Abstract
Background
Group A Streptococcus (GAS) causes human diseases ranging in severity from uncomplicated pharyngitis to life-threatening necrotizing fasciitis and shows high rates of macrolide resistance in several countries. Our goal is to identify antimicrobial resistance in Spanish GAS isolates collected between 1994 and 2006 and to determine the molecular epidemiology (emm/T typing and PFGE) and resistance mechanisms of those resistant to erythromycin and tetracycline.
Results
Two hundred ninety-five out of 898 isolates (32.8%) were erythromycin resistant, with the predominance of emm 4T4, emm 75T25, and emm 28T28, accounting the 67.1% of the 21 emm/T types. Spread of emm 4T4, emm 75T25 and emm 28T28 resistant clones caused high rates of macrolide resistance. The distribution of the phenotypes was M (76.9%), cMLSB (20.3%), iMLSB (2.7%) with the involvement of the erythromycin resistance genes mef(A) (89.5%), msr(D) (81.7%), erm(B) (37.3%) and erm(A) (35.9%).
Sixty-one isolates were tetracycline resistant, with the main representation of the emm 77T28 among 20 emm/T types. To note, the combination of tet(M) and tet(O) tetracycline resistance genes were similar to tet(M) alone reaching values close to 40%. Resistance to both antibiotics was detected in 19 isolates of 7 emm/T types, being emm 11T11 and the cMLSB phenotype the most frequent ones. erm(B) and tet(M) were present in almost all the strains, while erm(A), mef(A), msr(D) and tet(O) appeared in less than half of them.
Conclusions
Spanish GAS were highly resistant to macrolides meanwhile showed minor resistance rate to tetracycline. A remarkable correlation between antimicrobial resistance and emm/T type was noticed. Clonal spread of emm 4T4, emm 75T25 and emm 28T28 was the main responsable for macrolide resistance where as that emm 77T28 clones were it to tetraclycline resistance. A wide variety of macrolide resistance genes were responsible for three macrolide resistance phenotypes.
Similar content being viewed by others
Background
Group A Streptococcus (GAS) causes a broad spectrum of illness in humans, ranging from pharyngitis to severe systemic diseases. A resurgence in serious GAS infections, such as rheumatic fever, and invasive diseases, such as bacteraemia, necrotising fasciitis, septic arthritis, sepsis, pneumonia and streptococcal toxic shock syndrome, has been observed since the mid 1980s. Indeed, these have become an important cause of morbidity and mortality all over the world [1].
Penicillin is the first choice treatment. Macrolides and tetracyclines are the most common alternative antibiotics used with penicillin-allergic patients or when first line therapy fails. Increases in macrolide resistance have been reported from many countries, being in Europe, very common in the Mediterranean countries [2, 3].
Streptococci have two main mechanisms of macrolide resistance: target site modification and macrolide efflux systems. The first is achieved through a family of enzymes (rRNA methylases) that methylate an adenine residue (A2058) of the 23S rRNA V domain. This leads to a conformational change that reduces the binding of macrolides, lincosamide and streptogramin B to ribosomes, conferring co-resistance to these antibiotics (the MLSB phenotype). The MLSB phenotype may be expressed constitutively (cMLSB) or inducibly (iMLSB). These methylases are encoded by erm (erythromycin ribosome methylation) genes, with the erm(B) and erm(A) the most common [3]. In the second mechanism (the efflux system), transport proteins pump C14 and C15 macrolides out of the cell (M phenotype). The M phenotype is associated with the presence of the mef(A) and msr(D) genes, which code for the transmembrane and ATP-binding domains of this pump respectively [4].
Less information is available on the characteristics of tetracycline resistance mechanisms. In streptococci, resistance to tetracycline is conferred by ribosome protection genes such as tet(M) and tet(O) and by efflux pumps encoded by the tet(K) or tet(L) genes, although these last genes are relatively rare [4].
