Abstract
Background
This study evaluates the existence of numerical alterations of chromosome 17 and TP53 gene deletion in gastric adenocarcinoma. The p53 protein expression was also evaluated, as well as, possible associations with clinicopathological characteristics.
Methods
Dual-color fluorescence in situ hybridization and immunostaining were performed in twenty gastric cancer samples of individuals from Northern Brazil.
Results
Deletion of TP53 was found in all samples. TP53 was inactivated mainly by single allelic deletion, varying to 7–39% of cells/case. Aneusomy of chromosome 17 was observed in 85% of cases. Chromosome 17 monosomy and gain were both observed in about half of cases. Cells with gain of chromosome 17 frequently presented TP53 deletion. The frequency of cells with two chr17 and one TP53 signals observed was higher in diffuse than in intestinal-type GC. Immunoreactivity of p53 was found only in intestinal-type samples. The frequency of cells with two chr17 and two TP53 signals found was higher in samples with positive p53 expression than in negative cases in intestinal-type GC.
Conclusion
We suggest that TP53 deletion and chromosome 17 aneusomy is a common event in GC and other TP53 alterations, as mutation, may be implicated in the distinct carcinogenesis process of diffuse and intestinal types.
Similar content being viewed by others
Background
Gastric cancer (GC) is the fourth most frequent malignancy and the second most common cause of cancer death in the World [1]. In the State of Pará (Northern Brazil), GC was the most common cause of cancer death in 2000. In Belém, State of Pará, the 5-year-survival rate is about 9–10% [2]. A better understanding of the biology of this neoplasia progression is crucial for the development of better tests to early neoplasia detection and also of new treatment strategies for GC.
Molecular events in the carcinogenesis of GC remain largely unknown [3]. A key feature in the pathogenesis of most GC, as in many other solid cancers, is chromosomal instability, resulting in gains and losses of parts or even whole chromosomes [4].
Gastrointestinal tract tumors are notorious for being difficult to be analyzed by standard cytogenetic techniques [5–9]. Fluorescence in situ hybridization (FISH) assay allows rapid detection of numerical genetics aberrations in interphase nuclei in tumor cells. FISH assay should be used to evaluate cell-to-cell heterogeneity in gene or loci copy number and detect small subpopulations of genetically aberrant cells [10]. FISH studies have shown numerical aberrations 1, 7, 8, 9, 17, 20, X and Y to be common in GC [[7, 11–19], see also review [20]]. There are some studies in literature concerning TP53, located at chromosome 17p13.1, and chromosome 17 (chr17) copy number alterations by FISH assay in GC [[21–24], see also review [20]].
The TP53 tumor suppressor plays a pivotal role in the coordination of the repair process or in the induction of apoptosis. TP53 somatic alteration is described in approximately 50% of human cancers, including GC [25]. Deregulation of the TP53 pathway has been shown to involve mutations, loss of heterozygosity (LOH), increased expression of the TP53 inhibitor HDM2, or epigenetic silencing of the TP53 promoter [26, 27].
The aim of this study was to investigate chr17 and TP53 numerical alterations in GC samples from Pará State by dual-color FISH technique. Immunostaining for p53 protein was also evaluated. These results were correlated with clinicopathological characteristics.
Methods
Samples
The study included 20 gastric adenocarcinoma samples. Samples of primary tumors submitted to surgical resection were obtained from João de Barros Barreto University Hospital (HUJBB). This study investigated cancer samples of patients from Pará State, where there is a mixed population composed of three main ethnic groups: Amerindian, African and European [28].
Patients' age, sex and tumor anatomical sites were obtained from tumor registries. The mean age of the twenty patients was 55 ± 14.67 years (range 24–77). The female/male ratio was 3:2. All samples were classified according to Laurén [29] and tumors were staged using standard criteria by TNM staging [30]. According to Laurén's classification, 6 were diffuse type (30%) and 14 were intestinal type (70%). Table 1 shows cases with their histopathological characteristics.
