Abstract
Background
Excessive loss of bile acids in stool has been reported in patients with cystic fibrosis. Some data suggest that a defect in mucosal bile acid transport may be the mechanism of bile acid malabsorption in these individuals. However, the molecular basis of this defect is unknown. This study examines the expression of the ileal bile acid transporter protein (IBAT) and rates of diffusional (sodium independent) and active (sodium dependent) uptake of the radiolabeled bile acid taurocholate in mice with targeted disruption of the cftr gene.
Methods
Wild-type, heterozygous cftr (+/-) and homozygous cftr (-/-) mice were studied. Five one-cm segments of terminal ileum were excised, everted and mounted onto thin stainless steel rods and incubated in buffer containing tracer 3H-taurocholate. Simultaneously, adjacent segments of terminal ileum were taken and processed for immunohistochemistry and Western blots using an antibody against the IBAT protein.
Results
In all ileal segments, taurocholate uptake rates were fourfold higher in cftr (-/-) and two-fold higher in cftr (+/-) mice compared to wild-type mice. Passive uptake was not significantly higher in cftr (-/-) mice than in controls. IBAT protein was comparably increased. Immuno-staining revealed that the greatest increases occurred in the crypts of cftr (-/-) animals.
Conclusions
In the ileum, IBAT protein densities and taurocholate uptake rates are elevated in cftr (-/-) mice > cftr (+/-) > wild-type mice. These findings indicate that bile acid malabsorption in cystic fibrosis is not caused by a decrease in IBAT activity at the brush border. Alternative mechanisms are proposed, such as impaired bile acid uptake caused by the thick mucus barrier in the distal small bowel, coupled with a direct negative regulatory role for cftr in IBAT function.
Similar content being viewed by others
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the gene encoding the CFTR chloride channel [1]. CFTR is known to possess multiple cellular functions beyond its role as an apical membrane chloride channel [2]. For example, regulation of other ion channels, such as the epithelial sodium channel and the outwardly rectified chloride channel, are well-described functions of CFTR [3, 4]. Numerous other functions, such as regulation of intracellular vesicular pH, regulation of membrane recycling, and regulation of mucin exocytosis are less firmly established. Phenotypic expression in CF is variable, with multiple organs affected. The disease is commonly but variably expressed in the lungs, the exocrine pancreas, and the gastrointestinal mucosa [5, 6].
Significant intestinal malabsorption of various substrates, including bile acids, is a consistent finding in CF patients. The total bile acid pool is contracted unless supplementation with pancreatic enzymes is provided. In children, bile acid losses can be so severe that a deficiency of the amino acid taurine can occur due to excessive losses of taurine-conjugated bile acids [7, 8].
Bile acid malabsorption in CF has been attributed to an inhibitory effect of un-hydrolyzed, intraluminal triglycerides on the intestinal absorption of bile acids. However, in several studies the correlation between fecal fat excretion and fecal bile acid loss could not be demonstrated, suggesting that additional factors are responsible for bile acid malabsorption in cystic fibrosis [9, 10]. In vitro studies using brush border membrane vesicles or bathed tissue samples from patients with cystic fibrosis have shown that total ileal bile acid uptake is diminished [11, 12].
The present study was designed to determine if the activity and density of the ileal bile acid transporter IBAT is diminished in heterozygous and homozygous cftr knockout mice. The results indicate that bile acid malabsorption in CF is not caused by a defect in sodium-dependent bile acid transport at the brush border membrane suggesting another mechanism is operative.
Materials and Methods
Animals
Cftr knockout mice, with targeted disruption of the cftr gene created at the University of North Carolina [13], were maintained at the University of Washington Animal Care Facility under the auspices of the UW Cystic Fibrosis Research Center. Thirty-five post-weanling mice more than 20 days old (strain V129Xl/SvJ) were used, including 6 homozygous cftr (-/-), 23 heterozygous cftr (+/-) and 6 wild-type cftr (+/+) mice. All mice had been kept on a standard liquid diet (Peptamen®, Nestle Deerfield, IL)- for 3 weeks until immediately prior to sacrifice. On this diet, the mice showed no evidence of obstruction. Mice underwent inhalation anesthesia with 1.5% methoxyflurane (Metophane®, Mallinckrodt, Mundelein, IL) for the harvest of the intestines and were sacrificed subsequently by cervical dislocation under a protocol approved by the Institutional Animal Use Committee.
