethanol
-
4.
History of an exit site, tunnel or blood stream infection associated with the current catheter
Study Design
The study will follow a prospective, open-label, randomised, controlled design. Adequate allocation concealment will be ensured using a centralised computer-generated block randomisation procedure. Randomisation will occur on the day that trial consent is obtained and will be conducted by calling a centralised number to allocate the patient to a trial arm. Stratification will occur for incident versus prevalent catheters.
Experimental Intervention
Patients in the experimental arm will receive weekly instillation of 3 mL intravenous-grade 70% ethanol into each lumen of the catheter. Ethanol (Dehydrated alcohol USP injection, Phebra Pty Ltd, NSW 2066, Australia) is registered in Australia as an adjunct in the treatment of acute methanol and ethylene glycol poisoning and is supplied as a sterile solution of no less than 96.8% w/w of ethanol. Ethanol is diluted to 70% with sterile Water for Injections BP (Pfizer Australia Pty Ltd, NSW 2114 Australia) in a syringe prior to insertion into the catheter lumen. Dual lumen catheters receive 2 × 3 mL 70% ethanol, equivalent to 0.42 standard alcohol units. The ethanol lock is inserted immediately at the end of a haemodialysis session and left in situ until the next dialysis session (48 hours). When the patient returns for a further dialysis session, the ethanol lock will be aspirated out of each lumen of the catheter. For the remainder of the week patients in the experimental arm will receive standard heparin locks (Heparin sodium 5000 U/mL, Hospira, Germany).
Control Intervention
Patients in the control arm will only receive the standard heparin lock into each lumen.
Concurrent Treatments
Patients in each trial arm will undergo standard exit site and other management, as per the haemodialysis unit protocol. This will include thrice weekly exit site application of Medihoney (Medihoney™ Antibacterial Wound Gel, Medihoney Pty Ltd, Brisbane, Australia).
Blinding
Blinding of investigators and patients is not possible as the distinctive odour of 70% ethanol is immediately recognisable and unable to be concealed. Outcome assessment based on standardised and objective criteria will be performed by persons blinded to treatment allocation.
Outcome Measures
The primary outcome measure will be time to the first episode of CRB. CRB will be defined using standard objective criteria [30–32]: (a) probable catheter-related bacteraemia requires the isolation of a recognised pathogen from at least one set of blood cultures (or a potential skin contaminant from at least two sets of blood cultures) in the setting of clinical manifestations of sepsis with no other apparent source of sepsis other than the catheter; (b) definitive catheter-related bacteraemia requires the criteria of probable catheter-related bacteraemia and one of: purulence at the exit site; clinical sepsis that responds to antibiotic therapy upon catheter removal (after being refractory prior to catheter removal); or isolation of the same organism from the removed catheter tip.
Secondary outcomes will include:
-
a.
Incidence of CRB caused by different pathogens
-
b.
Time to exit site infection
-
c.
Time to infection-related catheter removal
-
d.
Time to catheter removal
-
e.
Adverse reactions
-
f.
Quality of life
-
g.
Economic cost
Clinical Assessment of Outcome
Reasons for removal of catheters will be assessed by medical staff. Blood cultures will only be taken as clinically indicated. The results of blood cultures collected during the study period will be recorded by research staff. Complications associated with lock therapy will be assessed on a weekly basis by nursing assessment. Patients will also be assessed for quality of life using the EQ-5D questionnaire, which will be administered by the study nurse on a monthly basis. They will also complete this questionnaire if admitted to hospital during their involvement in the trial. Data will be collected on health care service utilization external to the hospital (such as general practitioner visits) to allow economic evaluation in addition to the hospital-associated costs during the trial.
Monitoring for Adverse Events
The number and proportion of subjections who report treatment-emergent adverse events will be summarised for each group.
A trial Safety and Data Monitoring Committee has been set up to allow external evaluation of catheter-related problems at 6 months and annually after that period. This will consist of Renal and Infectious Diseases Physicians not otherwise involved in the study, who will assess the significance of catheter-associated problems in the treatment vs control group.
Sample Size Calculations
Prospective power calculations indicate that the study will have adequate statistical power (80% probability) to detect a clinically significant increase in infection-free survival from 200 days to 400 days if 56 patients are recruited into each arm (total 112 patients). These assumptions are based on local data in haemodialysis patients at the Princess Alexandra Hospital. A recruitment period of 3 years is anticipated.
Statistical Analyses
Infection-free survival curves, survival probabilities and estimated survival times will be generated using Kaplan-Meier methodology. Data will be censored at the time of study completion and removal of catheter for non-infective reasons. The first occurrence of a relevant infective event will be considered an event. Differences in the survival curves will be assessed using the log rank test. All data will be analysed on an intention-to-treat basis. P values < 0.05 will be considered significant.
