Abstract
Background
Pulmonary vascular structure remodeling (PVSR) is a hallmark of pulmonary hypertension. P27kip1, one of critical cyclin-dependent kinase inhibitors, has been shown to mediate anti-proliferation effects on various vascular cells. Beta-estradiol (β-E2) has numerous biological protective effects including attenuation of hypoxic pulmonary hypertension (HPH). In the present study, we employed β-E2 to investigate the roles of p27kip1 and its closely-related kinase (Skp-2) in the progression of PVSR and HPH.
Methods
Sprague-Dawley rats treated with or without β-E2 were challenged by intermittent chronic hypoxia exposure for 4 weeks to establish hypoxic pulmonary hypertension models, which resemble moderate severity of hypoxia-induced PH in humans. Subsequently, hemodynamic and pulmonary pathomorphology data were gathered. Additionally, pulmonary artery smooth muscle cells (PASMCs) were cultured to determine the anti-proliferation effect of β-E2 under hypoxia exposure. Western blotting or reverse transcriptional polymerase chain reaction (RT-PCR) were adopted to test p27kip1, Skp-2 and Akt-P changes in rat lung tissue and cultured PASMCs.
Results
Chronic hypoxia significantly increased right ventricular systolic pressures (RVSP), weight of right ventricle/left ventricle plus septum (RV/LV+S) ratio, medial width of pulmonary arterioles, accompanied with decreased expression of p27kip1 in rats. Whereas, β-E2 treatment repressed the elevation of RVSP, RV/LV+S, attenuated the PVSR of pulmonary arterioles induced by chronic hypoxia, and stabilized the expression of p27kip1. Study also showed that β-E2 application suppressed the proliferation of PASMCs and elevated the expression of p27kip1 under hypoxia exposure. In addition, experiments both in vivo and in vitro consistently indicated an escalation of Skp-2 and phosphorylated Akt under hypoxia condition. Besides, all these changes were alleviated in the presence of β-E2.
Conclusions
Our results suggest that β-E2 can effectively attenuate PVSR and HPH. The underlying mechanism may partially be through the increased p27kip1 by inhibiting Skp-2 through Akt signal pathway. Therefore, targeting up-regulation of p27kip1 or down-regulation of Skp-2 might provide new strategies for treatment of HPH.
Similar content being viewed by others
Background
Pulmonary hypertension is a common complication of chronic hypoxic lung diseases, characterized by sustained elevation of pulmonary artery pressure and vascular resistance [1]. Pulmonary vascular structure remodeling (PVSR) is a hallmark of severe and advanced pulmonary hypertension, presenting several histological changes such as intima thickening, media hyperplasia and adventitia widening, peripheral vessels muscularization and vasoocclusive plexiform lesions [1, 2]. Those changes finally lead to severe pulmonary hypertension, right ventricular hypertrophy and right heart failure, resulting in high morbidity and mortality [3]. Generally, there are 5 subsets of pulmonary hypertension classified according to the latest guidelines for the diagnosis and treatment of pulmonary hypertension [4]. Among these 5 subsets, pulmonary arterial hypertension (PAH), whose inducements including idiopathic, heritable, and connective tissue diseases, is predominantly suffered by women. According to epidemiological studies, there are about 2-3 times as many female as male patients [5–7]. The pathological lesions of PAH mainly affect the small pulmonary arteries (<500 μm of diameter), and are featured by medial hypertrophy, intimal proliferation and fibrotic changes, adventitial thickening, complex lesions, and thrombotic lesions [3, 8]. Compared with PAH, the pathological changes of pulmonary hypertension due to lung diseases and/or hypoxia are characterized by medial hypertrophy and intimal obstructive proliferation of the distal pulmonary arteries [4, 9]. In general, the severity of pulmonary hypertension due to lung diseases and/or hypoxia is usually from mild to moderate compared with PAH [4, 10].
Despite the fact that PAH associated with idiopathic, heritable, and connective tissue diseases largely occurred to women, researchers reported that women with chronic obstructive pulmonary diseases exhibited a lower risk of mortality than men [11]. Consistent with this observation, earlier studies on HPH animal models have demonstrated that female rats developed less severe PH than male [12, 13].
Current therapeutic strategies to pulmonary hypertension mainly include anticoagulation, prostacyclin, lung transplantation, atrial septostomy, and pulmonary endarterectomy [4, 8]. New therapeutic strategies, such as prostacyclin analogues, endothelin-1 receptor antagonists, phosphodiesterase inhibitors and L-arginine, are emerging [14]. Furthermore, genetic therapy, stem cell therapy, and anti-proliferative therapies are also being under explored in laboratories [15].