Geno-typing was performed using DNA isolated from the mouse tails. Following isolation of genomic DNA by standard techniques, PCR was performed using primers specific for wild-type mice and for cftr knockout mice. The primer sets used and the expected size products were as follows:
WT: CAGTGAAGCTGAGACTGTGA/GCATAATCCAAGAAAATTGAG(1.1 kb) CF: CGGTTCTTTTTGTCAAGAC/ATCCTCGCCGTCGGGCATGC (400 bp). Wild-type mice expressed cftr protein by immunoblotting, while cftr (-/-) did not [14]. All chemicals were from Sigma unless otherwise stated (Sigma-Aldrich, St. Louis, MO). The investigators performing the uptake and immunohistochemistry experiments were blinded to the results of the geno-typing.
Preparation of intestinal segments
Mice were anesthetized and the abdomen opened. A one-cm segment of jejunum was resected at the ligament of Treitz. The terminal 8 cm segment of ileum was removed and immediately transferred into 2°C cold mammalian Ringer solution (128 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM NaH2PO4 1.2 mM MgSO4, and 20 mM NaHCO3, pH at 37°C = 7.30 - 7.40, 290 mosm, gassed continuously with 95% O2/5% CO2). The intestinal length was measured and the tissue cut into 2 one-cm segments, 1 five-cm segment and another one-cm segment beginning distally. The five-cm gut segment located 2 cm to 7 cm proximal to ileocecal valve was used for uptake experiments. The segment was cut into five one-cm pieces. These specimens were everted and the intestinal sleeves secured onto 1 mm diameter stainless steel rods using 3–0 silk ties as described previously [15]. The distal ileal segments from 1–2 cm proximal to the ileocecal valve (immediately adjacent to the tissue used in the uptake experiments) and the jejunal segments were used for immunohistochemistry studies. The ileal segments from 7–8 cm and 0–1 cm proximal to the ileocecal valve, respectively, from each animal were pooled and used for Western blots.
Uptake measurements
All uptake measurements were performed between 1 and 2 hours after excision. Samples were randomized into 2 groups. Intestines from the first group were exposed to an identical radioactive taurocholate solution containing sodium. Samples from the second group were exposed to a sodium-free taurocholate solution. Mounted tissues were preincubated for 5 min. in 37°C warm, oxygenated Ringer's solution. Then rods were suspended with the tissue positioned 5 mm above stirring bars rotating at 1,200 rpm and uptake was measured for 2 min. in 37°C warm, oxygenated Ringer's solution with 1 mM taurocholate. To the uptake solutions tracer amounts of radiolabeled sodium taurocholate (3H-(G) sodium taurocholate, 74 GBq/mmol, NEN Research Products, Boston, MA) were added. 1,2-14C polyethylene glycol (MW 4000 Dalton; 9.3 MBq/mmol, NEN Research Products, Boston, MA), a marker substance not subject to carrier-mediated transport and with a very low diffusion coefficient, was added to these solutions to account for 3H-radiotracer in the adherent fluid. The incubation time was chosen based on validation experiments that showed that PEG equilibrated in the unstirred layer after two minutes. In contrast, taurocholate uptake rates remained linear for at least four minutes (data not shown).
To measure Na-independent uptake, Na-free Ringer's solution was prepared by replacing NaCl with Choline-Cl and NaHCO3 with KHCO3. In the Na-free solutions, taurocholate was added as a potassium salt. Potassium-taurocholate was prepared by ion exchange of a 1 M solution Na-taurocholate with potassium-loaded AmberLyte (Biorad, Hercules, CA). The potassium salt was subsequently recrystallized. Absence of sodium ions following the ion exchange was confirmed by flame-photometry.
