Abstract
Background
Tunnelled dialysis catheter (TC) infections are a major health complication and are associated with increased antibiotic consumption, hospital stays, health costs and mortality. Experimental data provide evidence that Ethenox, a mixture of enoxaparine 1000 U/mL in 40% v/v ethanol, could be a promising lock solution. The aim of the study is to compare an interdialytic lock solution of Ethenox with reference lock solutions, unfractionated heparin (UFH) or citrate 4% for the prevention of TCI in hemodialysis patients.
Method
This study will monitor a multicentre, prospective, single blind, randomized, controlled, parallel group trial. The main inclusion criteria are patients > 18 years old with end-stage renal disease, treated with chronic hemodialysis/hemodiafiltration three times a week, with incident or prevalent non-impregnated internal jugular TCs inserted for at least 2 weeks and able to give informed consent. Exclusion criteria are TCI in the previous 4 weeks and anti-infective treatment for TCI in the previous 2 weeks. Patients will be randomized to receive either study treatment Ethenox in the intervention group or reference solutions in the control group, unfractionated heparin (UFH) or citrate 4% w/v according to usual practice. The primary outcome measure will be time to first TCIs assessed by an endpoint adjudication committee blinded to the study arm according to predefined criteria. Patients will receive the study treatment for up to 12 months. Intention-to-treat analysis of the primary endpoint will be performed with a marginal Cox proportional hazard model. Prospective power calculations indicate that the study will have 90% statistical power to detect a clinical significant two-fold increase in median infection-free survival if 200 patients are recruited into each arm over a period of 24 months.
Discussion
Firm evidence of the efficacy of the Ethenox lock in preventing TCI could be of major clinical benefit for patients. The results of this study will allow the development of new guidelines based on a high level of evidence.
Trial registration
ClinicalTrials.gov Identifier: NCT03083184, date of registration March 17 2017 and European Clinical Trials Database Identifier: EudraCT 2016-A00180-51), date of registration July 11 2016.
Similar content being viewed by others
Background
Infections are the second cause of death in end-stage renal disease patients treated by hemodialysis. The risk of infection of vascular access is ten times greater in chronic hemodialysis patients with a tunnelled catheter (TC) than in those with an arteriovenous fistula. In patients with TC infection (TCI), bacteremia and secondary septic foci occur in 3 to 44% of cases [1]. In chronic hemodialysis patients, TCI results in antibiotics prescription, frequent hospitalizations, and high health costs, and reduces survival [2, 3]. Thus, the prevention of TCI is a major means of improving the prognosis of hemodialysis patients.
Unfractionated heparin (UFH), is the standard interdialytic lock solution for the prevention of TC thrombosis. However, UFH has no antibiofilm properties and could even promote bacteria development [4]. Antibiotic locks decrease the rate of TCI including TC-related bloodstream infections (TCBSI) and exit-site infection (ESI). The widespread use of antibiotic lock solutions raises concerns about the development of adverse events and the emergence of antimicrobial resistance [5, 6]. Antiseptic locks could help to overcome the problem of acquired antibiotic resistance. Hypertonic citrate lock solutions were initially shown to reduce the risk of TCBSI [7, 8]. However, they lead to increased risk of severe pulmonary embolism and even cardiac arrhythmia due to hypocalcaemia, probably as the result of systemic diffusion of concentrated citrate [9]. Other studies found no significant difference in TCBSI incidence between citrate 4% w/v and UFH lock [10, 11]. A recent meta-analysis reported that antimicrobial-containing citrate locks but not citrate alone reduce the risk of TCI [12]. The use of taurolidine-citrate 4% w/v locks reduces the rate of TCI but increases that of TC dysfunction and TC removal for occlusion [13]. When taurolidine citrate is combined with UFH twice weekly and urokinase once weekly, TC dysfunction does not increase relative to UFH alone. However, the decrease in TCIs caused by Gram-positive cocci, which are the most prevalent cause of TCI in France, is not significant [14]. Hence, it is necessary to develop alternative lock solutions containing an antithrombotic and an additional non-antibiotic substance with antibiofilm properties.