All patients had negative histories of exposure to either chemotherapy or radiotherapy prior to surgery; there was no other diagnosed cancer. An informed consent with approval of the ethics committee of HUJBB was obtained from the studied patients.
Fish
FISH was applied on cells fixed in methanol/acetic acid using recently made slides according to modified protocols [31]. The slides were washed in 2× saline sodium citrate (SSC)/0.5% NP-40 (pH 7.0) solution and dehydrated in 70%, 80% and 95% ethanol. To determine the chr17 and TP53 copy numbers, cells were hybridized with 10 μL dual-color direct labeled probe (Qbiogene®, CA, USA) specific for chr17 α-satellite and TP53 gene region, labeled with fluorescein and rhodamine respectively. The probe applied to the slide under a glass coverslip. The probe and sample were denatured at 75°C for 5 minutes and. In situ hybridization occurred at 37°C in a moist chamber overnight. Post-hybridization washings were done and the nuclei were counterstained with DAPI/antifade. Molecular cytogenetic analysis was carried out under an Olympus BX41 fluorescence microscope with triple DAPI/FITC/TRICT filter (Olympus, Japan) and the FISHView® of Applied Spectral Imaging® image analysis system (ASI Ldt., Israel). For each case, 200 interphase/metaphase nuclei were analyzed and were scored using the Hopman's criteria [32]. In our study, the cut-off level for interphase-FISH was 5%. To avoid misinterpretation due to technical error, gastric mucosal tissue (nonneoplastic) and normal lymphocyte nuclei were used as negative control.
Immunohistochemical staining
Deparaffinized tissue sections (4 μm) were incubated with primary monoclonal antibody p53 (DO-7, dilution 1:50, DakoCytomation, CA, USA) and secondary antibody followed by streptavidin-biotin-peroxidase complex (DakoCytomation, CA, USA) as previously described [18]. Slides were visualized with diaminobenzidine-H2O2 and counterstained with Harry's hematoxylin. The results were interpreted using the Ozturk's et al. criteria [33]. Positive p53 expression was defined as clear nuclear staining, whereas negative p53 immunostaining was considered when no positive cell was seen or rare cells were stained (less than 10% weakly stained tumor cells). A breast adenocarcinoma sample with known p53 immunoreactivity was used as positive control and a normal gastric mucosa as negative control. Two pathologists evaluated the immunostaining results independently.
Statistical analyses
Statistical analyses were performed using Fisher's exact test and Mann-Whitney test. P value < 0.05 was considered to be statistically significant.
Results
FISH
Lymphocyte nucleus and normal gastric mucosa showed two signals to chr17 and TP53 in 97.5% and 96.5% of analyzed cells respectively. All cancer samples presented numerical alterations of chr17 and TP53 gene. Normal nuclei were observed in 50.7–88% of cells/case (Table 1).
The main TP53 alteration observed was the single allelic deletion. This alteration was present in all cases, varying to 7–39% of cells/case (Figure 1A). Chr17 monosomy observed in 45% of samples, ranging 5–12% of cells/case. Chr17 gain was also detected in 45% of cases. Chr17 trisomy was observed in 20% of the cases in a frequency up to 7.5% cells/case (case 19), in which 40% and 60% of these cases showed two and one single TP53 copy number respectively. Chr17 tetrasomy with two TP53 signals was detected in 35% of cases in a frequency up to 9% cells/case. Four signals to chr17 and TP53 was observed in one case (case 2) in 13.5% of cells (Table 1).
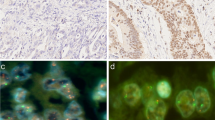
Cells submitted to FISH (A) and immunohistochemistry (B) assays. A1 (case 14), A2 (case 5) and A3 (case 6): interphase nuclei presenting chr17 monosomy (green signal) with one copy of TP53 (red signal), and nuclei presenting chr17 disomy with one or two copies of TP53 – 1000× magnification; B1 (case 14) and B2 (case 17): tissue with nuclear p53 immunoreactivity (brown stain) – 400× and 100× magnification, respectively; B3 (case 6): tissue without p53 immunoreactivity – 400× magnification.