Discussion
This trial has been designed to assess the efficacy of ethanol locks in the prevention of catheter-associated bacteremia in haemodialysis patients. Anecdotal and case series publications point to the potential efficacy of ethanol as a lock therapy in prevention and treatment of central venous catheter infection [33–35]. If effective, it will provide a cost-effective intervention which could substantially reduce patient morbidity and mortality, in addition to health-care associated costs. This trial will also prospectively assess adverse events that may be associated with ethanol use. In particular, the incidence of catheter-associated thrombosis has proven problematic in recent trials of sodium citrate locks [26]. There is a need for investigator-initiated trials of interventions such as this which do not attract pharmaceutical company funding or interest, but may provide important solutions to a clinical problems that confer substantial negative patient outcomes.
Abbreviations
- CKD:
-
Chronic kidney disease
- CRB:
-
Catheter-related bacteraemia
- ETOH:
-
Ethanol
- HD:
-
Haemodialysis
- HEALTHY-CATH:
-
Heparin versus EthAnol Lock THerapY for the prevention of Catheter Associated infecTion in Haemodialysis patients
- ICH:
-
International Conference on Harmonisation
- NHMRC:
-
National Health and Medical Research Council
- PAH:
-
Princess Alexandra Hospital
- TGA:
-
Therapeutic Goods Administration.
References
Marr KA, Sexton DJ, Conlon PJ, Corey GR, Schwab SJ, Kirkland KB: Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Ann Intern Med. 1997, 127: 275-280.
Sesso R, Barbosa D, Leme IL, Sader H, Canziani ME, Manfredi S, et al: Staphylococcus aureus prophylaxis in hemodialysis patients using central venous catheter: effect of mupirocin ointment. J Am Soc Nephrol. 1998, 9: 1085-1092.
Johnson DW, MacGinley R, Kay TD, Hawley CM, Campbell SB, Isbel NM, et al: A randomized, controlled trial of topical exit site mupirocin application in patients with tunnelled, cuffed haemodialysis catheters. Nephrol Dial Transplant. 2002, 17: 1802-1807. 10.1093/ndt/17.10.1802.
Johnson DW, van Eps C, Mudge DW, Wiggins KJ, Armstrong K, Hawley CM, et al: Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J Am Soc Nephrol. 2005, 16: 1456-1462. 10.1681/ASN.2004110997.
Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG: Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol. 2004, 15: 477-486. 10.1097/01.ASN.0000109668.05157.05.
USRDS: Excerpts from the USRDS 2002 annual data report: Atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2003, 41: S1-S260.
Pisoni RL: Vascular access use and outcomes: results from the DOPPS. Contrib Nephrol. 2002, 13-19. full_text.
Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG: Epidemiology of vascular access in the Australian hemodialysis population. Kidney Int. 2003, 64: 1893-1902. 10.1046/j.1523-1755.2003.00277.x.
Polkinghorne KR, McDonald SP, Marshall MR, Atkins RC, Kerr PG: Vascular access practice patterns in the New Zealand hemodialysis population. Am J Kidney Dis. 2004, 43: 696-704. 10.1053/j.ajkd.2003.11.023.
Di Benedetto A, Basci A, Cesare S, Marcelli D, Ponce P, Richards N: Increased use of catheters as vascular access: is it justified by patients' clinical conditions?. J Vasc Access. 2007, 8: 21-27.
Mendelssohn DC, Ethier J, Elder SJ, Saran R, Port FK, Pisoni RL: Haemodialysis vascular access problems in Canada: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II). Nephrol Dial Transplant. 2006, 21: 721-728. 10.1093/ndt/gfi281.
Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, et al: Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int. 2002, 61: 305-316. 10.1046/j.1523-1755.2002.00117.x.
Pisoni RL: Vascular access use and outcomes: results from the DOPPS. Contrib Nephrol. 2002, 13-19. full_text.
Schwab SJ, Beathard G: The hemodialysis catheter conundrum: hate living with them, but can't live without them. Kidney Int. 1999, 56: 1-17. 10.1046/j.1523-1755.1999.00512.x.
Allon M, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, et al: The spectrum of infection-related morbidity in hospitalized haemodialysis patients. Nephrol Dial Transplant. 2005, 20: 1180-1186. 10.1093/ndt/gfh729.
Ishani A, Collins AJ, Herzog CA, Foley RN: Septicemia, access and cardiovascular disease in dialysis patients: the USRDS Wave 2 study. Kidney Int. 2005, 68: 311-318. 10.1111/j.1523-1755.2005.00414.x.
Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK: Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001, 60: 1443-1451. 10.1046/j.1523-1755.2001.00947.x.
Pastan S, Soucie JM, McClellan WM: Vascular access and increased risk of death among hemodialysis patients. Kidney Int. 2002, 62: 620-626. 10.1046/j.1523-1755.2002.00460.x.
Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG: Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol. 2004, 15: 477-486. 10.1097/01.ASN.0000109668.05157.05.
Cheesbrough JS, Finch RG, Burden RP: A prospective study of the mechanisms of infection associated with hemodialysis catheters. J Infect Dis. 1986, 154: 579-589.
O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al: Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002, 51: 1-29.
Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al: Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005, 25: 107-131.