Ethanol is an inexpensive antiseptic agent with activity against a broad range of bacteria and fungi commonly involved in TCI. It acts by non-specific protein denaturation and thus is less likely to promote antimicrobial resistance. Ethanol concentration ≥ 40% v/v is highly effective in eradicating biofilm of Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Klebsiella pneumoniae and Candida albicans [15,16,17] whereas 46.7% citrate has no antibiofilm properties [18]. Studies have been performed on the integrity of TCs exposed to ethanol: several showed that prolonged exposure of polyurethane and silicon TCs to ethanol at 60% v/v and 70% v/v does not cause ultra-structural damage (electron microscopy studies) or modify their mechanical properties [19, 20]. Likewise, the risk of releasing substances from silicon [21] and polyurethane [22] TCs, as assessed by mass spectrometry, is low at concentrations of ethanol below 60% v/v and not different from that observed with a sodium chloride solution at 0.9%.
The HEALTHY-CATH study [23], involving 49 hemodialysis patients fitted with a TC, is the only randomized open label controlled trial to have assessed the efficacy of an ethanol lock strategy for preventing TCBSIs. In the intervention group a-70% v/v ethanol interdialytic lock solution was instilled once a week. The reduction of TCBSI risk in this group was not significantly lower than in the heparin group, a result that could have been due to the insufficient power generated by the small patient sample. More recently, a meta-analysis showed that ethanol lock conferred significant benefit in preventing catheter-related bacteraemia in patients with a TC [24].These results suggest that ethanol lock is effective in reducing the rate of TCBSI.
However, ethanol has no specific anticoagulant effect, and our group has previously reported an increase in the rates of TC dysfunction when a 60% v/v ethanol interdialytic lock solution is used [25]. Experimental data provide evidence that a mixture of ethanol and injectable anticoagulant may be a promising lock solution for preventing both TCI and TC dysfunctions. UFH, whatever its concentration, cannot be mixed in 40% v/v ethanol because of precipitation. ERA-EDTA recommends using low molecular weight heparins (LMWHs) for blood circuit anticoagulation during dialysis sessions. Like UFH, LMWHs have no antibiofilm properties. In contrast, they can be mixed in 40% v/v ethanol and among LMWHs enoxaparin has the highest solubility (up to 1300 U/mL). Our group has shown that 40% v/v ethanol - enoxaparin 400 U/mL is stable, compatible with TC materials and possesses antibiofilm and anticoagulant properties in vitro (patent). No clinical studies have previously assessed the efficacy of a combined solution of ethanol and LMWH in preventing TCI in chronic hemodialysis patients.
We assume that an interdialytic lock solution of Ethenox, a mixture of enoxaparin 1000 U/mL in 40% v/v ethanol, can be effectively and safely used to prevent TCI in chronic hemodialysis patients. The reduced concentration of ethanol in Ethenox compared to that in previous published reports and the aspiration of the lock before connection for dialysis will enhance tolerance while the addition of enoxaparin will effectively prevent the risk of TC dysfunction.
Methods/designs
Aims
The purpose of ETERNITY is to assess the efficacy of an interdialytic lock solution (Ethenox) as compared with reference lock solutions (UFH 5000 U/mL or Citrate 4% w/v), for preventing TCIs in chronic hemodialysis patients.
Ethical considerations and registrations
The study is promoted by the Centre Hospitalier Universitaire de Clermont-Ferrand. It was collaboratively designed by the dialysis unit, the medical intensive care unit and the department of clinical research and public health of the university hospital of Clermont-Ferrand, and Unit 6023 (Clermont-Ferrand, France) of the CNRS (French National Center for Scientific Research). The study complies with the principles of the Helsinki Declaration 2008. The whole protocol has been reviewed and approved by the Comité de Protection des Personnes—Sud-Est VI (no. 2016-AU1269). The investigator will explain the study to the eligible patient and give him/her the information form. Patients will be allowed the time they require to make a decision, after which the investigator will collect two initialed copies of the information form and two signed copies of the written consent form. The study is registered on Clinical Trials (NCT03083184) and on the European Clinical Trials Database (EudraCT 2016-A00180-51).
Design and setting of the study
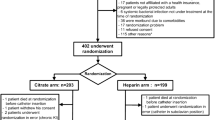
ETERNITY is a prospective phase III multi-centre, single blind, randomized, controlled, parallel group study (Fig. 1). It will be carried out in 14 dialysis units in France (Additional file 2). The planned duration of the study will be 36 months. The enrolment period will be 24 months. Patients will receive the study treatment for up to 12 months or until 1 month before the end of the study. To evaluate safety, all the patients included will be followed for adverse events for 4 additional weeks after they have received their last treatment dose in the study (Fig. 2).