Studies about estrogen have shown its various cardiovascular protective effects including prevention of coronary artery atherosclerosis [16], vasodilation of vessels [17], reduction of heart attacks [18] and attenuation of heart remodeling [19], etc. The molecular mechanisms of estrogen's cardiovascular protective effects involve decreasing induction of erythropoietin [20], inhibiting endothelin-1 expression [21], initiating nitride oxide synthesis [22], activating prostacyclin synthesis [23], and downregulating various adhension molecules [24]. Investigations also showed that estrogen can effectively relieve pulmonary hypertension of various etiologies including drugs, sclerosis, idiopathic, and other systematic diseases [25, 26]. Additionally, Earley and Mukundan et al found estrogen markedly alleviated chronic hypoxia-induced pulmonary hypertension through modulating different molecules' expression [20, 27]. Moreover, studies also revealed the anti-proliferation effects of estrogen in other proliferative vascular diseases [28, 29].
Pulmonary vascular proliferation and remodeling are considered to be the central pathogenesis in the process of chronic hypoxia-induced pulmonary hypertension (HPH) [30]. In normal situation, most of the PASMCs in healthy adult are in a quiescent state [31], while proliferative PASMCs are found in the pulmonary hypertension arterioles which contribute to media thickening and vascular resistance [30, 32]. Recent experimental studies indicate that cell cycle inhibition holds great potential as a therapeutic strategy for vascular proliferative diseases [33, 34]. The cell cycle progression is regulated by kinds of cyclin-dependent kinases (CDKs) and their specific regulatory cyclins. The cyclin-dependent kinases inhibitors include INK4 family and cip family which can inhibit the activity of the CDK-cyclin complexes. The Cip/Kip family can inhibit all cyclin/CDK complexes in vitro, in which p27kip1 is one of the core members controlling the cell cycle progression [35, 36].
Though p27kip1 was found years ago [37], it was recently recognized as an anti-oncogene protein [38, 39]. Subsequent studies revealed p27 kip1 as an inhibitor of vascular smooth muscle cell, which might participate in vascular proliferative diseases [40]. Fouty and colleagues forward demonstrated that p27 kip1 is important in modulating PASMCs [41]. Moreover, Yu et al found that decreased functional p27kip1 may contribute to PVSR associated with pulmonary hypertension, and up-regulated p27kip1 mediate the inhibition effect on HPH [42].
The S phase kinase associated protein 2 (Skp-2) was found as a member of the large eukaryotic family of F-box proteins which functions specifically for the degradation of p27kip1 [43]. Studies in these years demonstrated Skp-2 as a proto-oncogene and a positive regulator of cell cycle which involved in many diseases [44, 45]. After activated by the phosphorylated Akt (Akt-P), Skp-2 mediates the proteolysis of p27kip1 [46]. Now cancer therapeutic strategies targeting Skp-2 are becoming a hot topic [47, 48].
In view of the mentioned above, we hypothesized that β-E2 may act on the Akt signal pathway and keep p27kip1 from being degraded through down-regulation of Skp-2 under hypoxia condition. In this way, β-E2 may ameliorate chronic hypoxia-induced PVSR and pulmonary hypertension. Therefore, we adopted β-E2 to explore the roles of p27kip1 and Skp-2 in the evolution of PVSR and HPH in vivo. Further, we verified their effects on cultured PASMCs in vitro.
Methods
Experimental groups
All animal experiments were approved by the Animal Care and Use Committee of the Fourth Military Medical University and complied with the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). Male Sprague-Dawley rats (body weight 180-230 g) from the animal center of the Fourth Military Medical University (Xi'an, China) were used for all the experiments in our study.
Animals were randomly divided into 4 groups: 1) normoxia group, n = 7, 2) normoxia group treated with β-E2 (100 μg/kg via intraperitoneal injection), n = 7, 3) chronic hypoxia group, n = 7, and 4) chronic hypoxia group treated with β-E2 (100 μg/kg via intraperitoneal injection), n = 7. Animals designated for exposure to chronic hypoxia were housed intermittently in a hypobaric hypoxia chamber depressurized to 380 mmHg (oxygen concentration reduced to about 10%) and under hypoxia exposure for 10 h/d continuing 4 weeks. The normoxic control rats were housed at ambient barometric pressure (~718 mmHg, 21% oxygen). All animals were maintained in a 12:12-h light-dark cycle condition. The room temperature was air-conditioned at 25°C.
