Abstract
Background
Bacillus cereus is a foodborne pathogen that causes emetic or diarrheal types of food poisoning. The incidence of B. cereus food poisoning has been gradually increasing over the past few years, therefore, biocontrol agents effective against B. cereus need to be developed. Endolysins are phage-encoded bacterial peptidoglycan hydrolases and have received considerable attention as promising antibacterial agents.
Results
The endolysin from B. cereus phage B4, designated LysB4, was identified and characterized. In silico analysis revealed that this endolysin had the VanY domain at the N terminus as the catalytic domain, and the SH3_5 domain at the C terminus that appears to be the cell wall binding domain. Biochemical characterization of LysB4 enzymatic activity showed that it had optimal peptidoglycan hydrolase activity at pH 8.0-10.0 and 50°C. The lytic activity was dependent on divalent metal ions, especially Zn2+. The antimicrobial spectrum was relatively broad because LysB4 lysed Gram-positive bacteria such as B. cereus, Bacillus subtilis and Listeria monocytogenes and some Gram-negative bacteria when treated with EDTA. LC-MS analysis of the cell wall cleavage products showed that LysB4 was an L-alanoyl-D-glutamate endopeptidase, making LysB4 the first characterized endopeptidase of this type to target B. cereus.
Conclusions
LysB4 is believed to be the first reported L-alanoyl-D-glutamate endopeptidase from B. cereus-infecting bacteriophages. The properties of LysB4 showed that this endolysin has strong lytic activity against a broad range of pathogenic bacteria, which makes LysB4 a good candidate as a biocontrol agent against B. cereus and other pathogenic bacteria.
Similar content being viewed by others
Background
Bacillus cereus is a Gram-positive, spore-forming, rod-shape bacterium that grows well in aerobic and anaerobic environments [1]. It causes food poisoning by producing two different types of toxins: an emetic toxin and a diarrheal toxin [2]. Although the symptoms caused by B. cereus food poisoning are relatively mild, the incidence of the disease is gradually increasing, and it can develop into severe disease [3]. In addition, B. cereus can survive at a wide temperature range and form spores in harsh environments, especially during food processing; therefore, measures to control B. cereus effectively in the food industry are necessary [4, 5].
Recently, endolysins have been explored as promising antibacterial agents. Endolysins are phage-encoded enzymes that hydrolyze the peptidoglycan bacterial cell wall [6]. Endolysins are synthesized at the end of the phage replication cycle and allow liberation of progeny phage particles from the host cell [7]. Most endolysins lack secretory signal sequences, therefore, holins are needed for endolysins to pass through the inner membrane and reach peptidoglycan, defined as the canonical holin-endolysin lysis system [6, 8].
Endolysins are expected to be more effective biocontrol agents toward Gram-positive than Gram-negative bacteria, because the latter have an outer membrane that blocks access of endolysins to the peptidoglycan layer, when applied exogenously [9]. In addition, other advantages of endolysins as biocontrol agents include: (i) low chance of developing bacterial resistance; (ii) species-specific lytic activity without affecting other bacteria; and (iii) high enzymatic activity that enables bacterial cells lysis within minutes or even seconds [7, 10, 11]. Endolysins are successfully applied in food products, such as milk and banana juice, to prevent contamination of Staphylococcus aureus or Gram-negative bacteria [12, 13]. Besides, many reports already have shown that endolysins have high potential as strong therapeutic agents against a number of human pathogens through animal model studies [7, 14–16].
To date, only three endolysins from B. cereus bacteriophages have been characterized, all of which are N-acetylmuramoyl-L-alanine amidase-type endolysins [17]. Moreover, only a few reported phages can infect B. cereus, although many Bacillus-targeting bacteriophages have been reported [18, 19]. Thus, more bacteriophages and endolysins targeting B. cereus should be isolated and characterized to provide additional candidates for B. cereus biocontrol agents.