Schema for the Eternity trial. Enrolled patients will be randomly assigned in a 1:1 ratio either to the intervention group (Ethenox) or to the control group (reference solution: UFH 5000 U/mL or citrate 4% w/v depending on which solution is generally used). Random allocation will be performed by minimization using a computer algorithm. The study will be performed single blind for the patients and the analysts. The primary outcome will be time to first TCIs assessed by an endpoint adjudication committee blinded from study arm according to predefined criteria
Study population
Eligible patients are adult (≥18 years of age) patients undergoing chronic hemodialysis in the study dialysis centres and treated with hemodialysis/haemodiafiltration via a TC inserted in a jugular vein three times a week for at least 2 weeks.
Exclusion criteria are intended use of the catheter for less than 3 months, intolerance of or hypersensitivity to ethanol, enoxaparin or UFH and citrate, previous history of type II heparin-induced thrombocytopenia, local or systemic antibiotic treatment for prevention or treatment of a TCI in the previous 2 weeks, presence of TCI in the previous 4 weeks, previous history of bacteraemia without a negative blood culture evidence of healing, TC impregnated with anti-infective agents or anticoagulants, pregnancy or risk of pregnancy (i.e. pre-menopausal woman not using a reliable method of contraception) and breastfeeding.
Endpoints
Primary endpoints
The primary outcome will be time to first TCI. In the study, TCI is a composite endpoint defined by the occurrence of at least one of the three following events: definitive TCBSI, probable TCBSI or ESI. TCI diagnosis and its type (definitive or probable TCBSI or ESI) will be assessed by an endpoint adjudication committee (EAC, see Additional file 1) blinded to study arm according to predefined criteria (Tables 1 and 2).
Secondary endpoints
Secondary endpoints will comprise:
-
1.
Other TCI prevention criteria
-
Time to first definitive or probable TCBSI
-
Time to first ESI
-
Incidence rate of definitive or probable TCBSI
-
Incidence rate of ESI
-
-
2.
Prevention of TC colonizations: rates of colonization of removed TCs
-
3.
Catheter patency
-
Time to first TC dysfunction
-
Incidence rate of TC dysfunctions
-
Dialysis dose: monthly (Kt/V)sp and (KT/V)e (2nd generation Daugirdas equation) and Kt/V measured by the dialysis machine (ionic dialysance or optical measurement of urea in the effluent dialysate)
-
-
4.
TC survival: time to TC removal
-
5.
Health care consumption
-
Time to first systemic antibiotic treatment for TCI
-
Total duration and type of antibiotic treatment
-
Time to first hospitalization for TCI
-
Total duration of hospital stays for TCI
-
Total number of TCs replaced during the study
-
-
6.
Safety
-
Incidence rate of breaches in TC integrity (TC leakage or disruption)
-
Incidence rate of clinical adverse events related to ethanol exposure (tiredness, ethanol taste, headaches, dizziness, nausea, light-headedness, and increase in serum aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase or alkaline phosphatase).
-
Incidence rate of type II heparin-induced thrombocytopenia.
-
Incidence rate of haemorrhages: bleeding or suspected bleeding, qualified as major if associated with a decrease in haemoglobin levels of more than 2 g/dL or the need to transfuse at least two units of red blood cells.
-
-
7.
Overall mortality: time to death from any cause
-
8.
Inflammation: CRP value (measured every 3 months).
Description of the products
Ethenox solution is obtained by mixing a pre-packaged ethanol-saline solution with 0.6 mL of enoxaparin (10,000 U/mL) to achieve 6 mL of the required ethanol content and enoxaparin concentration (enoxaparin 1000 U/mL in 40% v/v ethanol). The pharmacy department of the university hospital of Clermont-Ferrand will prepare the ethanol-saline solution in flasks and will send them with enoxaparin syringes every 3 months.to the study centres in boxes containing 14 treatments.
UFH lock solution is pure sodium heparinate 5000 U/mL packaged in the form of a single-use ampoule, i.e. heparin 25,000 U / 5 mL. Citrate solution at 4% is packaged in the form of a single-use ampoule, i.e. sodium citrate 4% 5 mL.