After incubation, tissues were removed from the rods, placed in scintillation vials and solubilized in TS-2 tissue solubilizer (Research Products International, Mount Prospect, IL). Then 5 ml Safety-Solve scintillation cocktail (Research Products International) were added. Beta emissions (in disintegrations per minute {DPM}) from tritium and 14C were calculated from CPM counted in a dual channel liquid scintillation counter (Tricarb 2200 CA, Packard Instrument Co., Downers Grove, IL) based on appropriate standard spectra and quench curves for the dual-labeled samples. Calculations of uptake rates were performed as described previously [15, 16].
Immunohistochemistry studies
Polyclonal rabbit antibodies were obtained from a commercial source (Research Genetics, Huntsville, AL) against a C-terminal 14 amino acid oligopeptide fragment of IBAT and purified by immuno-affinity chromatography as described [17]. The ileal tissue segments were snap-frozen in -70°C cold isopentane and then stored in -70°C until the tissues were processed. Frozen sequential 10 micron cross-sections were cut in the cryostat at -25°C. The cryo-sections were air-dried at room temperature, fixed in acetone for 5 min., washed for 15 min in Tris-buffered saline (TBS; 20 mM Tris-HCl, 500 mM NaCl, pH 7.4) and incubated for 1 hour with 1% purified milk protein blocking solution (Boehringer-Mannheim, Indianapolis, IN) in TBS to reduce background staining. A 1:20 dilution of anti-IBAT protein antibodies was applied for one hour. Pre-immune serum served as a negative control. Sections were washed and incubated for one hour with a 1:200 dilution of a secondary goat anti-rabbit Cy3-IgG antibody (Jackson Immuno-Research Laboratories, West Grove, PA), then washed in 1 × TBS for 2 × 10 min. Slides were mounted using an aqueous medium containing Hoechst 33258 dye as a nuclear stain (Sigma, St. Louis, MO). The distribution of the fluorescence signal was evaluated under a fluorescence microscope (Zeiss, Jena, Germany) with photometer electronic multiplier (Hamamatsu, Billerica, MA) equipped with an intensity measuring program for fluorescence. The signal density was quantified as fluorescence intensity over background in circular image fields extending from the villus tip to the crypt base using an MS2 image analysis system (Imaging Research, St. Catherines, Ontario, Canada).
Western blots
Brush border membrane vesicles were prepared as described by deRooij [11]. Briefly, frozen ileal tissue fragments (20–25 mg) were thawed and homogenized in 100 μl of isotonic buffer (300 mM mannitol, 12 mM Tris-HCl, pH 7.1) using a Polytron homogenizer (Kinematica AG, Littau, Switzerland). This suspension was cleared form debris and diluted with five volumes of ice cold water and MgCl2 was added to a final concentration of 10 mM. After 40 minutes this suspension was centrifuged at 3,000 × g for 15 min. The supernatant was decanted into another vial and stored at 4°C. The pellet was resuspended in buffer and after 40 minutes this suspension was centrifuged as before. The two supernatant fractions were combined and centrifuged for 30 min at 27,000 × g to spin down the brush border membrane vesicles. 10 μl of the brush border membrane preparation was diluted in 90 μl of Laemmli buffer, boiled for 5 min and separated on a denaturing polyacrylamide gel (5 % stacking gel/10 % resolving gel) at 120 V over 2 hours [18]. The separated proteins were transferred onto nitrocellulose membrane using a Trans-blot instrument (Biorad, Hercules, CA) in glycine-methanol buffer following the manufacturer's suggestions. Blots were developed using a 1:100 dilution of the rabbit anti-hamster IBAT antibodies and an amplified alkaline phosphatase goat-anti-rabbit immunoblot assay kit (Biorad, Hercules, CA) as recommended by the manufacturer.
Statistical Analysis
All data were collected from individual tissues and processed as independent data points as well as grouped by their origin (2 cm, 3 cm from the ileocecal valve, etc.) Results were expressed as mean +/- standard error of the mean (SEM). Comparisons were made between multiple groups using ANOVA and the unpaired student t-test as a post-hoc test. Significance was assumed at p < 0.05.