The frequency of cells with two chr17 and one TP53 signals observed was higher in diffuse than in intestinal-type GC (p = 0.026). Chromosome alterations were not associated with other clinicopathological characteristics.
p53 protein expression
In the present study, breast adenocarcinoma (positive control) showed p53 overexpression and the normal gastric mucosa (negative control) showed lack of p53 immunoreactivity.
Seventeen GC samples were analyzed by immunostaining. Immunoreactivity of p53 (positive) was found in 8 cases (47%) (Figure 1B). All of these cases were intestinal-type (8 of 12 samples). Statistical analysis showed an association between intestinal-type GC and p53 expression (p = 0.0294). In our sample, p53 immunoreactivity was not associated with age, gender, location and TNM status (p > 0.05) (Table 1).
The frequency of cells with two chr17 and one TP53 signals observed was higher in samples with immunoreactivity of p53 negative than in cases with positive p53 expression (p = 0.016). In intestinal-type GC, the frequency of cells with two chr17 and two TP53 signals found was higher in samples with positive p53 expression than in negative cases (p = 0.027).
Discussion
Aneusomy is one of the most common findings in human cancer. Chromosome copy number changes encompass a continuum ranging from sporadic events to a change of chromosome numbers with each cell division. Although aneusomy can be detected at early stages of transformation and even in certain premalignant lesions, the degree of numerical chromosomal aberrations generally increases with tumor progression, and tumors with aggressive clinical behavior are more likely to be aneusomy than less malignant tumors. Aneusomy has also been found to be associated with poor treatment outcome in cancer patients [34].
Regarding chr17 and TP53 copy number, we observed normal nuclei in 50.7–88% of cells/case. This result corroborates our previous conventional and molecular cytogenetic studies, which demonstrated that chr17 aneusomy is not a frequent in GC samples of individual from Northern Brazil [7, 35, 36].
There are few studies in literature concerning chr17 and TP53 copy number alterations. Our findings corroborated Kobayashi et al. [21] that found deletion of TP53 in 39% of 67 tumors and all of these samples showed chr17 polysomy. Takahashi et al. [22] also observed that p53 signal count was lower than the chromosome 17 signal count in 1 of 3 intestinal-type GC.
Gomyo et al. [23] demonstrated 3 or 4 signals for chr17 in 46% of 13 intestinal-type GC samples and 77% of these cases showed TP53 deletion by FISH assay. In our sample, 45% of all cases presented 3 or 4 signals for chr17 and TP53 deletion was detected in all cases.
Suzuki et al. [24] observed an increased of chr17 polysomy frequency and the incidence of TP53 deletion ranged from 55% to 90% in ten GC samples. They also described that TP53 deletion was significantly higher in intestinal than in diffuse-type cancers. However, in our sample we found TP53 deletion in up to 49% cells/case and the frequency of cells with two chr17 and one TP53 signals observed was higher in diffuse than in intestinal-type GC. Inconsistencies regarding the frequency of TP53 deletion in GC between our study and Suzuki et al. [24] may be suggestive of distinct gastric carcinogenesis pathways in different ethnic composition or differences in stage when the analysis was done. It is widely reported that differences between carcinogenesis processes can be the result of distinct environmental and genetic factors.
Suzuki et al. [24] also observed that chr17 monosomy was present in 70% of 10 cases and the most frequent pattern in these cases was the combination of one copy of chr17 and one of TP53. On the other hand, in our sample we observed chr17 monosomy in 45% of 20 cases (cut-off level of 5%) and the more frequent pattern was the combination of two copies of chr17 with one TP53 copy by cell.
In the present study, chr17 tetrasomy with two TP53 signals was frequently observed. We also could observe that chr17 gain tended to be more frequently found in tumors with higher extension (T3 or T4 stages). This finding suggests that tetrasomy event is a subsequent step after gene deletion, which could justify the higher frequency of cells with two copies of chr17 and one TP53 copy and also the tendency of increased level of chr17 gain in tumors with higher extension. Galipeau et al. [37] suggested that increased polysomy level is associated with inactivation of the TP53 in Barrett's esophagus in vivo, supporting our hypothesis.