Each centre is free to choose the antiseptic solution used but cannot change it during the course of the study. The use of an antiseptic ointment at the exit-site until wounds have healed or in the event of ESI is authorized in accordance with guidelines [26]. The use of a fibrinolytic lock solution is not allowed except in the event of TC dysfunction. The use of other antimicrobial locks is not allowed and nor is that of dressings impregnated with an antibiotic or antiseptic.
Study description
Screening
Patients eligible to participate in the study will be identified by a clinical research assistant in each study centre at the beginning of the study and then weekly.
Enrolment
Eligible patients will be recruited during a routine hemodialysis session by an investigator from centres participating in the study. The investigator will explain the study to the patient and give him/her the information form. The patients willing to participate in the study will sign the written consent form.
Randomization
Enrolled patients will be randomly assigned in a 1:1 ratio either to the intervention group (Ethenox) or to the control group (reference solution: UFH 5000 U/mL or citrate 4% w/v depending on the solution instilled in the patient’s TCs prior to inclusion). Random allocation will be performed by minimization using a computer algorithm. Minimization strata will be the study centre, the incident or prevalent nature of the TC and in the prevalent group the existence or not of a previous infection of the TC in place (Fig. 1).
Blinding
The recognisable smell of ethanol and the need to prepare the Ethenox lock solution makes it difficult to ensure the blinding of lock solutions by nurses despite the use of masks. The study will be performed single blind for the patients and the analysts.
Study intervention
TC handling protocol
TC will be handled according to the protocol established previously in each dialysis centre in compliance with the recommendations of the French Society for Hospital Hygiene [27]. In particular, the dressing of the TC must be changed systematically at least once a week or when the dressing is no longer occlusive or in the event of signs of TCI (shivering, pain caused by the catheter or fever). The use of TCs is exclusively reserved for renal replacement therapy. Outside the dialysis centre, apart from emergency procedures, it is prohibited to use TCs for the infusion of solution or for blood sampling. If this were to occur, the patient will be included only in the intent-to-treat (ITT) analysis.
Treatment under study
Ethenox in the intervention group and reference solutions in the control group will be used by the hemodialysis nurse as TC lock solution after each hemodialysis session with all TCs inserted in patients during the study period. After the patient is disconnected from the hemodialysis machine, the lumens of the TC will be rinsed with 20 mL of NaCl solution at 0.9%. The lock solution under study (Ethenox, UFH or Citrate 4% w/v) will be instilled slowly with a syringe into each lumen of the TC. The volume injected corresponds to the intraluminal volume of the TC branch determined at the time of inserting the TC according to its length (Canaud TC) or as inscribed on the catheter (palindrome TC). The lumens of the TC are then blocked with a sterile plug and the occlusive dressing of the TC is performed. The lock is left in place until the next hemodialysis session. The interdialytic lock (Ethenox or control) will be aspirated before connection at the start of the following hemodialysis session according to the routine procedure of the centres.
The physician in charge of the patient will suspend or discontinue the study treatment if he/she considers that the patient’s health requires it. Patients who will not be administered their study lock more than once a month will be nevertheless included in the per-protocol analysis. In the event of temporary change of dialysis centre (hospitalization, holiday, etc.) the study lock will be suspended and resumed when the patients return to their dialysis centre. These patients will be included only in the ITT analysis.
Conduct to be followed in the event of signs of TCI
In patients presenting signs of TCI, the lock studied will be maintained and patient management will be implemented according to the decision taken by the physician responsible for the patient in accordance with the practice of each centre. The diagnosis of TCI and its type will be confirmed or not a posteriori by the EAC.
Conduct to be followed in the event of catheter dysfunction
At each dialysis session, when extracorporeal blood flow of ≥300 ml/min cannot be achieved with an arterial line pressure higher than − 300 mmHg, the strategy to follow for restoring catheter patency will be to use successively, if necessary, flushing of catheter lumens with saline using a 10 mL syringe, reposition of the patient, reversal of the lines, and fibrinolytic therapy in the absence of contra-indications (administered, for example, at the start of the following dialysis session). The value of blood flow rate and pressure on the arterial line just before each reversal of the lines or fibrinolytic therapy prescription will be noted on the nurse’s record. The time of reversal of the line or fibrinolytic therapy use will be noted on the nurse’s record. Except when a fibrinolytic interdialytic lock is prescribed, the study lock will be maintained.