In previous work, we isolated the bacteriophage B4 (accession no. JN790865), which is a lytic phage infecting B. cereus, from forest mud, and its genome was sequenced and analyzed to annotate important features (Shin et al., unpublished). In the present study, an endolysin gene was identified in the B4 bacteriophage genome. This endolysin gene was cloned and expressed in Escherichia coli, and the purified endolysin was characterized for its biochemical properties. To the best of our knowledge, LysB4 is the first endolysin belonging to the L-alanoyl-D-glutamate endopeptidases originating from B. cereus bacteriophages.
Results
Identification and expression of the LysB4 phage endolysin
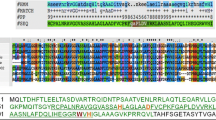
Annotation of bacteriophage B4 genome sequence identified a predicted open reading frame (ORF) for a putative endolysin gene (Shin et al., unpublished). This 789-bp-long ORF was designated lysB4. Using the InterProScan program (http://www.ebi.ac.uk/Tools/pfa/iprscan/), LysB4 was predicted to have the VanY domain (PF02557) at the N terminus and SH3_5 domain (PF08460) at the C terminus (Figure 1a). According to BLASTP analysis [20], the N terminus of LysB4 had high similarity to L-alanoyl-D-glutamate peptidases of Listeria monocytogenes FSL J1-175 (ZP 05387674), Bacillus subtilis subsp. subtilis str. 168 (CwlK, NP 388163) , the Listeria phage A500 (Ply500, YP 001488411) and the Bacillus phage SPO1 (YP 001487954), and the C terminus had high similarity to proteins belonging to B. cereus AH676 (ZP 0419059), Bacillus phages TP21-L (Ply21, CAA72267) and bg1 (LysBG1, ABX56141), and the Lactobacillus phage LL-Ku (AAV30211). Among these proteins, Ply500 of Listeria phage A500 needs Zn2+ in its active site according to a structural analysis [21]. The three Zn2+-coordinating residues (His80, Asp87 and His133) characterized in PlyA500 were conserved in the amino acid sequence of LysB4 [21], and the Zn2+ binding domain (SxHxxGxAxD) reported in Enterococcus VanX was found in LysB4 (Figure 1b) [22].
Sequence analysis of LysB4. (a) Domain structures of LysB4 compared with four other peptidoglycan hydrolases. CwlK, the cell wall hydrolase in B. subtilis (ZP 08507241); Ply500, an endolysin in a L. monocytogenes phage (CAA59365); Ply21, an endolysin in a B. cereus phage (CAA72267); and LysBG1, an endolysin from a Bacillus phage (ABX56141). The grey shadows indicate conserved regions between proteins. (b) Alignment of amino acid sequences of LysB4, Ply500 and CwlK in their VanY domains. Three small triangles indicate Zn2+ binding residues, and the zinc binding motif was boxed.
Recombinant LysB4 was cloned and expressed in E. coli with an N-terminal His-tag followed by purification using affinity chromatography. SDS-PAGE showed a single band between 26 and 34 kDa, which was consistent with the calculated molecular mass (28 kDa; Figure 2a). Only 5 μg of purified LysB4 could lyse B. cereus ATCC 10876 cells substantially in 5 min (Figure 2b). Viable cell counting revealed that 5 μg of LysB4 under this reaction condition could reduce the viable cell number by 3 to 4-log after 15 min (data not shown). Moreover, typical optical microscopy showed that most bacilli were ruptured and disappeared by addition of LysB4 within 15 min (Figure 2c).
Purification of LysB4 and lytic activity of LysB4. (a) Purified LysB4 was loaded on an SDS-PAGE gel. Lane M, molecular weight marker; lane 1, the purified LysB4 fraction. (b) Different concentration of LysB4 was added to the suspension of B.cereus ATCC 10876, and decrease in turbidity was monitored. (c) Diluted suspension of B. cereus ATCC10876 (100 μl) was mixed with 5 μg of LysB4 and observed under optical microscope (× 1,000 magnification).