Williams et al. [38] described that TP53 deletion was the most common aberration in gastritis, intestinal metaplasia, dysplasia e GC by FISH assay. The author suggested that this abnormality may exist in the initiation and progression to gastric cancer.
TP53 deletion, as well as chromosome 17 aneusomy, was observed in all analyzed samples, despite Laurén's histopathologic types. However, differential p53 expression was detected between these groups.
Increased immunostaining of p53 can depend on either increased synthesis of wild-type protein or accumulation of mutated protein in the cell, since the antibody recognizes both types of the protein [39]. In the present study, we observed an increased frequency of immunostained nuclei and the greater staining intensity in 47% of GC samples, as compared to normal gastric mucosa. We suggest that the p53 overexpression may be related to the mutated type of this protein. The frequency of p53 overexpression in GC has been described varying from 19% to 57.5% of cases [23, 40–44] and some studies also described TP53 mutations related with its protein overexpression [23, 41].
In the present study, only intestinal-type GC presented p53 immunoreactivity. Our research suggests that, beside TP53 loss by allelic deletion or chr17 aneusomy, a mutation in the remaining TP53 allele may exist in intestinal-type GC samples, which would explain the protein immunoreactivity. On the other hand, two possibilities might be considered to the absence of immunoreactivity in diffuse-type GC: this absence was not due to mutations in TP53 gene or an eventual mutation in this gene would not interfere in the protein accumulation. In both situations the immunoreactivity cannot be detected.
The p53 expression was also associated with a higher frequency of cells with two chr17 and two TP53 signals in intestinal-type GC. We hypothesize that these cells may present TP53 with mutations and this event could be occurring earlier than allelic deletion in intestinal-type gastric carcinogenesis. Further investigations concerning TP53 mutations and expression should be done in larger samples, also including early GC specimens.
Conclusion
Our findings showed that TP53 deletion and chromosome 17 aneusomy are common events in GC. Our results also suggest that LOH is an important TP53 alteration in GC. However, other TP53 alterations than allelic deletion may be implicated in the carcinogenesis process.
References
Parkin DM, Bray F, Ferlay J, Pisani P: Global cancer statistics, 2002. CA Cancer J Clin. 2005, 55: 74-108. 10.3322/canjclin.55.2.74.
Mortalidade por Câncer Gástrico no Estado do Pará, 1980–1997. Arq Gastroenterol. Edited by: Resende ALS, Mattos IE, Koifman S. 2006, DATASUS/Ministério da Saúde, Brasil, Informações de saúde, 43: 247-52.
Kimura Y, Noguchi T, Kawahara K, Kashima K, Daa T, Yokoyama S: Genetic alterations in 102 primary gastric cancers by comparative genomic hybridization: gain of 20q and loss of 18q are associated with tumor progression. Mod Pathol. 2004, 17: 1328-37. 10.1038/modpathol.3800180.
Lengauer C, Kinzler KW, Vogelstein B: Genetic instabilities in human cancers. Nature. 1998, 396: 643-9. 10.1038/25292.
Ferti-Passantonopoulou AD, Panani AD, Vlachos JD, Raptis SA: Common cytogenetic findings in gastric cancer. Cancer Genet Cytogenet. 1987, 24: 63-73. 10.1016/0165-4608(87)90083-5.
Xia JC, Lu S, Geng JS, Fu SB, Li P, Liu QZ: Direct chromosome analysis of ten primary gastric cancers. Cancer Genet Cytogenet. 1998, 102: 88-90. 10.1016/S0165-4608(97)00293-8.