Results
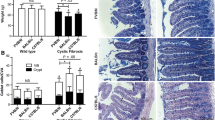
Active rates of taurocholate uptake were twice as high in the heterozygous cftr (+/-) mice (n = 23) compared to wild-type mice (n = 6) (average of all data points in the terminal ileum 109 ± 8 vs. 64 ± 13 cm pmol/mm2; p < 0.05) and fourfold higher in the homozygous cftr (-/-) mice (n = 6) (212 ± 32 pmol/mm2; p < 0.05 vs. wild-type). Increases occurred similarly at different sites in the terminal ileum in locations from 2 cm to 7 cm from the ileocecal valve (Fig. 1a). However, only differences in the section at 2 and 3 cm reached a level of significance of p < 0.05. Differences in rates of passive uptake of taurocholate were not statistically significant among groups (Fig. 1b). Microscopic morphology of the mucosa was similar in the different study groups.
Sodium-Dependent Taurocholate Uptake Rates by Ileal Segments from Wild-Type, Heterozygous and Cystic Fibrosis Mice. An increase in active taurocholate uptake was observed in all examined sections of the terminal ileum of knock-out mice. Uptakes rates approximately doubled in heterozygous knock-out mice (CFTR +/-; n = 23) and quadrupled in homozygous mutants (CFTR -/-; n = 6) compared to wild-type controls (WT; n = 6). Differences were statistically significant in the 2 cm and 3 cm groups (# = significant difference when comparing this group to the wild-type group at p < 0.05; ** = significant difference when comparing this group to the heterozygous group at p < 0.05). The axial distribution curves of taurocholate uptake were similar and had peaks at 3–4 cm before the ileocecal valve in all three groups.
Prior to use for Western blotting, brush border membrane vesicle suspensions were tested for enrichment of brush border enzymes and showed a 4 – 5-fold increase of alkaline phosphatase and a 10 – 11-fold increase in alpha-glucosidase activity using standard methods [19]. On densitometric evaluations of Western blots, IBAT concentrations were twice as high in heterozygotes as in controls (17 ± 3 vs. 5 ± 1 density units; p < 0.05) and four times as high in homozygotes (30 ± 4 vs. 5 ± 1 density units; p < 0.05) (Fig. 2 and 3). A similar relationship was found in intensity of IBAT immunostaining on examinations of microscopic sections treated with anti-IBAT antibodies. The highest increases in signal density were noted at the base of the crypts of cftr (-/-) animals (arrow in Fig. 4). These unusual changes were never found in any jejunal samples and occurred only occasionally as weak signals in ileal samples from wild-type animals.
Western Blot of Ileal Brush Border Proteins from Wild-Type, Heterozygous and Cystic Fibrosis Mice. An increase in IBAT protein concentration was observed in all examined brush border membrane vesicle preparations of the terminal ileum in knock-out mice. IBAT content approximately doubled in heterozygous knock-out mice and quadrupled in homozygous mutants compared to wild-type controls.
Quantification of Western Blot for IBAT in Figure 3 from Wild-Type, Heterozygous and Cystic Fibrosis Mice. IBAT band density approximately doubled in heterozygous knock-out mice (n = 23) and quadrupled in homozygous mutants (n = 6) compared to wild-type controls (n = 6) (# = significant difference when comparing this group to the wild-type group at p < 0.05; ** = significant difference when comparing this group to the heterozygous group at p < 0.05).
Staining of IBAT protein in the Ileal Mucosal Brush Border from Wild-Type, Heterozygous and Cystic Fibrosis Mice. An increase in IBAT protein staining intensity was observed in microscopic sections of the terminal ileum of knock-out mice. IBAT signal intensities were higher in heterozygous knock-out mice and still higher in homozygous mutants compared to wild-type controls. Jejunal mucosa showed no IBAT signals.