Conduct to be followed in the event of protocol violation
Anticipated violations to study protocol have been described above. Violations not mentioned can be recorded by the CRA as required by the protocol. Any questions regarding protocol violations should be directed to the steering committee (Additional file 1) by phone.
Study assessment
At each session, the nurse in charge of the patient will search for signs indicating the presence of a TCI or dysfunction using a check list (comprising clinical signs of infection and bacteriological samples, antibiotic treatment, TC lines inversion and fibrinolytic treatments) according to the usual practice of the dialysis centres and clinical practice guidelines [26, 28]. Data will be collected weekly in an electronic case report form specifically developed for the study. Data for adjudication of the primary outcomes and serious adverse events will be recorded continuously.
Organization of the trial
Funding/support
ETERNITY is sponsored by the university hospital of Clermont-Ferrand (CHU de Clermont Ferrand, Clermont-Ferrand, France) and supported by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique National 2015, Study sponsor code: PHRC N 2015 ANIORT).
Coordination and conduct of the trial
Before the start of the study, all health-care workers and clinical research assistants in the 14 participating study centres will attend a formal training session on the study protocol, patient randomization and data collection in the Clinsight Clinical Data Monitoring Software (CDMS) electronic case report form. CDMS is a secure, interactive, web response system available at each study centre. The data manager will have access to the dataset. He will send login with limited access to each investigators ans local clinical research assistant. All documents required for the study will be available in each dialysis centre. Data monitoring in each center will be made by the same coordinating clinical research assistant independent from each study center. The principal investigator and a clinical research assistant will be available to discuss any problem concerning data collection, treatment invoices and protocol compliance and to assess study progress. Informations relative to important protocol modifications will be communicated to investigators using email and CDMS.
Interim analysis and independent data and safety monitoring committee
An independent data and safety monitoring committee (Additional file 1) will analyse the adverse events, give its opinion on major amendments of the protocol, proceed to the interpretation of an intermediate analysis (at 1/3 of the inclusions) and if deemed necessary announce the suspension or early termination of the study.
Sample size calculation
The working hypothesis of this randomized controlled trial is that Ethenox will double the median time to TCI. On the basis of the data collected in DIALIN, median TC duration use is 6 months [29]. The median survival time without TCI reported in recent clinical trials ranges between 690 and 1150 days. However, to achieve a statistical power of 90% with a risk of type I error of 5%, a total of 400 patients need to be enrolled over a period of 24 months.
Statistical analysis
General consideration
The statistical unit of the primary analysis and of most secondary analyses will be the TC. When a new TC is inserted in a patient participating in the study, the new TC will be analysed for the occurrence of a TCI or TC dysfunction only from the time when the patient fulfils the eligibility criteria. This will rule out ongoing TCI or early dysfunction of the TC due to poor positioning at the time of insertion or an anatomical abnormality of the patient.
The principal analysis will be performed on the intention-to-treat principle with Stata software (version13, StataCorp, College Station, TX). The tests will be two-sided, with a type I error set at α = 0.05. Quantitative variables will be presented as mean ± SD when normally distributed (assumption of normality studied by Shapiro-Wilk test), and for non-normal distributions as median, quartiles and range. Qualitative variables will be expressed as numbers and associated percentages.
Primary analysis
To evidence the efficacy of the Ethenox lock solution in preventing TCIs in comparison with the reference lock solutions (UFH 5000 U/mL or Citrate 4%), the censored data will be estimated by the Kaplan-Meier method. The comparison between groups regarding the primary endpoint will be performed with a marginal Cox proportional hazard model. Results will be expressed as hazard ratios and 95% confidence interval. Second, owing to the possible non-random censoring process the proposition to right-censor patients at the time of TC removal is debatable. The previous models will be performed with the Inverse Censoring Probability Weighting estimator. Finally, because of possible competing events, which prevent an event of interest from occurring rather than just preventing it from being seen to happen (censoring), Fine and Gray’s approach will be used.