The prevalence of antimicrobial resistance is due to several circulating clones associated with certain emm types. The aim of the present study was to identify antimicrobial resistance in Spanish group A Streptococcus (GAS) isolates and to determine the molecular epidemiology (emm/T typing and PFGE) and resistance mechanisms of those resistant to erythromycin and tetracycline. This study is focused on Spanish GAS population collected from a wide spectrum of clinical backgrounds and not only from carriers as occurs for other studies. The long term studied period (13 years) and the different geographical origin may allow us to obtain an approach more real to susceptibility, phenotypes, genotypes, emm-types and PFGE profiles distribution in Spain.
Results
Overall GAS susceptibility rates
All 898 Spanish GAS isolates showed susceptibility to penicillin and vancomycin. In addition, a 32.8% (295 isolates) rate of resistance to erythromycin was seen, along with 6.5% (59) resistance to clindamycin, 6.8% (61) resistance to tetracycline, and 0.3% (3) resistance to rifampin.
Macrolide resistance phenotypes and genotypes
Two hundred ninety five (32.8%) erythromycin resistant isolates were detected among the 898 GAS isolates gathered over the 13-year collection period. The M phenotype was clearly predominant (227 isolates, 76.9%), followed by the cMLSB (60 isolates, 20.3%) and iMLSB phenotypes (8 isolates, 2.7%) (Table 1). The isolates with the cMLSB phenotype showed high-level resistance to erythromycin and clindamycin (MIC90 ≥256 mg/L), whereas those with the iMLSB and M phenotypes showed lower erythromycin resistance values and susceptibility to clindamycin (Table 1). To highlight, the cMLSB phenotype was more predominant among invasive that in non-invasive, 43.8 and 12.6%, respectively.
In the present work, the mef(A) (89.5%) and msr(D) (81.7%) genes were the most prevalent macrolide resistance determinants. erm(B) and erm(A) were observed in just 37.3% and 35.9% of isolates respectively (Table 1). Fourteen macrolide resistance genotypes were identified among the 295 erythromycin-resistant isolates (Table 2), with msr(D)/mef(A) (38%) and msr(D)/mef(A)/erm(A)(19.7%) the two most common combination. Both genotypes were associated with the M phenotype.
Tetracycline resistance phenotypes and genotypes
Tetracycline-resistant phenotype was observed in 61 isolates (6.8%), showed MICs ranging from 8 to 64 mg/L (MIC50 16 mg/L, MIC90 32 mg/L) with a genotype distribution of tet(M)/tet(O) (42.6%), tet(M) (39.3%) and tet(O) (18.0%).
Erythromycin and tetracycline co-resistance
Co-resistance was detected in 19 isolates (2.1%). The erythromycin MIC was >256 mg/L for 18 isolates and just 32 mg/L for one isolate. The clindamycin MICs were also high at >256 mg/L for 14 of the 19 isolates. All isolates except one (iMLSB) had the cMLSB macrolide resistance phenotype. The resistance genes detected were erm(B) (94.7%), erm(A) (42.1%), mef(A) (47.4%), msr(D) (36.8%), tet(M) (100.0%) and tet(O) (36.8%), with tet(M) the only tetracycline resistance determinant in 13 isolates, while in 6 it was simultaneously detected with tet(O) (Table 3).
T- serotypes and emm types (emm/T types) distribution
Twenty one emm/ T types were observed in the erythromycin-resistant population (295) (Table 3), the 6 most common being emm 4T4 (39.3%), emm 75T25 (14.6%), emm 28T28 (13.2%), emm 6T6 (9.8%), emm 12T12 (6.8%) and emm 11T11 (4.1%) which represented 87.8% of the erythromycin-resistant isolates. High macrolide resistance rates were associated with the above emm/T types: emm 75T25 (93.5%), emm 4T4 (84.7%), emm 11T11 (50%), emm 28T28 (50%), emm 6T6 (43.3%) and emm 12T12 (29.4%).
In the present tetracycline-resistant population (61), 20 different emm/T types were identified (Table 3). emm 77T28 (37.3%) was the main emm/T type associated with tetracycline resistance; all emm 77T28 isolates detected over the 13 years of the study were resistant to this antibiotic.