Discussion
Untreated children and adults with cystic fibrosis excrete amounts of fecal bile acids similar to patients with ileal resections [20, 21]. Based on kinetic studies, this increased fecal loss appears to be secondary to an interrupted enterohepatic circulation of bile acids [20, 21]. Bile acid malabsorption in cystic fibrosis appears to be due in part to diminished secretion of pancreatic enzymes and the presence of un-hydrolyzed triglycerides. Supplementation with pancreatic enzymes and a low-fat diet can markedly reduce the fecal excretion of bile acids [9, 10, 20, 23]. Other evidence, however, suggests that a diminished capacity of the ileal mucosa for bile acid uptake plays a significant role in bile acid malabsorption associated with cystic fibrosis that is independent of triglyceride concentrations. Findings supporting this notion include: 1. The absence of a correlation between fecal fat excretion and fecal bile acid loss; 2. The normal fecal bile acid losses in patients with liver disease compared to excessive losses in those without liver disease despite similar fecal fat excretion; and 3. The finding that patients with almost normal fat excretion continue to lose large amounts of bile acids.
Based on these findings, a defect in ileal bile acid transport mechanisms in cystic fibrosis has been suspected. Data in support of this hypothesis are contradictory. Fondacaro et al. bathed ileal biopsies from CF patients in taurocholate solutions. They found a decrease in total (apical + basolateral) bile acid uptake compared to normal controls [12] and hypothesized that bile acid malabsorption in cystic fibrosis was due to a primary mucosal defect although they had not specifically studied apical, Na+-dependent absorption. De Rooij et al. found significant decreases in active bile acid uptake when examining ileal brush border membrane vesicles [11] and proposed that altered viscosity of the ileal mucus was likely not a factor in ileal bile acid malabsorption associated with cystic fibrosis. In another study using marker perfusions with taurocholate and glycocholate in three infants, however, the bile acid uptake rates with cystic fibrosis were similar to control subjects [24].
The results from our study stand in contrast to these earlier studies. We found a significant increase in bile acid transport in cftr (-/-) and cftr (+/-) mice. This increase in IBAT function was paralleled by increases in IBAT protein concentrations. Similar discrepancies between measurements of solute uptake with brush border membrane vesicles or tissue biopsies and in vivo experiments have been observed in studies examining sodium/glucose cotransport in cystic fibrosis. Jejunal glucose absorption was enhanced in patients with cystic fibrosis when measured with catheter perfusion techniques [25]. Ussing chamber studies of intact CF biopsy specimens suggest that glucose and alanine uptake rates are markedly increased in cystic fibrosis [26]. Taylor also studied intact jejunal biopsies and found that the rate of active sodium/glucose transport approximately doubled in CF compared to normal jejunum [27].
Contrary to these findings, other investigations have been unable to demonstrate any changes in sodium/glucose co-transport when examining brush border membrane vesicles [28]. The up-regulation of intestinal sodium-linked nutrient absorption by cftr may occur via a mechanism that does not involve changes of the brush border membrane but rather intracellular components [28]. Intestinal sodium-linked nutrient absorption would then be undetectable in brush border membrane vesicle studies. However, a number of studies using biopsies in Ussing chambers have also been unable to demonstrate enhanced glucose absorption in cystic fibrosis [29–31].
Phenotypic expression is variable in patients carrying homozygous or heterozygous mutations of the CFTR gene. The ΔF508 mutation is the most common genetic disturbance affecting the CFTR gene. ΔF508 heterozygotes are affected to a lesser degree than are homozygotes. In heterozygous infants with the WT/ΔF508 genotype, sweat chloride excretion is elevated 1.5-fold over normal values compared to a tenfold increase in homozygous patients with the ΔF508/ΔF508 genotype [32]. Adult heterozygous patients have fewer problems with respiratory infections compared to homozygotes [5]. Our findings that bile acid absorption was moderately increased in heterozygotes and markedly increased in homozygotes were therefore not unexpected. Heterozygotes are able to produce a limited number of intact, correctly localized CFTR protein molecules resulting in partial phenotypic expression. The correlation between zygosity and level of IBAT protein expression suggests a close relationship between the function of the CFTR chloride channel and the sodium-dependent ileal bile acid transporter.