Secondary analysis
For the secondary endpoint, the censored data will be estimated by the Kaplan-Meier method and compared between groups by a marginal Cox proportional hazard model. Concerning the parameters collected longitudinally, mixed models will be performed to take into account inter- and intra-patient variability. The effects of group, time and their interaction (time x group) will be studied as fixed effects, with the patient effect considered as a random effect (data repeated for the same subject). Lastly, regarding comparisons for which the statistical unit will be the patient, the usual tests will be applied: Student t-test or Mann-Whitney test if the conditions of the t-test are not competed (normality, homoscedasticity studied by the Fisher-Snedecor test) for parameters of a quantitative nature and the Chi-squared test or Fisher’s exact test for categorical variables.
Method of taking into account missing and invalid data
Regarding the longitudinal data, a sensitivity analysis will be performed to study the attrition bias to ascertain the quantity (level of attrition) and nature (independence of the study group) of missing data to propose the most appropriate method of data imputation such as that of Verbeke and Molenberghs.
Discussion
TC use is frequent in chronic hemodialysis patients [30, 31]. Thus, prevention of TCIs is essential. TCIs lead to systemic antibiotic administration in more than 70% of cases [29]. This contributes to an increase in the prevalence of multi-resistant pathogens. Decreasing TCI rates by the use of Ethenox solution will reduce antibiotic consumption and the emergence of multi-resistant pathogens.
TCIs are responsible for high morbidity and mortality in chronic hemodialysis patients [1]. TCBSI leads to hospitalization for 75% of patients. Mortality related to TCBSI is about 20%. The harmful effects of TCIs are also indirect. Recurrent TCIs are associated with a chronic low-grade inflammatory state [32] that is involved in the pathogenesis of protein-energy wasting syndrome. TCIs have also been associated with an increased risk of cardio-vascular events [33]. Thus, inflammation due to infection may cause accelerated atherosclerosis. Prevention of TCI is therefore of paramount importance for improving endpoints in chronic hemodialysis patients fitted with TCs.
Ethenox solution is less expensive than the other antimicrobial solutions (€3.20 for Ethenox made with enoxaparine as against €30 for taurolidine citrate) and of equal cost as citrate 4% w/v (€2.80). The price of enoxaparin will decrease once enoxaparin generics, which are already on the market in several countries, are made available in France. Several studies have estimated the cost of treating a TCBSI at about €20,000 [34]. Thus, preventing TCI could lead to a significant reduction in health expenditure.
Finally, the results of this study will help to establish with a high level of evidence national and international recommendations for the prevention of TCIs in chronic hemodialysis patients.
Abbreviations
- ESI:
-
Exit-site infection
- ITT:
-
Intention to treat
- LMWH:
-
Low molecular weight heparins
- TC:
-
Tunnelled dialysis catheter
- TCBSI:
-
TC-related bloodstream infections
- TCI:
-
TC infection
- UFH:
-
Unfractionated heparin
References
Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011;79(6):587–98.
Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, Pannu NI, Thomas C, Hemmelgarn BR, Craig JC, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol. 2013;24(3):465–73.
Engemann JJ, Friedman JY, Reed SD, Griffiths RI, Szczech LA, Kaye KS, Stryjewski ME, Reller LB, Schulman KA, Corey GR, et al. Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect Control Hosp Epidemiol. 2005;26(6):534–9.
Shanks RM, Donegan NP, Graber ML, Buckingham SE, Zegans ME, Cheung AL, O'Toole GA. Heparin stimulates Staphylococcus aureus biofilm formation. Infect Immun. 2005;73(8):4596–606.
Dixon JJ, Steele M, Makanjuola AD. Anti-microbial locks increase the prevalence of Staphylococcus aureus and antibiotic-resistant Enterobacter: observational retrospective cohort study. Nephrol Dial Transplant. 2012;27(9):3575–81.
Landry DL, Braden GL, Gobeille SL, Haessler SD, Vaidya CK, Sweet SJ. Emergence of gentamicin-resistant bacteremia in hemodialysis patients receiving gentamicin lock catheter prophylaxis. Clin J Am Soc Nephrol. 2010;5(10):1799–804.
Weijmer MC, van den Dorpel MA, Van de Ven PJ, ter Wee PM, van Geelen JA, Groeneveld JO, van Jaarsveld BC, Koopmans MG, le Poole CY, Schrander-Van der Meer AM, et al. Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter-locking solution in hemodialysis patients. J Am Soc Nephrol. 2005;16(9):2769–77.