Effect of pH, temperature and ionic strength
Analysis of lytic activity at different pH showed that LysB4 had the highest lytic activity at pH 8.0-10.0 (Figure 3a). This endolysin was relatively stable under a wide range of pH values, as incubation at pH 2.0-10.5 for 30 min did not inactivate the lytic activity (data not shown). In addition, although this endolysin was active to lyse the susceptible bacteria between 37 and 75°C, the maximal activity was shown at 50°C (Figure 3b). However, LysB4 was inactivated when it was incubated at > 55°C for 30 min (data not shown). The influence of NaCl on the lytic activity of LysB4 was determined from 0-200 mM NaCl. As the NaCl concentrations increased, LysB4 lytic activity was reduced, resulting in approximately 60% decrease in the presence of 200 mM NaCl (Figure 3c).
Effect of pH, temperature, and NaCl on the lytic activity of LysB4. The effect of pH (a), temperature (b), and NaCl concentration (C) on the lytic activity of LysB4 against B. cereus ATCC 10876 cells was shown. Relative lytic activity was obtained by comparing the lytic activity of each test with the maximal lytic activity among the dataset. Each column represents the mean of triplicate experiments, and error bars indicate the standard deviation.
Effect of divalent metal ions
To examine the effects of divalent metal ions to LysB4 enzymatic activity, we first removed metal ions from the protein using 5.0 mM EDTA. As seen in Table 1 incubation of endolysin with 5 mM EDTA significantly decreased the lytic activity, which suggests LysB4 required metal ions for its full lytic activity. When 0.1 mM Zn2+or Mn2+ was added to the EDTA-treated endolysin, the lytic activity of the enzyme was restored (Table 1). In the case of other divalent metal ions, such as Ca2+ and Mg2+, addition of higher concentration (1 mM) restored LysB4 enzymatic activity. However, addition of Hg2+ and Cu2+ did not resort activity of the EDTA-treated endolysin. Taken together, LysB4 requires divalent metal ions, particularly Zn2+ or Mn2+ for its enzymatic activity.
Antimicrobial spectrum of LysB4
Antimicrobial activity against several Gram-positive and Gram-negative bacteria (Table 2) was examined. Six B. cereus strains, B. subtilis, and two L. monocytogenes strains were susceptible to 5 μg LysB4, showing complete lysis in the reaction buffer within 5 min. This enzyme did not show lytic activity against other Gram-positive bacteria such as Enterococcus faecalis, Staphylococcus aureus strains, Streptococcus thermophilus and Lactococcus lactis. Furthermore, LysB4 lytic activity was not detected with Gram-negative bacteria, since they have a different cell wall composition (e.g., outer membrane) from Gram-positive bacteria. However, when cells were washed with 0.1 M EDTA to increase the cell wall permeability, LysB4-mediated cell lysis was detected for all tested Gram-negative bacteria including E. coli, Pseudomonas aeruginosa, Cronobacter sakazakii, Salmonella Typhimurium strains, Salmonella Enteritidis, Shigella flexneri, and Shigella boydii. In particular, E. coli O157:H7 strains were lysed efficiently by LysB4.
Endopeptidase activity of LysB4
LysB4 had the VanY domain at its N terminus. The VanY domain encoded an L-alanoyl-D-glutamate endopeptidase and therefore LysB4 was expected to have endopeptidase activity. This was confirmed using the trinitrobenzene sulfonic acid (TNBS) method that detects the liberated free amino groups from B. cereus peptidoglycan caused by hydrolysis of LysB4. Pre-existing amino groups were eliminated by acetylating the peptidoglycan. We detected a high concentration of free amino groups (0.37 mM) released from the acetylated peptidoglycan after incubation with LysB4 (15 μg) for 1 h, whereas only 0.04 mM was released from the peptidoglycan in the absence of LysB4. Moreover, this enzyme did not show any N-acetylmuramoyl-L-alanine amidase or glycosidase activity (data not shown). Therefore, LysB4 belongs to the endopeptidases.