In the erythromycin- and tetracycline-resistant population population (19), 7 emm/T types were observed, the majority being emm 11T11 (57.8%) (Table 3); indeed, 45.8% of all emm 11T11 recovered from the initial GAS population (898) were co-resistant.
The correlation between the different emm/ T types and macrolide resistance genotypes is shown in Table 2. The mef(A)/msr(D) gene complex was the most common in almost all emm/T types, either alone or in combination with other genes. The mef(A)/msr(D) genotype was the most common in the emm 1T1 (6/10), emm4 T4 (62/116), emm 6T6 (26/29) and emm 12T12 (10/20) types. The msr(D)/mef(A)/erm(A)(36/116) was the most common genotype among the emm 4T4 (36/116) and emm 75T25 (17/43) types.
PFGE typing
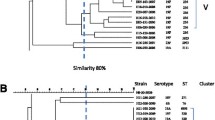
In the erythromycin-resistant population (295 isolates), 79 (26.8%) SmaI-restricted and 216 (73.2%) SmaI-non-restricted isolates were identified. SmaI-restricted isolates generated 30 pulsotypes with a similarity range of 38.8% to 94.7% (Figure 1). Their distribution by phenotype was: M (11 isolates), cMLSB (58) and iMLSB (6).
The 216 SmaI-non-restricted isolates (Table 4) were typed with Sfi I, generating 22 pulsotypes with a similarity range of 12.2% to 88.9% (Figure 2). The M phenotype (212 isolates) predominated over the cMLSB (2) and iMLSB (2) phenotypes. In addition, 11 different emm/T types were detected (Table 4) among 216 SmaI-non-restricted isolates, the most common being emm 4T4 and emm 75T25. All emm 4T4 and all emm 75T25 erythromycin-resistant isolates but one were Sma I non-restricted and had the M phenotype; together these accounted for 53.9% of the macrolide-resistant isolates in our study.
In the case of tetracycline-resistant isolates, all were SmaI-restricted, generating 30 pulsotypes with a similarity range of 42.16 to 100.0% (Figure 1). The Sma 10a emm 77T28 and Sma 64 emm 11T11 pulsotypes may be associated with tetracycline resistance since 100% of these isolates were resistant to this antibiotic. All co-resistant (erythromycin and tetracycline) isolates were Sma I-restricted.
Discussion
Several reports show that GAS resistance to macrolides and tetracyclines are high some countries such Spain and continue to increase; indeed, they have become clinically problematic.
In Europe, the most northerly countries (with the exception of Finland) have reported low levels of resistance (<4%) [5] while strong resistance has been reported from Mediterranean countries such as Italy (22,6%), France (22.4%), Greece (24.0%), Spain (21.3%) and Portugal (26.6%) [6–10]. This values contrast with those of Israel (1.8%) and Iran (0.2%) [11, 12].
In our study, 32.8% of isolates showed resistance to macrolides. Efflux pumps (M phenotype) are one of the major mechanisms conferring resistance to macrolide antibiotics, and streptococci making use of this system have been commonly reported from European countries, Argentina, the USA and Canada [5, 13–15]. The M phenotype has been identified as predominant in several Spanish studies, reaching a rate of 95.6% in a multicentre study undertaken in 1998 or 64.5% in an extensive national multicenter surveillance study in 2006–2007 [16, 17]. In the present population, the efflux system was also the main macrolide resistance mechanism seen, being manifested by 76.9% of isolates.
cMLSB phenotype, another common phenotype reported in Europe [18], was displaced by the M phenotype in several European countries from 1990 [10, 19]. In our study, cMLSB phenotype was the second most commonly encountered (20.3%) like SAUCE project carried out in 2006–2007 [17]. In this last report, flutuations in the rates of resistance to macrolides are observed (1996–1997: 26.7%; 1998–1999: 20.4%; 2001–2002: 24.3; 2006–2007: 19%) meanwhile there is an increasing trend in the prevalence of MLSB phenotype from 14% in 2001–2002 to 35.5% in 2006–2007 [17].