Assumpção PP, Ishak G, Chen ES, Takeno SS, Leal MF, Guimarães AC, Calcagno DQ, Khayat AS, Demachki S, Smith Mde A, et al: Numerical aberrations of chromosome 8 detected by classic cytogenetic and Fluorescence in situ Hybridization in individuals from Northern Brazil with gastric adenocarcinomas. Cancer Genet Cytogenet. 2006, 169: 45-9. 10.1016/j.cancergencyto.2006.03.019.
Ochi H, Douglass HO, Sandberg AA: Cytogenetic studies in primary gastric cancer. Cancer Genet Cytogenet. 1986, 22: 295-307. 10.1016/0165-4608(86)90022-1.
Kitayama Y, Igarashi H, Sugimura H: Different vulnerability among chromosomes to numerical instability in gastric carcinogenesis: stage-dependent analysis by FISH with the use of microwave irradiation. Clin Cancer Res. 2000, 6: 3139-46.
Kallioniemi A, Visakorpi T, Karhu R, Pinkel D, Kallioniemi OP: Gene Copy Number Analysis by Fluorescence in Situ Hybridization and Comparative Genomic Hybridization. Methods. 1996, 9: 113-21. 10.1006/meth.1996.0015.
Panani AD, Ferti AD, Avgerinos A, Raptis SA: Numerical aberrations of chromosome 8 in gastric cancer detected by fluorescence in situ hybridization. Anticancer Res. 2004, 24: 155-9.
van Dekken H, Pizzolo JG, Kelsen DP, Melamed MR: Targeted cytogenetic analysis of gastric tumors by in situ hybridization with a set of chromosome-specific DNA probes. Cancer. 1990, 66: 491-7. 10.1002/1097-0142(19900801)66:3<491::AID-CNCR2820660315>3.0.CO;2-Q.
Han K, Oh EJ, Kim YS, Kim YG, Lee KY, Kang CS, Kim BK, Kim WI, Shim SI, Kim SM: Chromosomal numerical aberrations in gastric carcinoma: analysis of eighteen cases using in situ hybridization. Cancer Genet Cytogenet. 1996, 92: 122-9. 10.1016/S0165-4608(96)00165-3.
Beuzen F, Dubois S, Flejou JF: Chromosomal numerical aberrations are frequent in oesophageal and gastric adenocarcinomas: a study using in-situ hybridization. Histopathology. 2000, 37: 241-9. 10.1046/j.1365-2559.2000.00887.x.
Fringes B, Mayhew TM, Reith A, Gates J, Ward DC: Numerical aberrations of chromosomes 1 and 17 correlate with tumor site in human gastric carcinoma of the diffuse and intestinal types. Fluorescence in situ hybridization analysis on gastric biopsies. Lab Invest. 2000, 80: 1501-8.
Kitayama Y, Igarashi H, Watanabe F, Maruyama Y, Kanamori M, Sugimura H: Nonrandom chromosomal numerical abnormality predicting prognosis of gastric cancer: a retrospective study of 51 cases using pathology archives. Lab Invest. 2003, 83: 1311-20. 10.1097/01.LAB.0000087622.80751.C5.
Calcagno DQ, Leal MF, Taken SS, Assumpção PP, Demachki S, Smith MA, Burbano RR: Aneuploidy of chromosome 8 and C-MYC amplification in individuals from northern Brazil with gastric adenocarcinoma. Anticancer Res. 2005, 25: 4069-74.
Calcagno DQ, Leal MF, Seabra AD, Khayat AS, Chen ES, Demachki S, Assumpção PP, Faria MH, Rabenhorst SH, Ferreira MV, et al: Interrelationship between chromosome 8 aneuploidy, C-MYC amplification and increased expression in individuals from northern Brazil with gastric adenocarcinoma. World J Gastroenterol. 2006, 12: 6207-11.
Guimarães AC, Quintana LG, Leal MF, Takeno SS, Assumpção PP, Lima EM, Khayat AS, Chen ES, Smith MdeAC, Burbano RR: Aneuploidy of chromosome 8 detected by fluorescence in situ hybridisation in ACP01 cell line gastric adenocarcinomas. Clin Exp Med. 2006, 6: 129-33. 10.1007/s10238-006-0108-5.