Determination of the cleavage site by LysB4 in the peptidoglycan
The specific LysB4 cleavage site in the peptidoglycan was determined by reverse-phase (RP)-HPLC and LC-MS (Figure 4). A peak that was absent from the control reaction (Figure 4a) and had a retention time of 31.03 min was observed in cell wall samples digested with LysB4 (arrow, Figure 4b). This peak corresponded to a fragment ion at m/z of 311.86, which seemed to be the [M-H]- of 2,4-dinitrophenol (DNP)-D-Glu (Mr, 313). Both peaks at 31.75 min in Figure 4a and at 31.79 min in Figure 4b corresponded to DNP. When non-acetylated and acetylated peptidoglycan substrate were hydrolyzed by 4 N HCl and analyzed by RP-HPLC, the peak corresponding to DNP-D-diaminopimelic acid (Mr, 355) appeared only with the non-acetylated peptidoglycan sample, which showed that free amino groups of diaminopimelic acid in non-cross-linked peptide stem were labeled with DNP in this sample (data not shown). The lack of this peak with the acetylated peptidoglycan sample indicated that all the free amino groups were successfully acetylated. These results suggested that LysB4 acts as an L-alanoyl-D-glutamate endopeptidase to cut the peptide bond between the L-Ala and D-Glu (arrow, Figure 4c).
Discussion
In this study, LysB4, a newly identified endolysin from the B. cereus-specific bacteriophage B4, was expressed, purified, and characterized. We showed that LysB4 was an L-alanoyl-D-glutamate endopeptidase. These endopeptidases have been reported in L. monocytogenes phages, the E. coli bacteriophage T5, and a B. subtilis strain [21, 23, 24]. In contrast, all the characterized endolysins found in bacteriophages infecting Bacillus species are amidases (Ply21, Ply12, and PlyBa) [17]. Thus, LysB4 is the first characterized L-alanoyl-D-glutamate endopeptidase originating from B. cereus phages.
LysB4 has two domains; the VanY domain at the N-terminus and SH3_5 domain at the C-terminus. The majority of the endolysins have two domains connected by a short linker: the N-terminal catalytic domain is responsible for cell lytic activity and the C-terminal cell wall binding domain that recognizes and binds a specific substrate, such as carbohydrate in the cell wall of target bacteria [10]. The catalytic VanY domain is conserved in other L-alanoyl-D-glutamate endopeptidases, including CwlK in B. subtilis and Ply500 in L. monocytogenes bacteriophage A500 [23, 25] and D-alanoyl-D-alanine carboxypeptidases [26]. The SH3_5 domain at the C-terminus was found in the putative lysins of Bacillus bacterial strains, Bacillus phages and Lactobacillus phages (Figure 1a), suggesting that this domain is the cell wall binding domain.
Biochemical characterization showed that the LysB4 endolysin was slightly alkalophilic, because activity was optimal at pH 8.0-10.0. It was also slightly thermophilic, with an optimal temperature of 50°C. The maximal lytic activity occurred in the absence of NaCl. This enzyme required a divalent metal ion, such as Zn2+ or Mn2+, for full enzymatic activity. A similar requirement for divalent cations was seen for Ply500 in L. monocytogenes bacteriophage A500 [23]. The other characterized L-alanoyl-D-glutamate peptidase, T5 endolysin requires Ca2+ instead of Zn2+ or Mn2+ [24]. The requirement of Zn2+ or Mn2+ is supported by protein sequence analysis, because LysB4 has the three Zn2+-coordinating residues (His80, Asp87, His133) of Ply500, and the Zn2+-binding domain (SxHxxGxAxD) [22].
Endolysins are generally known to be highly specific against particular species of bacteria. However, LysB4 showed lytic activity against a broad range of bacterial species. LysB4 showed similar activity toward susceptible Gram-positive and Gram-negative bacteria, whereas other reported L-alanoyl-D-glutamate endopeptidases have a much narrower target host range [23]. LysB4 could lyse not only B. cereus strains but also other Gram-positive bacteria such as B. subtilis and L. monocytogenes strains. In addition, this enzyme also showed lytic activity toward Gram-negative bacteria when treated with EDTA. Most Gram-negative bacteria contain the Alγ type peptidoglycan, and Bacillus species and L. monocytogenes have the Alγ type cell wall as well [23, 24, 27, 28]. Thus, LysB4 probably targets Alγ type peptidoglycan. This relatively broad antibacterial spectrum of LysB4 was surprising, given the narrow host range of the bacteriophage B4. Bacteriophage B4 only targets one strain of B. cereus (strain ATCC 10876) of five tested B. cereus strains and other Gram-positive bacterial species including L. monocytogenes strains, S. aureus, and Ent. faecalis (Shin et al. unpublished). This suggests that there are more bacterial species with the LysB4 cell wall recognition site than those containing the bacteriophage B4 receptor. Therefore, further studies are needed to determine the moiety targeted by the LysB4 cell-wall binding SH3_5 domain.