Among Spanish isolates of this work, iMLSB phenotype was minority (2.7%) in contrast to Norway (75%) (1993–2002) or Bulgaria (57.7%) (1993 – 2002) where it was reported the most prevalent phenotype [5].
A gene-phenotype correlation previously described was also noticed [3, 9]. mef(A) and erm(B) were predominant in isolates with the M and cMLSB phenotype respectively, whereas all isolates with the iMLSB phenotype harboured the erm(A) gene.
The mef(A) gene responsible for the M phenotype was detected in all but three of the present Spanish isolates with that phenotype. One of these three isolates showed none of the genes studied. In the remaining two, msr(D) was observed alone or in combination with erm(A). In these last two cases, the msr(D) gene might be only one of the determinants responsible for the M phenotype. msr(D) and mef(A) have been placed in the same genetic element [8, 20], suggesting that the proteins they encode may act as a dual efflux system. However, it has also been suggested that the msr(D)-encoded pump can function independently of the mef-encoded protein [20].
The erm(B) gene responsible for the cMLSB phenotype was identified in all but three of the present isolates with this phenotype. None of genes tested could be amplified in two isolates, indicating that other resistance genes must be involved. The remaining isolate harboured erm(A) and mef(A). In this case, erm(A) may be responsible for the cMLSB phenotype since alterations in the regulatory region of the gene have been identified that induce constitutive expression [21].
An ample macrolide resistance genes combination was identified, specifically fourteen genotypes. Interestingly, single genotypes could show one or several phenotypes, a phenomenon reported by other authors [5, 10]. One of these, erm(B)/msr(D)/mef(A) genotype showed M and MLSB phenotypes in 25 and 8 isolates respectively, while the erm(B)/erm(TR)/msr(D)/mef(A) genotype showed all three macrolide resistance phenotypes. Nowadays, this correlation between genotype and phenotype is not well understood.
In our erythromycin-resistant population (295), the 6 most common emm/types: emm 4T4 (39.3%), emm 75T25 (14.6%), emm 28T28 (13.2%), emm 6T6 (9.8%), emm 12T12 (6.8%) and emm 11T11 (4.1%) have been previously associated with macrolide resistance in numerous reports [6, 10, 12, 14]. emm 28 and emm 4 have been reported the most common in Europe (2003–2004) [18], and to be responsible for an increase in erythromycin resistance among GAS in Spain, Finland and Quebec [6]. emm 12 is the main resistant emm type in Germany, Greece, Italy, Portugal, Israel [10, 12, 13] and the second one in the United States, being surpassed only by emm 75 [14].
Most of erythromycin-resistant isolates were Sma- non-restricted (73.2%) due to the presence prophage-like elements that confer the M phenotype and harbour the mef(A) and msr(D) genes. These genetic elements encode a DNA-modifying methyltransferase that acts on the Sma I recognition sequence and renders DNA refractory to cleavage by Sma I [21]. All but four of the present Sma I non-restricted isolates were susceptible to tetracycline and had an M phenotype. This suggests that these isolates carry mef(A) and msr(D) contained within a Tn1207.1 transposon inserted into a larger genetic element such as the Tn1207.3 or 58.8 kb chimeric element, flanked by the comEC gene from the Tn1207.3/Φ10394.4 family [22]. In our study, all emm 4T4 and all emm 75T25 erythromycin-resistant isolates but one were Sma I non-restricted and had the M phenotype; together these accounted for 53.9% of the Spanish macrolide-resistant isolates. Several resistant clones previously described in Spain were identified [9, 10]. The emm 4T4 Sfi 1 (79) clone resembles to clone B described in 1999 [10]. It was the most common in the present study, indicating it to still be circulating in Spain. This clone has a wide distribution, and it has recently been identified in Finland, Greece, Italy, England and Sweden [23]. Clone C, previously identified in Spain, the United Kingdom and the United States [23] was not detected among the present isolates, although it might be related to the present clones emm 4T4 Sfi 4 and emm 4T4 Sfi 5.