Abbas SA, Haloob IA, Taylor SL, Curry EM, King BB, Merwe Van der WM, et al: Effect of antimicrobial locks for tunneled hemodialysis catheters on bloodstream infection and bacterial resistance: a quality improvement report. Am J Kidney Dis. 2009, 53: 492-502. 10.1053/j.ajkd.2008.09.019.
Dogra GK, Herson H, Hutchison B, Irish AB, Heath CH, Golledge C, et al: Prevention of tunneled hemodialysis catheter-related infections using catheter-restricted filling with gentamicin and citrate: a randomized controlled study. J Am Soc Nephrol. 2002, 13: 2133-2139. 10.1097/01.ASN.0000022890.29656.22.
Yahav D, Rozen-Zvi B, Gafter-Gvili A, Leibovici L, Gafter U, Paul M: Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta-analysis of randomized, controlled trials. Clin Infect Dis. 2008, 47: 83-93. 10.1086/588667.
Power A, Duncan N, Singh SK, Brown W, Dalby E, Edwards C, et al: Sodium citrate versus heparin catheter locks for cuffed central venous catheters: a single-center randomized controlled trial. Am J Kidney Dis. 2009, 53: 1034-1041. 10.1053/j.ajkd.2009.01.259.
Chambers ST, Peddie B, Pithie A: Ethanol disinfection of plastic-adherent micro-organisms. J Hosp Infect. 2006, 63: 193-196. 10.1016/j.jhin.2006.01.009.
Guenu S, Heng AE, Charbonne F, Galmier MJ, Charles F, Deteix P, et al: Mass spectrometry and scanning electron microscopy study of silicone tunneled dialysis catheter integrity after an exposure of 15 days to 60% ethanol solution. Rapid Commun Mass Spectrom. 2007, 21: 229-236. 10.1002/rcm.2837.
Crnich CJ, Halfmann JA, Crone WC, Maki DG: The effects of prolonged ethanol exposure on the mechanical properties of polyurethane and silicone catheters used for intravascular access. Infect Control Hosp Epidemiol. 2005, 26: 708-714. 10.1086/502607.
Bureau of Infectious Diseases Division of Nosocomial and Occupational Infections: Infection control guidelines: Preventing infections associated with indwelling intravascular access devices. Health Canada. 1997, 23: S8-S11.
Randolph AG, Cook DJ, Gonzales CA, Brun-Buisson C: Tunneling short-term central venous catheters to prevent catheter-related infection: a meta-analysis of randomized, controlled trials. Crit Care Med. 1998, 26: 1452-1457. 10.1097/00003246-199808000-00038.
Maki DG, Weise CE, Sarafin HW: A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977, 296: 1305-1309.
Metcalf SC, Chambers ST, Pithie AD: Use of ethanol locks to prevent recurrent central line sepsis. J Infect. 2004, 49: 20-22. 10.1016/j.jinf.2003.08.010.
Broom J, Woods M, Allworth A, McCarthy J, Faoagali J, Macdonald S, et al: Ethanol lock therapy to treat tunnelled central venous catheter-associated blood stream infections: results from a prospective trial. Scand J Infect Dis. 2008, 40: 399-406. 10.1080/00365540701756953.
Ackoundou-N'guessan C, Heng AE, Guenu S, Charbonne F, Traore O, Deteix P, et al: Ethanol lock solution as an adjunct treatment for preventing recurrent catheter-related sepsis – first case report in dialysis setting. Nephrol Dial Transplant. 2006, 21: 3339-3340. 10.1093/ndt/gfl358.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2369/10/23/prepub
Acknowledgements
Participation of patients at the Princess Alexandra Hospital (PAH) Renal Dialysis Unit is greatly appreciated. The assistance of the nursing staff of the PAH Renal Dialysis Unit is invaluable, as is the patient recruitment, data collection, and research coordination of Neil Underwood and Barbara Johnson of Infection Management Services, Princess Alexandra Hospital. This study is funded by a grant from the Princess Alexandra Private Practice Trust Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JB was the principal investigator; conceived study; participated in design and co-ordination; helped to draft manuscript; read and approved the final manuscript. SO participated in co-ordination; helped to draft manuscript; read and approved the final manuscript. SG participated in co-ordination; helped to draft manuscript; read and approved the final manuscript. GP participated in design and co-ordination; helped to draft manuscript; read and approved the final manuscript. CH participated in design and co-ordination; helped to draft manuscript; read and approved the final manuscript. NI participated in design and co-ordination; helped to draft manuscript; read and approved the final manuscript. SC participated in design and co-ordination; helped to draft manuscript; read and approved the final manuscript. DM participated in design and co-ordination; helped to draft manuscript; read and approved the final manuscript. DJ participated in design and co-ordination; helped to draft manuscript; read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Broom, J.K., O'Shea, S., Govindarajulu, S. et al. Rationale and design of the HEALTHY-CATH trial: A randomised controlled trial of Heparin versus EthAnol Lock THerapY for the prevention of Catheter Associated infecTion in Haemodialysis patients. BMC Nephrol 10, 23 (2009). https://doi.org/10.1186/1471-2369-10-23
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2369-10-23