Conclusions
LysB4 is the first characterized L-alanoyl-D-glutamate endopeptidase originating from a B. cereus bacteriophage. Although LysB4 has similar enzymatic and genetic properties to Ply500 from L. monocytogenes bacteriophage, LysB4 has broader spectrum and can lyse both Gram-positive and Gram-negative bacteria, including a number of foodborne pathogens. As this enzyme also shows strong lytic activity and stability in wide range of pH and temperature, LysB4 has high potential as an effective antibacterial agent to control foodborne pathogens. In the presence of agents such as EDTA, which permeabilize the outer cell membrane [29], LysB4 could be successfully applied exogenously to control Gram-negative bacteria as well as Gram-positive bacterial pathogens.
Methods
Bacterial strains, phage and growth conditions
B. cereus ATCC 10876 was used as the host for bacteriophage B4 (KCTC 12013BP) and the substrate for the LysB4 endolysin. E. coli BL21 (DE3) was used as the host for expression of the recombinant LysB4. Bacterial strains that were used for antimicrobial spectrum determination are described in Table 2 along with the results. All the bacterial strains were routinely grown at 37°C in Luria-Bertani (LB) broth medium (Difco). Ampicillin (50 μg/ml) was added when necessary.
Cloning, expression, and purification of LysB4
The endolysin gene (lysB4) was amplified from the genomic DNA of the bacteriophage B4 by polymerase chain reaction (PCR) using primers lysB4F (5'-AGTGGAAGTCATATGGCAATGGCATTA-3') and lysB4R (5'-TAAAAAAAGGATCCCCGAAGGACTTCC). The PCR product was cloned into pET15b (Novagen), which has an N-terminal hexahistidine (His)-tag sequence. The correctly cloned plasmid was transformed into competent E. coli BL21 (DE3). Expression of the recombinant LysB4 was induced with 0.1 mM isopropyl-β-D-thiogalactopyranoside at OD600 1.0, followed by incubation for an additional 5 h at 30°C. Bacterial cells were suspended in lysis buffer (50 mM potassium phosphate, 200 mM sodium chloride, pH 7.0) and disrupted by sonication (Branson Ultrasonics). After centrifugation at 15,000 × g for 20 min, the supernatant was passed through a Ni-NTA Superflow column (Qiagen), and purification of the recombinant LysB4 was performed according to the manufacturer's instructions. The purified protein was stored at -80°C until use after the buffer was changed to the storage buffer (50 mM potassium phosphate, pH 8.0, 200 mM NaCl, 30% glycerol) using PD Miditrap G-25 (GE Healthcare).
Lytic activity assay
The lytic activity of the endolysin against bacterial cells was assayed by monitoring the decrease in OD600 [30]. B. cereus ATCC 10876 or other bacteria were cultivated to exponential phase. Cells were harvested and resuspended with the reaction buffer (50 mM Tris-HCl, pH 8.0) to adjust OD600 to 0.8-1.0. When needed, 0.1 M EDTA was used to treat the Gram-negative bacteria after harvesting, as described previously [31]. The endolysin (100 μl) was added to the cell suspension (900 μl) followed by incubation at room temperature, unless indicated otherwise. OD600 values were monitored over time. The lytic activity was calculated after 5 min as followed; {ΔOD600 test (endolysin added) - ΔOD600 control (buffer only)}/initial OD600.