The major macrolide-resistant clone emm 75T25 Sfi 12(41) was similar (additional band between 48.5 and 97 kb) to clone D described by Perez-Trallero et al. [10]. The emm 6T6 Sfi 17 and emm 84T25 Sfi 22 clones might be associated with resistance since they were only observed in isolates resistant to erythromycin.
Regarding tetracycline resistance, we detected values of 6.8% between 1994 and 2006, indicating there to be no trend towards increased tetracycline in Spain. However, higher rates have been found in other countries such as Israel (23.6%), Denmark (33.7%), Portugal (38.7%) or Iran (42%) [10–12].
In this study, a predominance of genotype with both genes tet(M) and tet(O) (42.6%) was observed. But no Spanish reports citing the predominance of both genes appears to exist, tet( M) alone is usually the most common resistance determinant followed by tet(O) [9].
In the present tetracycline-population, emm 77T28 was the main emm/T type. emm 77 has been previously associated with resistance to tetracycline in Israel and Europe [12]. In Italy and Norway, an emm 77 clone has been reported that is characterised by its carrying tet(O) linked to erm(A)and being associated with the iMLSB phenotype [2]. In the present study, the two co-resistant emm 77T28 isolates showed genotypes different to those described by Palmieri et al. [2].
With regard to co-resistance, we found that all isolates (19) except one had the cMLSB macrolide resistance phenotype such as Greece (Athens) and Norway [5, 15]. In contrast, in Finland, iMLSB isolates showing co-resistance have reached rates of 93% [19]. A correlation between the M phenotype and co-resistance has been also reported [23], but this was not detected in the present study.
Of the 19 co-resistant isolates, five carried tet(M)/erm(B) as their only resistance genes, suggesting they may carry conjugative transposons of the Tn916 family in which erm(B) and tet(M) are linked [24],whereas 13 harboured tet(M)/erm(B) associated with other resistance genes. In the remaining isolate, the erm(B), mef(A), tet(M) and tet(O) genes were all detected. mef(A) and tet(O) linkage has been previously reported in co-resistant isolates [22, 25]. In the present work, mef(A) appeared associated with other macrolide resistance genes and linked to tet(M) (1 isolate) or to tet(M)/tet(O) (5). The main emm/T type detected in coresistant isolates was emm 11T11 (57.8%). This emm/T type has previously been associated with co-resistance [9, 11] with an erm(B)/tet(M) clone prevalent among Spanish MLSB isolates [9]. Four isolates with this genotype were found in the present work, but we can not confirm whether they belong to the above clone.
Conclusion
In summary, the resistance against erythromycin, single or together to tetracycline, is due to a wide combination of resistance genes in Spanish GAS. Erythromycin resistance is mainly consequence of clonal spread of emm 4T4, emm 75T25, both associated with M phenotype and SmaI non-restricted, and emm 28T28. Whereas tetracycline resistance and coresistance is due to clonal spread of emm 77T28 and emm 11T11, respectively, all Sma I restricted.
Methods
Bacterial isolates
Between 1994 and 2006, 898 GAS isolates were submitted for their characterisation to the Streptococcal Reference Laboratory from 75 Hospitals and Public Health Laboratories in 32 Spanish provinces. GAS identification was confirmed by colony morphology, β-haemolysis on blood agar, a latex agglutination assay (Slidex, Streptokit, BioMerieux, Marcy-L´Etoile, France), and by using the rapid ID 32 STREP kit (BioMerieux, Marcy-L´Etoile, France). The erythromycin- and tetracycline-resistant isolates were selected as the study population (see section antimicrobial susceptibility tests). This population (337 isolates) was collected from a wide spectrum of clinical backgrounds, including necrotising fasciitis (3), cellulitis and other skin infections (67), streptococcal toxic shock syndrome (13), sepsis and meningitis (17), respiratory infection (5), bone infection and rheumatic fever (4), genital infection (20), otitis (12),conjunctivitis (1), scarlet fever (70) and pharyngotonsillitis (80), as well as from asymptomatic carriers (45). For the latter status, the GAS isolates were recovered from oropharyngeal swabs. A limitation of the study was due to the voluntary nature of the submission of these strains, producing a bias in the annual number.