Winnett G, Nolan J, Miller M, Ashman N. Trisodium citrate 46.7% selectively and safely reduces staphylococcal catheter-related bacteraemia. Nephrol Dial Transplant. 2008;23(11):3592–8.
Polaschegg HD, Sodemann K. Risks related to catheter locking solutions containing concentrated citrate. Nephrol Dial Transplant. 2003;18(12):2688–90.
Power A, Duncan N, Singh SK, Brown W, Dalby E, Edwards C, Lynch K, Prout V, Cairns T, Griffith M, et al. Sodium citrate versus heparin catheter locks for cuffed central venous catheters: a single-center randomized controlled trial. Am J Kidney Dis. 2009;53(6):1034–41.
Macrae JM, Dojcinovic I, Djurdjev O, Jung B, Shalansky S, Levin A, Kiaii M. Citrate 4% versus heparin and the reduction of thrombosis study (CHARTS). Clin J Am Soc Nephrol. 2008;3(2):369–74.
Zhao Y, Li Z, Zhang L, Yang J, Yang Y, Tang Y, Fu P. Citrate versus heparin lock for hemodialysis catheters: a systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis. 2014;63(3):479–90.
Solomon LR, Cheesbrough JS, Ebah L, Al-Sayed T, Heap M, Millband N, Waterhouse D, Mitra S, Curry A, Saxena R, et al. A randomized double-blind controlled trial of taurolidine-citrate catheter locks for the prevention of bacteremia in patients treated with hemodialysis. Am J Kidney Dis. 2010;55(6):1060–8.
Labriola L, Jadoul M. Taurolidine-based lock solutions for hemodialysis catheters: the enthusiasm should be tempered. Kidney Int. 2018;93(4):1015–6.
Qu Y, Istivan TS, Daley AJ, Rouch DA, Deighton MA. Comparison of various antimicrobial agents as catheter lock solutions: preference for ethanol in eradication of coagulase-negative staphylococcal biofilms. J Med Microbiol. 2009;58(Pt 4:442–50.
Oncu S. Optimal dosage and dwell time of ethanol lock therapy on catheters infected with Candida species. Clin Nutr. 2014;33(2):360–2.
Lesens O, Balestrino D, Charbonnel N, Aumeran C, Traore O, Forestier C, Souweine B. Effectiveness of ethanol-based lock solutions on catheter biofilm microorganisms, Presented at the ICAAC Congress; 2013. p. 952.
Balestrino D, Souweine B, Charbonnel N, Lautrette A, Aumeran C, Traore O, Forestier C. Eradication of microorganisms embedded in biofilm by an ethanol-based catheter lock solution. Nephrol Dial Transplant. 2009;24(10):3204–9.
Crnich CJ, Halfmann JA, Crone WC, Maki DG. The effects of prolonged ethanol exposure on the mechanical properties of polyurethane and silicone catheters used for intravascular access. Infect Control Hosp Epidemiol. 2005;26(8):708–14.
Landry DL, Jaber RA, Hanumanthappa N, Lipkowitz GS, O'Shea MH, Bermudez H, Hathorne AP, Braden GL. Effects of prolonged ethanol lock exposure to carbothane- and silicone-based hemodialysis catheters: a 26-week study. J Vasc Access. 2015;0(0):0.
Guenu S, Heng AE, Charbonne F, Galmier MJ, Charles F, Deteix P, Souweine B, Lartigue C. Mass spectrometry and scanning electron microscopy study of silicone tunneled dialysis catheter integrity after an exposure of 15 days to 60% ethanol solution. Rapid Commun Mass Spectrom. 2007;21(2):229–36.
Balestrino D, Quintana M, Charbonnel N, Forestier C, Souweine B, Lartigue C. Ethanol 40% / enoxaparin 400 UI/mL lock solution: in vitro antibiofilm activity and compatibility with polyurethane catheter. In: Submission; 2015.
Broom JK, Krishnasamy R, Hawley CM, Playford EG, Johnson DW. A randomised controlled trial of heparin versus EthAnol lock THerapY for the prevention of catheter associated infecTion in hemodialysis patients--the HEALTHY-CATH trial. BMC Nephrol. 2012;13:146.
Zhao T, Liu H, Han J. Ethanol lock is effective on reducing the incidence of tunneled catheter-related bloodstream infections in hemodialysis patients: a systematic review and meta-analysis. Int Urol Nephrol. 2018;50(9):1643.