To evaluate the effect of pH on LysB4 enzymatic activity, the endolysin (5 μg) was added to B. cereus cells suspended with a variety of buffers: 0.1% trifluoroacetic acid (TFA) for pH 2.0; 50 mM sodium acetate for pH 4.3; 50 mM 2-(N-morpholino)ethanesulfonic acid for pH 6.0; 50 mM Bis-Tris for pH 7.0; 50 mM Tris-HCl for pH 8.0-8.5; 50 mM glycine for pH 9.0-9.5; and 50 mM N-cyclohexyl-3-aminopropanesulfonic acid for pH 10.0-10.5. Different temperatures (25-72°C) were applied to test the effect of temperature on LysB4 (0.1 μg) enzymatic activity. To evaluate the stability of the endolysin, the lysis assays were performed against B. cereus ATCC 10876 at room temperature and pH 8.0 after the enzyme was incubated for 30 min under the selected pH conditions or at different temperatures. The influence of NaCl on lytic activity of LysB4 (1 μg) was tested with addition of various concentrations of 0, 50, 100, 150 and 200 mM NaCl.
The effects of metal ions on the lysis activity were determined as previously reported [32]. To chelate metal ions attached to the endolysin, EDTA (5.0 mM) was added to the enzyme (5 μg) and incubated at 37°C for 1 h. EDTA was removed by exchanging the buffer to reaction buffer using PD trap G-25 (GE Healthcare). The EDTA-treated enzyme was added to the cell resuspension with metal ions (ZnCl2, MgCl2, MnCl2, CuCl2, HgCl2 or CaCl2 0.1 or 1.0 mM) and the lysis activity was assayed in the reaction buffer.
Assays for endopeptidases, glycosidases, and amidases
Endopeptidase activity was measured by quantification of liberated free amino groups from the peptidoglycan by the endolysin reaction. A crude cell wall of B. cereus was prepared by the method described by Kuroda and Sekiguchi [33], and to block pre-existing free amino groups in the peptidoglycan, B. cereus cell wall was acetylated as described by Pritchard et al. [34]. Free amino groups generated by digestion of the cell wall by LysB4 endolysin were assayed by the TNBS method [35]. Serine was used as the standard [36]. Glycosidase activity was confirmed by the method of Pritchard et al. [34] and amidase assay was performed as described by Hazenberg et al. [37].
Determination of the cleavage site in peptidoglycan
The LysB4 cleavage site in the peptidoglycan was determined as described by Fukushima et al. [28]. Briefly, the acetylated peptidoglycan was digested with LysB4 for 0 and 60 min, and the released free amino groups detected by addition of 1-fluoro-2,4-dinitrobenzene, which forms 2,4-dinitrophenol (DNP) amino acid derivatives. These mixtures were hydrolyzed with 4 N HCl for 12 h at 97°C to digest glycosidic and peptide bonds. The DNP-labeled compounds were separated by RP-HPLC (HP1100) with Vydac C18 column (4.6 × 250 mm), using 365 nm for detection of the eluted products. Using two elution buffers (A, 0.025% TFA in water; B, 0.025% TFA in acetonitrile), elution was performed with a linear gradient of buffer B (0-100%) for 60 min at 40°C. After identifying the peaks, LC-MS analysis was performed to confirm the molecular mass of the peaks using Finnigan TSQ Quantum Ultra EMR (Thermo Scientific). This experiment was performed by Korea Basic Science Institute, Seoul Center (Seoul, Republic of Korea).
Nucleotide sequence accession number
The nucleotide sequence of lysB4 was deposited to GenBank under the accession number JN616385.
References
Schoeni JL, Wong AC: Bacillus cereus food poisoning and its toxins. J Food Prot. 2005, 68: 636-648.
Beecher DJ, Wong AC: Identification and analysis of the antigens detected by two commercial Bacillus cereus diarrheal enterotoxin immunoassay kits. Appl Environ Microbiol. 1994, 60: 4614-4616.
Dierick K, Van Coillie E, Swiecicka I, Meyfroidt G, Devlieger H, Meulemans A, Hoedemaekers G, Fourie L, Heyndrickx M, Mahillon J: Fatal family outbreak of Bacillus cereus-associated food poisoning. J Clin Microbiol. 2005, 43: 4277-4279. 10.1128/JCM.43.8.4277-4279.2005.
King NJ, Whyte R, Hudson JA: Presence and significance of Bacillus cereus in dehydrated potato products. J Food Prot. 2007, 70: 514-520.