Panani AD: Cytogenetic and molecular aspects of gastric cancer: clinical implications. Cancer Lett. 2008, 266: 99-115. 10.1016/j.canlet.2008.02.053.
Kobayashi M, Kawashima A, Mai M, Ooi A: Analysis of chromosome 17p13 (p53 locus) alterations in gastric carcinoma cells by dual-color fluorescence in situ hybridization. Am J Pathol. 1996, 149: 1575-84.
Takahashi Y, Nagata T, Asai S, Shintaku K, Eguchi T, Ishii Y, Fujii M, Ishikawa K: Detection of aberrations of 17p and p53 gene in gastrointestinal cancers by dual (two-color) fluorescence in situ hybridization and GeneChip p53 assay. Cancer Genet Cytogenet. 2000, 121: 38-43. 10.1016/S0165-4608(00)00231-4.
Gomyo Y, Osaki M, Kaibara N, Ito H: Numerical aberration and point mutation of p53 gene in human gastric intestinal metaplasia and well-differentiated adenocarcinoma: analysis by fluorescence in situ hybridization (FISH) and PCR-SSCP. Int J Cancer. 1996, 66: 594-9. 10.1002/(SICI)1097-0215(19960529)66:5<594::AID-IJC2>3.0.CO;2-O.
Suzuki S, Tenjin T, Shibuya T, Tanaka S: Chromosome 17 copy numbers and incidence of p53 gene deletion in gastric cancer cells. Dual color fluorescence in situ hybridization analysis. Nippon Ika Daigaku Zasshi. 1997, 64: 22-9.
Szymanska K, Hainaut P: TP53 and mutations in human cancer. Acta Biochim Pol. 2003, 50: 231-8.
Fenoglio-Preiser CM, Wang J, Stemmermann GN, Noffsinger A: TP53 and gastric carcinoma: a review. Hum Mutat. 2003, 21: 258-70. 10.1002/humu.10180.
Hurt EM, Thomas SB, Peng B, Farrar WL: Reversal of p53 epigenetic silencing in multiple myeloma permits apoptosis by a p53 activator. Cancer Biol Ther. 2006, 5: 1154-60. 10.1158/1535-7163.MCT-05-0446.
Batista dos Santos SE, Rodrigues JD, Ribeiro-dos-Santos AK, Zago MA: Differential Contribution of Indigenous Men and Women to the Formation of an Urban Population in the Amazon Region as Revealed by mtDNA and y-DNA. Am J Phys Anthropol. 1999, 109: 175-80. 10.1002/(SICI)1096-8644(199906)109:2<175::AID-AJPA3>3.0.CO;2-#.
Laurén P: The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965, 64: 31-49.
Sobin LH, Wittekind CH, eds: TNM: classification of malignant tumours. 2002, New York: Wiley-Liss, 6
Pinkel D, Straume T, Gray JW: Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA. 1986, 83: 2934-8. 10.1073/pnas.83.9.2934.
Hopman AH, Ramaekers FC, Raap AK, Beck JL, Devilee P, Ploeg van der M, Vooijs GP: In situ hybridization as a tool to study numerical chromosome aberrations in solid bladder tumors. Histochemistry. 1988, 89: 307-16. 10.1007/BF00500631.
Ozturk Y, Ozer E, Lebe B, Bekem O, Buyukgebiz B: Immunohistochemical evaluation of p53 expression and proliferative activity in children with Helicobacter pylori associated gastritis. J Pediatr Gastroenterol Nutr. 2005, 40: 467-70. 10.1097/01.MPG.0000148832.22130.D7.
Duensing A, Duensing S: Guilt by association? p53 and the development of aneuploidy in cancer. Biochem Biophys Res Commun. 2005, 331: 694-700. 10.1016/j.bbrc.2005.03.157.