Our study is the first that correlates changes in function of another intestinal solute transporter with the expression level of CFTR. Increased bile acid transport in cftr (-/-) mice is associated with an increase in the abundance of IBAT protein in the brush border membrane. Similar associations exist in normal rodents. In hamsters, rats and mice, increases and decreases in bile acid absorption at the brush border membrane are mediated by an up- or down-regulation in IBAT gene transcription [33, 34]. In several rodent species including mice, regulation of IBAT is clearly dependent on intraluminal bile acid concentrations and occurs via a negative feedback mechanism [33–35]. Feeding of bile acids suppresses transcription of IBAT mRNA and reduces IBAT protein abundance; feeding of the bile acid binder cholestyramine has the opposite effect. In normal animals, this negative feedback mechanism keeps the enterohepatic bile acid pool in equilibrium. As bile acids are lost, increased absorption by a greater number of transporters, in combination with increased hepatic bile acid synthesis from cholesterol, helps restore normal bile acid levels. In contrast, cftr (-/-) mice may experience bile acid malabsorption even though their ileal mucosal cells express four-fold more IBAT transporters than normal cells. This apparently paradoxical finding would suggest a luminal factor that prevents bile acids from entering the mucosal cells in the ileum.
Based on this evidence, we postulate that the more viscous mucus layer in the ileal crypts of individuals with cystic fibrosis limits access of luminal bile acids and micelles to the ileal crypt cells. This may result in an altered programming of ileal stem cells and transit cells that give rise to absorptive enterocytes over-expressing IBAT protein.
Further investigations are needed to corroborate this hypothesis. Additional studies should examine changes in mucus layer thickness, mucus viscosity and permeability for bile acids associated with hetero- and homozygosity for CFTR mutations. An alternative mechanism for the upregulation of IBAT in cystic fibrosis is that CFTR acts as a negative regulator of IBAT, as it does for the epithelial sodium channel [36]. As IBAT is a sodium-dependent apical membrane transporter, it might be regulated by CFTR, as is the epithelial sodium channel (ENaC) present in the apical membrane of the ileal enterocyte. CFTR regulation of ENaC has been described in the colon in humans [37] and in cftr knockout mice [37]. Co-localization of CFTR and ENaC suggests that direct protein-protein interactions allow CFTR to closely regulate the activity of this channel [39, 40]. Our results suggest that knockout of CFTR leads to an analogous increase in expression and function of IBAT. Of course, a more widespread perturbation of ion channel regulation in the absence of CFTR is also a possibility, as CFTR has been demonstrated to be a master regulator of such diverse proteins as the Na/K pump [41], the basolateral Na/K/2Cl co-transporter [42], and the inducible nitric oxide synthase [43].
Conclusions
In summary, in the widely studied cftr knockout mouse model (which clearly demonstrates intestinal pathology), the apical sodium dependent ileal bile acid transporter is expressed and functions at higher levels than in wild-type animals, with intermediate levels of IBAT expression and function in heterozygous cftr (+/-) mice. The results of this study open up further avenues of investigation with respect to the role of the mucus layer in causing a secondary up-regulation of IBAT expression and function. In addition, IBAT may be yet another apical membrane transport protein that is regulated by CFTR.
Grant Support
Part of this work was supported by a VA Merit Review Grant
Abbreviations
- IBAT:
-
ileal bile acid transporter
- CFTR:
-
cystic fibrosis transmembrane conductance regulator. This is the protein product of the Cystic Fibrosis gene and is considered to act as a Cl channel. TBS: Tris-buffered saline.
References
Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL: Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989, 245: 1066-1073.
Jentsch TJ: Chloride channels: a molecular perspective. Curr Opin Neurobiol. 1996, 6: 303-310. 10.1016/S0959-4388(96)80112-7.
Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB: CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995, 81: 1063-1073.
Kunzelmann K, Schreiber R, Nitschke R, Mall M: Control of epithelial Na+ conductance by the cystic fibrosis transmembrane conductance regulator. Pflugers Arch. 2000, 440: 193-201. 10.1007/s004240051039.
Rosenecker J: Relations between the frequency of the DeltaF 508 mutation and the course of pulmonary disease in cystic fibrosis patients infected with Pseudomonas aeruginosa. Eur J Med Res. 2000, 5: 356-359.
Colombo C, Roda A, Roda E, Piceni Sereni L, Brega A, Fugazza R, Giunta A: Bile acid malabsorption in cystic fibrosis with and without pancreatic insufficiency. J Pediatr Gastroenterol Nutr. 1984, 3: 556-562.