Kim SK, Kim KP, Jang SS, Shin EM, Kim MJ, Oh S, Ryu S: Prevalence and toxigenic profiles of Bacillus cereus isolated from dried red peppers, rice, and Sunsik in Korea. J Food Prot. 2009, 72: 578-582.
Young I, Wang I, Roof WD: Phages will out: strategies of host cell lysis. Trends Microbiol. 2000, 8: 120-128. 10.1016/S0966-842X(00)01705-4.
Schuch R, Nelson D, Fischetti VA: A bacteriolytic agent that detects and kills Bacillus anthracis. Nature. 2002, 418: 884-889. 10.1038/nature01026.
Ziedaite G, Daugelavicius R, Bamford JK, Bamford DH: The holin protein of bacteriophage PRD1 forms a pore for small-molecule and endolysin translocation. J Bacteriol. 2005, 187: 5397-5405. 10.1128/JB.187.15.5397-5405.2005.
Borysowski J, Weber-Dabrowska B, Gorski A: Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med (Maywood). 2006, 231: 366-377.
Fischetti VA: Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol. 2008, 11: 393-400. 10.1016/j.mib.2008.09.012.
Loessner MJ: Bacteriophage endolysins-current state of research and applications. Curr Opin Microbiol. 2005, 8: 480-487. 10.1016/j.mib.2005.06.002.
Garcia P, Martinez B, Rodriguez L, Rodriguez A: Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. Int J Food Microbiol. 2010, 141: 151-155. 10.1016/j.ijfoodmicro.2010.04.029.
Nakimbugwe D, Masschalck B, Anim G, Michiels CW: Inactivation of gram-negative bacteria in milk and banana juice by hen egg white and lambda lysozyme under high hydrostatic pressure. Int J Food Microbiol. 2006, 112: 19-25. 10.1016/j.ijfoodmicro.2006.06.010.
Cheng Q, Nelson D, Zhu S, Fischetti VA: Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother. 2005, 49: 111-117. 10.1128/AAC.49.1.111-117.2005.
Witzenrath M, Schmeck B, Doehn JM, Tschernig T, Zahlten J, Loeffler JM, Zemlin M, Muller H, Gutbier B, Schutte H, et al: Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit Care Med. 2009, 37: 642-649. 10.1097/CCM.0b013e31819586a6.
Gupta R, Prasad Y: P-27/HP endolysin as antibacterial agent for antibiotic resistant Staphylococcus aureus of human infections. Curr Microbiol. 2011, 63: 39-45. 10.1007/s00284-011-9939-8.
Loessner MJ, Maier SK, Daubek-Puza H, Wendlinger G, Scherer S: Three Bacillus cereus bacteriophage endolysins are unrelated but reveal high homology to cell wall hydrolases from different bacilli. J Bacteriol. 1997, 179: 2845-2851.
Lee WJ, Billington C, Hudson JA, Heinemann JA: Isolation and characterization of phages infecting Bacillus cereus. Lett Appl Microbiol. 2011, 52: 456-464. 10.1111/j.1472-765X.2011.03023.x.
Shin H, Bandara N, Shin E, Ryu S, Kim KP: Prevalence of Bacillus cereus bacteriophages in fermented foods and characterization of phage JBP901. Res Microbiol. 2011, 162: 791-797. 10.1016/j.resmic.2011.07.001.
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ: Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997, 25: 3389-3402. 10.1093/nar/25.17.3389.
Korndorfer IP, Kanitz A, Danzer J, Zimmer M, Loessner MJ, Skerra A: Structural analysis of the L-alanoyl-D-glutamate endopeptidase domain of Listeria bacteriophage endolysin Ply500 reveals a new member of the LAS peptidase family. Acta Crystallogr D: Biol Crystallogr. 2008, 64: 644-650. 10.1107/S0907444908007890.
McCafferty DG, Lessard IAD, Walsh CT: Mutational analysis of potential zinc-binding residues in the active site of the enterococcal D-Ala-D -Ala dipeptidase VanX. Biochemistry. 1997, 36: 10498-10505. 10.1021/bi970543u.