Antimicrobial susceptibility tests
The minimum inhibitory concentrations (MICs) of penicillin, vancomycin, erythromycin, clindamycin, tetracycline and rifampin were determined using the E-test (AB Biodisk, Solna, Sweden) following the standard method [26]. Susceptibility results were categorized according to the criteria of the Clinical and Laboratory Standards Institute [26]. The erythromycin- (MIC ≥ 1 mg/L) and tetracycline-resistant (MIC ≥ 8 mg/L) isolates were then selected as the study population. Streptococcus pneumoniae ATCC 49619 was used as control.
Detection of the macrolide resistance phenotype
Erythromycin-resistant isolates were classified on the basis of their susceptibility patterns as shown by double-disk tests involving erythromycin (15 μg) and clindamycin (2 μg ) disks (Becton Dickinson Microbiology Systems, Cockeysville, MD, USA) [27]. Three phenotypes were assigned: M (erythromycin resistant and clindamycin susceptible), cMLSB (constitutive erythromycin and clindamycin resistant), and iMLSB (erythromycin resistant and clindamycin inducible). Blunting of the clindamycin inhibition zone near to the erythromycin disk indicated an iMLSB phenotype, whereas susceptibility to clindamycin with no blunting indicated the M phenotype.
Detection of erythromycin and tetracycline resistance genes
All erythromycin-resistant isolates were screened by PCR for the erythromycin resistance genes erm(B) [28], erm(A) [3], mef(A) [4], and msr(D) [29]. Tetracycline-resistant isolates were tested for the tetracycline resistance genes tet(M) and tet(O) [4]. PCR assays were carried out according to previously described conditions for each individual primer pairs.
T-serotype and emm type (emm/T types)
The T-serotype was determined by slide agglutination using type-specific antisera (Seiken-Oxoid, Cambridge, UK). emm sequencing was performed according to the protocol of the CDC International Streptococcal Reference Laboratory (http://www.cdc.gov/ncidod/biotech/strep/protocols.htlm).
Pulsed field gel electrophoresis (PFGE) analysis
PFGE was performed as previously described [30] with slight modifications. Chromosomal DNA was digested with the Sma I (40U) restriction enzyme (Fermentas, Vilnius, Lithuania) for 4 h at 30°C and the electrophoresis conditions were 22 h with an 0.5 to 40s switch time ramp at a 120° angle and 6 V/cm. Sma I non-restricted isolates were typed by PFGE using the SfiI restriction enzyme (Fermentas, Vilnius, Lithuania) under previously described conditions [31]. The PFGE profiles were analysed using InfoQuest FP software v.4.5 (Bio-Rad Laboratories, Hercules, CA, USA), employing the UPGMA method with the Dice coefficient and a position tolerance of 1.2%. Sma- and Sfi-profiles were number-coded. For closely related Sma- types (1–2 bands of difference) a letter was added.
References
Cunningham MW: Pathogenesis of group a streptococcal infections. Clin Microbiol Rev. 2000, 13: 470-511. 10.1128/CMR.13.3.470-511.2000.
Palmieri C, Vecchi M, Littauer P: Clonal spread of macrolide- and tetracycline-resistant [erm(A) tet(O)] emm77 Streptococcus pyogenes isolates in Italy and Norway. Antimicrob Agents Chemother. 2006, 50: 4229-4230. 10.1128/AAC.00943-06.
Seppala H, Skurnik M, Soini H: A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob Agents Chemother. 1998, 42: 257-262. 10.1093/jac/42.2.257.
Malhotra-Kumar S, Lammens C, Piessens J: Multiplex PCR for simultaneous detection of macrolide and tetracycline resistance determinants in streptococci. Antimicrob Agents Chemother. 2005, 49: 4798-4800. 10.1128/AAC.49.11.4798-4800.2005.
Littauer P, Caugant DA, Sangvik M: Macrolide-resistant Streptococcus pyogenes in Norway: population structure and resistance determinants. Antimicrob Agents Chemother. 2006, 50: 1896-1899. 10.1128/AAC.50.5.1896-1899.2006.