Thompson GN: Excessive fecal taurine loss predisposes to taurine deficiency in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1988, 7: 214-219.
Carrasco S, Codoceo R, Prieto G, Lama R, Polanco I: Effect of taurine supplements on growth, fat absorption and bile acid on cystic fibrosis. Acta Univ Carol [Med] (Praha). 1990, 36: 152-156.
Watkins JB, Tercyak AM, Szczepanik P, Klein PD: Bile salt kinetics in cystic fibrosis: influence of pancreatic enzyme replacement. Gastroenterology. 1977, 73: 1023-1028.
Smalley CA, Brown GA, Parkes ME, Tease H, Brookes V, Anderson CM: Reduction of bile acid loss in cystic fibrosis by dietary means. Arch Dis Child. 1978, 53: 477-482.
de Rooij FW, van den Berg JW, Sinaasappel M, Bosman-Jacobs EP, Touw-Blommesteijn AC: Bile acid malabsorption in cystic fibrosis; membrane vesicles, a tool for revealing the role of the ileal brush border membrane. Acta Paediatr Scand Suppl. 1985, 317: 28-30.
Fondacaro JD, Heubi JE, Kellogg FW: Intestinal bile acid malabsorption in cystic fibrosis: a primary mucosal cell defect. Pediatr Res. 1982, 16: 494-498.
Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH: An animal model for cystic fibrosis made by gene targeting. Science. 1992, 257: 1083-1088.
Kuver R, Savard C, Nguyen TD, Osborne WR, Lee SP: Isolation and long-term culture of gallbladder epithelial cells from wild-type and CF mice. In Vitro Cell Dev Biol Anim. 1997, 33: 104-109.
Karasov WH, Diamond JM: A simple method for measuring intestinal solute uptake in vitro. J Comp Physiol. 1983, 152: 105-116.
Stelzner M, Hoagland V, Somasundaram S: A simple method for measuring of intestinal solute transport in mucosal biopsy specimens. Dig Dis Sci. 2001, 46: 451-461. 10.1023/A:1005681624873.
Stelzner M, Hoagland V, Somasundaram S: Distribution of bile acid absorption and bile acid transporter gene message in the hamster ileum. Pflugers Arch – Europ J Physiol. 2000, 440: 157-162.
Sambrook J, Fritsch E, Maniatis T: Molecular cloning. A laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press;. 1989
Weber AM, Roy CC, Morin CL, Lasalle R: Malabsorption of bile acids in children with cystic fibrosis. N Engl J Med. 1973, 289: 1001-1005.
Peters T, Heath J, Wansbury-Jones M, Doe W: Enzyme activities and properties of lysosomes and brush border in jejunal biopsies of control subjects and patients with coeliac disease. Clin Sci and Molecular Med. 1975, 48: 250-267.
O'Brien S, Mulcahy H, Fenlon H, O'Broin A, Casey M, Burke A, FitzGerald MX, Hegarty JE: Intestinal bile acid malabsorption in cystic fibrosis. Gut. 1993, 34: 1137-1141.
Harries JT, Muller DP, McCollum JP, Lipson A, Roma E, Norman AP: Intestinal bile salts in cystic fibrosis: studies in the patient and experimental animal. Arch Dis Child. 1979, 54: 19-24.
Roller RJ, Kern F: Minimal bile acid malabsorption and normal bile acid breath tests in cystic fibrosis and acquired pancreatic insufficiency. Gastroenterology. 1977, 72: 661-665.
Thompson GN, Davidson GP: In vivo bile acid uptake from terminal ileum in cystic fibrosis. Pediatr Res. 1988, 23: 323-328.
Frase LL, Strickland AD, Kachel GW, Krejs GJ: Enhanced glucose absorption in the jejunum of patients with cystic fibrosis. Gastroenterology. 1985, 88: 478-484.
Baxter P, Goldhill J, Hardcastle J, Hardcastle PT, Taylor CJ: Enhanced intestinal glucose and alanine transport in cystic fibrosis. Gut. 1990, 31: 817-820.