Takeno SS, Leal MF, Lisboa LC, Lipay MV, Khayat AS, Assumpção PP, Burbano RR, Smith Mde A: Genomic alterations in diffuse-type gastric cancer as shown by high-resolution comparative genomic hybridization. Cancer Genet Cytogenet. 2009, 190: 1-7. 10.1016/j.cancergencyto.2008.09.007.
Burbano RR, Assumpção PP, Leal MF, Calcagno DQ, Guimarães AC, Khayat AS, Takeno SS, Chen ES, De Arruda Cardoso Smith M: C-MYC locus amplification as metastasis predictor in intestinal-type gastric adenocarcinomas: CGH study in Brazil. Anticancer Res. 2006, 26: 2909-14.
Galipeau PC, Cowan DS, Sanchez CA, Barrett MT, Emond MJ, Levine DS, Rabinovitch PS, Reid BJ: 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett's esophagus. Proc Natl Acad Sci USA. 1996, 93: 7081-4. 10.1073/pnas.93.14.7081.
Williams L, Jenkins GJ, Doak SH, Fowler P, Parry EM, Brown TH, Griffiths AP, Williams JG, Parry JM: Fluorescence in situ hybridisation analysis of chromosomal aberrations in gastric tissue: the potential involvement of Helicobacter pylori. Br J Cancer. 2005, 92: 1759-66. 10.1038/sj.bjc.6602533.
César AC, Borim AA, Caetano A, Cury PM, Silva AE: Aneuploidies, deletion, and overexpression of TP53 gene in intestinal metaplasia of patients without gastric cancer. Cancer Genet Cytogenet. 2004, 153: 127-32. 10.1016/j.cancergencyto.2004.01.017.
Gabbert HE, Müller W, Schneiders A, Meier S, Hommel G: The relationship of p53 expression to the prognosis of 418 patients with gastric carcinoma. Cancer. 1995, 76: 720-6. 10.1002/1097-0142(19950901)76:5<720::AID-CNCR2820760503>3.0.CO;2-E.
Poremba C, Yandell DW, Huang Q, Little JB, Mellin W, Schmid KW, Böcker W, Dockhorn-Dworniczak B: Frequency and spectrum of p53 mutations in gastric cancer–a molecular genetic and immunohistochemical study. Virchows Arch. 1995, 426: 447-55. 10.1007/BF00193167.
Kim JH, Uhm HD, Gong SJ, Shin DH, Choi JH, Lee HR, Noh SH, Kim BS, Cho JY, Rha SY, et al: Relationship between p53 overexpression and gastric cancer progression. Oncology. 1997, 54: 166-70. 10.1159/000227682.
Gürel S, Dolar E, Yerci O, Samli B, Oztürk H, Nak SG, Gülten M, Memik F: Expression of p53 protein and prognosis in gastric carcinoma. J Int Med Res. 1999, 27: 85-9.
Lee DY, Park CS, Kim HS, Kim JY, Kim YC, Lee S: Maspin and p53 protein expression in gastric adenocarcinoma and its clinical applications. Appl Immunohistochem Mol Morphol. 2008, 16: 13-8.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-230X/9/55/prepub
Acknowledgements
Supported by Financiadora de Estudos e Projetos (FINEP CT-INFRA/FADESP) grant No. 0927-03. RRB and MACS have a PQ fellowship granted by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). MFL has a fellowship granted by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP). ASK had a doctorate fellowship granted by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and is grateful to Hannah and Tarek for technical supports.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RRB and MACS designed the study. ASK, ACG, DQC, ADS, EML, MFL were involved in data collection, literature searches, genetic and statistical analysis. MHGF, SHBR, SD were involved in pathological analysis. PPA recruited patients and was responsible by samples collection. ASK wrote the first draft of the manuscript. All authors listed have contributed to all subsequent drafts, and have approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Khayat, A.S., Guimarães, A.C., Calcagno, D.Q. et al. Interrelationship between TP53gene deletion, protein expression and chromosome 17 aneusomy in gastric adenocarcinoma. BMC Gastroenterol 9, 55 (2009). https://doi.org/10.1186/1471-230X-9-55
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-230X-9-55