Bingen E, Bidet P, Mihaila-Amrouche L: Emergence of macrolide-resistant Streptococcus pyogenes strains in French children. Antimicrob Agents Chemother. 2004, 48: 3559-3562. 10.1128/AAC.48.9.3559-3562.2004.
Grivea IN, Al Lahham A, Katopodis GD: Resistance to erythromycin and telithromycin in Streptococcus pyogenes isolates obtained between 1999 and 2002 from Greek children with tonsillopharyngitis: phenotypic and genotypic analysis. Antimicrob Agents Chemother. 2006, 50: 256-261. 10.1128/AAC.50.1.256-261.2006.
Montagnani F, Stolzuoli L, Croci L: Erythromycin resistance in Streptococcus pyogenes and macrolide consumption in a central Italian region. Infection. 2009, 37: 353-357. 10.1007/s15010-008-8023-1.
Perez-Trallero E, Montes M, Orden B: Phenotypic and genotypic characterization of Streptococcus pyogenes isolates displaying the MLSB phenotype of macrolide resistance in Spain, 1999 to 2005. Antimicrob Agents Chemother. 2007, 51: 1228-1233. 10.1128/AAC.01054-06.
Silva-Costa C, Ramirez M, Melo-Cristino J: Rapid inversion of the prevalences of macrolide resistance phenotypes paralleled by a diversification of T and emm types among Streptococcus pyogenes in Portugal. Antimicrob Agents Chemother. 2005, 49: 2109-2111. 10.1128/AAC.49.5.2109-2111.2005.
Jasir A, Tanna A, Noorani A: High rate of tetracycline resistance in Streptococcus pyogenes in Iran: an epidemiological study. J Clin Microbiol. 2000, 38: 2103-2107.
Nir-Paz R, Block C, Shasha D: Macrolide, lincosamide and tetracycline susceptibility and emm characterisation of invasive Streptococcus pyogenes isolates in Israel. Int J Antimicrob Agents. 2006, 28: 313-319. 10.1016/j.ijantimicag.2006.07.005.
Reinert RR, Franken C, van Der LM: Molecular characterisation of macrolide resistance mechanisms of Streptococcus pneumoniae and Streptococcus pyogenes isolated in Germany, 2002–2003. Int J Antimicrob Agents. 2004, 24: 43-47. 10.1016/j.ijantimicag.2004.02.020.
Green MD, Beall B, Marcon MJ: Multicentre surveillance of the prevalence and molecular epidemiology of macrolide resistance among pharyngeal isolates of group a streptococci in the USA. J Antimicrob Chemother. 2006, 57: 1240-1243. 10.1093/jac/dkl101.
Michos AG, Bakoula CG, Braoudaki M: Macrolide resistance in Streptococcus pyogenes: prevalence, resistance determinants, and emm types. Diagn Microbiol Infect Dis. 2009, 64: 295-299. 10.1016/j.diagmicrobio.2009.03.004.
Alos JI, Aracil B, Oteo J: High prevalence of erythromycin-resistant, clindamycin/miocamycin-susceptible (M phenotype) streptococcus pyogenes: results of a Spanish multicentre study in 1998. Spanish group for the study of infection in the primary health care setting. J Antimicrob Chemother. 2000, 45: 605-609. 10.1093/jac/45.5.605.
Perez-Trallero E, Martin-Herrero JE, Mazon A: Antimicrobial resistance among respiratory pathogens in Spain: latest data and changes over 11 years (1996–1997 to 2006–2007). Antimicrob Agents Chemother. 2010, 54: 2953-2959. 10.1128/AAC.01548-09.
Luca B, Ekelund K, Darenberg J: Abstract of the XVII lancefield international symposium on streptococci and streptococcal diseases. Genetic determinants and epidemiology of antibiotic resistance among invasive isolates of Streptococcus pyogenes in Europe. 2008, Porto Heli, Greece: Federation European Microbiological Societies, 164-
Kataja J, Huovinen P, Skurnik M: Erythromycin resistance genes in group a streptococci in Finland. The Finnish Study Group for Antimicrobial Resistance. Antimicrob Agents Chemother. 1999, 43: 48-52.