Taylor CJ, Baxter PS, Hardcastle J, Hardcastle PT, Goldhill J: Glucose intolerance in cystic fibrosis [letter]. Arch Dis Child. 1989, 64: 759-
Beesley AH, Hardcastle J: Sodium/glucose cotransporter activity in cystic fibrosis. Arch Dis Childhood. 1996, 75: 169-172.
Berschneider HM, Knowles MR, Azizkhan RG, Boucher RC, Tobey NA, RC Orlando, Powell DW: Altered intestinal chloride transport in cystic fibrosis. Faseb J. 1988, 2: 2625-2629.
Taylor CJ, Baxter PS, Hardcastle J, Hardcastle PT: Absence of secretory response in jejunal biopsy samples from children with cystic fibrosis [letter]. Lancet. 1987, 2: 107-108. 10.1016/S0140-6736(87)92781-4.
Taylor CJ, Baxter PS, Hardcastle J, Hardcastle PT: Failure to induce secretion in jejunal biopsies from children with cystic fibrosis. Gut. 1988, 29: 957-962.
Farrell PM, Koscik RE: Sweat chloride concentrations in infants homozygous or heterozygous for F508 cystic fibrosis. Pediatrics. 1996, 97: 524-528.
Lillienau J, Crombie DL, Munoz J, Longmire-Cook SJ, Hagey LR, Hofmann AF: Negative feedback regulation of the ileal bile acid transport system in rodents. Gastroenterology. 1993, 104: 38-46.
Torchia EC, Cheema SK, Agellon LB: Coordinate Regulation of bile acid biosynthetic and recovery pathways. Biochem Biophys Res. 1996, 225: 128-133. 10.1006/bbrc.1996.1141.
Stelzner M: Unpublished data.
Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC: CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995, 269: 847-850.
Mall M, Bleich M, Kuehr J, Brandis M, Greger R, Kunzelmann K: CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. Am J Physiol. 1999, 277: G709-716.
Grubb BR, Boucher RC: Enhanced colonic Na+ absorption in cystic fibrosis mice versus normal mice. Am J Physiol. 1997, 272: G393-400.
Ji HL, Chalfant ML, Jovov B, Lockhart JP, Parker SB, Fuller CM, BA Stanton, Benos DJ: The cytosolic termini of the beta- and gamma-ENaC subunits are involved in the functional interactions between cystic fibrosis transmembrane conductance regulator and epithelial sodium channel. J Biol Chem. 2000, 275: 27947-27956.
Berdiev BK, Shlyonsky VG, Karlson KH, Stanton BA, Ismailov II: Gating of amiloride-sensitive Na(+) channels: subunit-subunit interactions and inhibition by the cystic fibrosis transmembrane conductance regulator. Biophys J. 2000, 78: 1881-1894.
Ito Y, Mizuno Y, Aoyama M, Kume H, Yamaki K: CFTR-Mediated anion conductance regulates Na(+)-K(+)-pump activity in Calu-3 human airway cells. Biochem Biophys Res Commun. 2000, 274: 230-235. 10.1006/bbrc.2000.3125.
Shumaker H, Soleimani M: CFTR upregulates the expression of the basolateral Na(+)-K(+)-2Cl(-) cotransporter in cultured pancreatic duct cells. Am J Physiol. 1999, 277: C1100-1110.
Steagall WK, Elmer HL, Brady KG, Kelley TJ: Cystic fibrosis transmembrane conductance regulator-dependent regulation of epithelial inducible nitric oxide synthase expression. Am J Respir Cell Mol Biol. 2000, 22: 45-50.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-230X/1/10/prepub
Acknowledgements
The authors wish to express their gratitude to J. Donald Ostrow for a critical review of the manuscript and for his helpful advice. The authors also thank Dr. Christopher Wilson, Dept. of Immunology, University of Washington, for providing mice from his colony.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing Interests
None declared.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Stelzner, M., Somasundaram, S., Lee, S.P. et al. Ileal mucosal bile acid absorption is increased in Cftr knockout mice. BMC Gastroenterol 1, 10 (2001). https://doi.org/10.1186/1471-230X-1-10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-230X-1-10