Loessner MJ, Wendlinger G, Scherer S: Heterogeneous endolysins in Listeria monocytogenes bacteriophages: a new class of enzymes and evidence for conserved holin genes within the siphoviral lysis cassettes. Mol Microbiol. 1995, 16: 1231-1241. 10.1111/j.1365-2958.1995.tb02345.x.
Mikoulinskaia GV, Odinokova IV, Zimin AA, Lysanskaya VY, Feofanov SA, Stepnaya OA: Identification and characterization of the metal ion-dependent L-alanoyl-D-glutamate peptidase encoded by bacteriophage T5. FEBS J. 2009, 276: 7329-7342. 10.1111/j.1742-4658.2009.07443.x.
Smith TJ, Blackman SA, Foster SJ: Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology. 2000, 146 (Pt 2): 249-262.
Reynolds PE, Ambur OH, Casadewall B, Courvalin P: The VanY(D) DD-carboxypeptidase of Enterococcus faecium BM4339 is a penicillin-binding protein. Microbiology. 2001, 147: 2571-2578.
Schleifer KH, Kandler O: Peptidoglycan types of bacterial cell walls and their taxonomic implications. Microbiol Mol Biol Rev. 1972, 36: 407-477.
Fukushima T, Yao Y, Kitajima T, Yamamoto H, Sekiguchi J: Characterization of new L, D-endopeptidase gene product CwlK (previous YcdD) that hydrolyzes peptidoglycan in Bacillus subtilis. Mol Genet Genomics. 2007, 278: 371-383. 10.1007/s00438-007-0255-8.
Begunova EA, Stepnaia OA, Tsfasman IM, Kulaev IS: The effect of the extracellular bacteriolytic enzymes of Lysobacte sp. on gram-negative bacteria. Mikrobiologiia. 2004, 73: 320-325.
Gaeng S, Scherer S, Neve H, Loessner MJ: Gene cloning and expression and secretion of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl Environ Microbiol. 2000, 66: 2951-2958. 10.1128/AEM.66.7.2951-2958.2000.
Leive L: Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968, 243: 2373-2380.
Schmelcher M, Waldherr F, Loessner MJ: Listeria bacteriophage peptidoglycan hydrolases feature high thermoresistance and reveal increased activity after divalent metal cation substitution. Appl Microbiol Biotechnol. 2012, 93: 633-943. 10.1007/s00253-011-3372-6.
Kuroda A, Sekiguchi J: Cloning, sequencing and genetic mapping of a Bacillus subtilis cell wall hydrolase gene. J Gen Microbiol. 1990, 136: 2209-2216.
Pritchard DG, Dong S, Baker JR, Engler JA: The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology. 2004, 150: 2079-2087. 10.1099/mic.0.27063-0.
Marschutz MK, Caliceti P, Bernkop-Schnurch A: Design and in vivo evaluation of an oral delivery system for insulin. Pharm Res. 2000, 17: 1468-1474. 10.1023/A:1007696723125.
Mokrasch LC: Use of 2,4,6-trinitrobenzenesulfonic acid for the coestimation of amines, amino acids, and proteins in mixtures. Anal Biochem. 1967, 18: 64-71. 10.1016/0003-2697(67)90057-7.
Hazenberg MP, de Visser H: Assay for N-acetylmuramyl-L-alanine amidase in serum by determination of muramic acid released from the peptidoglycan of Brevibacterium divaricatum. Eur J Clin Chem Clin Biochem. 1992, 30: 141-144.
Acknowledgements
This work was supported by grants to S. Ryu from the Agriculture Research Center program of the Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea. BS, JL and HS were the recipients of a graduate fellowship provided by the MEST through the Brain Korea 21 Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
BS, JL and SR designed the study. BS performed the experiments. HS carried out the sequence analysis. BS, JY, and SR analyzed the data and wrote the paper. SH critically reviewed the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Son, B., Yun, J., Lim, JA. et al. Characterization of LysB4, an endolysin from the Bacillus cereus-infecting bacteriophage B4. BMC Microbiol 12, 33 (2012). https://doi.org/10.1186/1471-2180-12-33
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2180-12-33