Heng AE, Abdelkader MH, Diaconita M, Nony A, Guerraoui A, Caillot N, Rince M, Deteix P, Souweine B. Impact of short term use of interdialytic 60% ethanol lock solution on tunneled silicone catheter dysfunction. Clin Nephrol. 2011;75(6):534–41.
Vanholder R, Canaud B, Fluck R, Jadoul M, Labriola L, Marti-Monros A, Tordoir J, Van Biesen W. Catheter-related blood stream infections (CRBSI): a European view. Nephrol Dial Transplant. 2010;25(6):1753–6.
Recommandations de la société française d’hygiène hospitalière. Bonnes pratiques d'hygiène en hémodialyse. Revue officielle de la société française d'hygiène hospitalière. 2005;13(2):136-9.
Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45.
Rapport annuel du réseau de surveillance des infections en hémodialyse (DIALIN) 2013. http://www.cpias-auvergnerhonealpes.fr/Reseaux/DIALIN/Resultats/Rapport_annuel_2013.pdf.
Agence de la biomédecine. Rapport annuel du Réseau Epidémiologie et Information en Néphrologie (REIN) 2012. https://www.agence-biomedecine.fr/IMG/pdf/rapport_rein_vdef_2012.pdf.
Noordzij M, Jager KJ, van der Veer SN, Kramar R, Collart F, Heaf JG, Stojceva-Taneva O, Leivestad T, Buturovic-Ponikvar J, Benitez Sanchez M, et al. Use of vascular access for hemodialysis in Europe: a report from the ERA-EDTA registry. Nephrol Dial Transplant. 2014;29(10):1956–64.
Venditto M, du Montcel ST, Robert J, Trystam D, Dighiero J, Hue D, Bessette C, Deray G, Mercadal L. Effect of catheter-lock solutions on catheter-related infection and inflammatory syndrome in hemodialysis patients: heparin versus citrate 46% versus heparin/gentamicin. Blood Purif. 2010;29(3):268–73.
Ishani A, Collins AJ, Herzog CA, Foley RN. Septicemia, access and cardiovascular disease in dialysis patients: the USRDS wave 2 study. Kidney Int. 2005;68(1):311–8.
Kosa SD, Lok CE. The economics of hemodialysis catheter-related infection prophylaxis. Semin Dial. 2013;26(4):482–93.
Acknowledgements
We are indebted to Mr. Jeffrey Watts for assistance in the preparation of the manuscript.
Funding
This study is funded by a grant from the French Ministry of Health (Protocole Hospitalier de Recherche Clinique PHRC national 2015). It is a public funder which has no role in protocol writing, data collection, analysis of results and their publication.
Trial Sponsor: Centre Hospitalier Universitaire de Clermont Ferrand, 58 rue Montalemenberg 63000 Clermont Ferrand
Study sponsor code: PHRC N 2015 ANIORT
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
JA is coordinating investigator, member of the steering committee, conceived the study and drafted the manuscript. AP, MA are coordinating clinical research assistants and helped to conceive the study. BP is methodologist and biostatistician. MB, JF, AG, EK, TK, HLM, DT, CGV, GV, HW are clinical investigator, read and approved the final manuscript. HW is clinical investigator, read and approved the final manuscript. LB is the pharmacist in charge of ethenox production. AEH, BS are member of the steering committee, conceived the study and drafted the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol, the information and consent forms, and the investigator’s brochure on Ethenox have received authorization from the “cpp-sudest6” (AU 1269) ethics committee and the Agence National de Sécurité du Médicament et des produits de santé (ANSM) (2016-A00180-51). The investigator explain the study to the patient and give him/her the information form. The patients willing to participate in the study sign a written consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Members of the steering committee, endpoint adjudication committee and data and safety monitoring committee. (DOCX 14 kb)
Additional file 2:
Study centers. List of the dialysis units/hospital that are involved in this study. (DOCX 13 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Aniort, J., Piraud, A., Adda, M. et al. Evaluation of the efficacy of an interdialytic “ethanol 40% v/v - enoxaparin 1000 U/mL” lock solution to prevent tunnelled catheter infections in chronic hemodialysis patients: a multi-centre, randomized, single blind, parallel group study. BMC Nephrol 20, 149 (2019). https://doi.org/10.1186/s12882-019-1338-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-019-1338-6