Daly MM, Doktor S, Flamm R: Characterization and prevalence of MefA, MefE, and the associated msr(D) gene in Streptococcus pneumoniae clinical isolates. J Clin Microbiol. 2004, 42: 3570-3574. 10.1128/JCM.42.8.3570-3574.2004.
Malhotra-Kumar S, Mazzariol A, Van Heirstraeten L: Unusual resistance patterns in macrolide-resistant Streptococcus pyogenes harbouring erm(A). J Antimicrob Chemother. 2009, 63: 42-46.
Bacciaglia A, Brenciani A, Varaldo PE: SmaI typeability and tetracycline susceptibility and resistance in Streptococcus pyogenes isolates with efflux-mediated erythromycin resistance. Antimicrob Agents Chemother. 2007, 51: 3042-3043. 10.1128/AAC.00249-07.
Kataja J, Huovinen P, Efstratiou A: Clonal relationships among isolates of erythromycin-resistant Streptococcus pyogenes of different geographical origin. Eur J Clin Microbiol Infect Dis. 2002, 21: 589-595. 10.1007/s10096-002-0771-8.
Brenciani A, Bacciaglia A, Vecchi M: Genetic elements carrying erm(B) in Streptococcus pyogenes and association with tet(M) tetracycline resistance gene. Antimicrob Agents Chemother. 2007, 51: 1209-1216. 10.1128/AAC.01484-06.
Giovanetti E, Brenciani A, Lupidi R: Presence of the tet(O) gene in erythromycin- and tetracycline-resistant strains of Streptococcus pyogenes and linkage with either the mef(A) or the erm(A) gene. Antimicrob Agents Chemother. 2003, 47: 2844-2849. 10.1128/AAC.47.9.2844-2849.2003.
Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing: twentieth informational supplement M100-S20. 2010, Wayne, PA, USA: CLSI
Seppala H, Nissinen A, Yu Q: Three different phenotypes of erythromycin-resistant Streptococcus pyogenes in Finland. J Antimicrob Chemother. 1993, 32: 885-891. 10.1093/jac/32.6.885.
Sutcliffe J, Grebe T, Tait-Kamradt A: Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996, 40: 2562-2566.
Luthje P, Schwarz S: Molecular basis of resistance to macrolides and lincosamides among staphylococci and streptococci from various animal sources collected in the resistance monitoring program BfT-GermVet. Int J Antimicrob Agents. 2007, 29: 528-535. 10.1016/j.ijantimicag.2006.12.016.
Nguyen L, Levy D, Ferroni A: Molecular epidemiology of Streptococcus pyogenes in an area where acute pharyngotonsillitis is endemic. J Clin Microbiol. 1997, 35: 2111-2114.
Perez-Trallero E, Marimon JM, Montes M: Clonal differences among erythromycin-resistant Streptococcus pyogenes in Spain. Emerg Infect Dis. 1999, 5: 235-240. 10.3201/eid0502.990207.
Acknowledgments
The authors thank the clinical microbiologists involved in the isolation and submission of GAS strains to Streptococcus Laboratory at the CNM, the Biopolymers Unit of the Centro Nacional de Microbiología for assistance in sequencing and Adrian Burton for revision of the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial competing interest
This research was funded by an intramural predoctoral fellowship from the Carlos III Health Institute (grant number 05/0030) and the Spanish Ministry of Science and Innovation.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PV, MJM, SV, JA and VR participated in the molecular data collection and analysis. DA, CS and VR conducted the microbiological methods and analysed data. SV, JA and VR interpreted data, and drafted the manuscript. SV and JA were involved in critically revising the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rubio-López, V., Valdezate, S., Álvarez, D. et al. Molecular epidemiology, antimicrobial susceptibilities and resistance mechanisms of Streptococcus pyogenes isolates resistant to erythromycin and tetracycline in Spain (1994–2006). BMC Microbiol 12, 215 (2012). https://doi.org/10.1186/1471-2180-12-215
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2180-12-215