Hemodynamic experiments and tissue preparation
After 4 weeks hypoxia exposure, the animals were anesthetized with 20% ethylurethanm (4 ml/kg i.p.), and a soft silicagel catheter linked to a Powerlab system (AD Instruments, Colorado Springs, CO, AUSTRALIA) was inserted into the right jugular vein. There were special pressure waveforms displayed on the monitor when the catheter arrived in the right ventricle chamber. The right ventricle peak systolic pressure (RVSP) was then recorded. Meanwhile, the mean carotid artery pressure (mCAP) was recorded via a special catheter inserted into the carotid artery.
After the hemodynamic data were recorded, sternotomy surgery was performed. Lungs together with heart were harvested and put in a culture plate with cold PBS. The weight of right ventricle (RV) and left ventricle plus septum (LV+S) were obtained, and the ratio of (RV/LV+S) was calculated as an index of RV hypertrophy. The lungs were dissected into 3-mm-thick slices at the same point (the lower lobe of the right lung) and placed in neutral buffer (pH 7.4) containing 10% formalin. The remained lungs were frozen in -80°C freezer for subsequent experiments.
Morphological investigation
After soaked in 10% formalin for 72 hours, the slices were embedded in paraffin and sectioned into 4-μm-thick sections and hematoxylin and eosin staining was done. The stained lung sections were processed by a pathologist for light microscopic observation and photo images analysis. Pulmonary arteries, external diameter of which ranged from 50 to 200 μm, 5-6 vessels with approximate round shape were obtained from each individual animal, total 40 arteries were got from every group. The average size of the obtained vessels was 78 μm. The outside diameter and inside diameter of pulmonary arterioles were measured by an image-processing program (Image-Pro Plus, Version 5.1, Media Cybernetics, USA). The medial wall thickness, the cross sectional area of medial wall, and the total cross sectional vessel area were obtained. Pulmonary vascular structure remodeling was assessed by percent medial wall thickness (MT%) and percent medial wall area (MA%) two indices: (MT%) = 100 × (medial wall thickness)/(vessel semi-diameter); (MA%) = 100 × (cross-sectional medial wall area)/(total cross-sectional vessel area). All the morphological analysis was conducted in a double-blind method.
Cell culture and treatment
Pulmonary artery smooth muscle cells (PASMCs) were obtained by tissue explant culturing method. Pulmonary arteries were isolated from adult male Sprague-Dawley rats as described above. After the adventitial layers together with surrounding tissue were removed, the pulmonary arteries were dissected into small pieces and placed in a culture flask. The flask was overturned placed and Dulbecco Eagle's minimum essential medium (DMEM) (HyClone, Logan, UT, USA) was added in. After 2-4 hours, the flask was carefully turned over, and the medium immersed the tissue pieces. The explanted tissue was cultured in DMEM supplemented with 100 U/ml penicillin, 0.1 mg/ml streptomycin, 2 mM L-glutamine, and 10% FBS and grown in humidified incubators (HH·CP-01W, Shanghai Boxun Industry & Commerce Co., Ltd. Medical Equipment Factory, Shanghai, China) at 37°C in 95% O2 , 5% CO2. The PASMCs grew out in about a week, and cell passage was performed when the cells grew to 70% confluence. Cells were used between passages 3 and 6. Smooth muscle cell identity was verified by positive staining for smooth muscle α-actin (mouse monoclonal antibody; Sigma, USA) at each passage (>95% of cells stained positive for smooth muscle α-actin). The cells were seeded at 1 × 107 cells per well in cell culture Petri dishes (JET BIOFIL inc, Canada) and allowed to grow for 2 days. Then the cells were undergone serum-starvation for 36 hours. The media was then changed to containing 5% FBS phenol-red-free DMEM (HyClone, Logan, UT, USA) with β-E2 in various dosages (10-5, 10-7, 10-9 mol/L). There were total 5 groups for cell study, one normoxia group, and three hypoxia exposure groups treated with three different dosages of β-E2, and one under hypoxia exposure group alone. Cells were cultured either in 21% oxygen or 2% oxygen condition (HERAcell 240, Heraeus Inc, Germany) for another 48 hours. After treatment the cell lysates were obtained as described below for the Western blotting and RT-PCR analysis.
Cell proliferation assay
To investigate the inhibition of β-E2 on hypoxia-induced proliferation of PASMCs, 3-(4,5-dimethylthiazal-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) tests were completed. In a word, cells were seeded in 96-well cell culture Petri dishes at 4 × 104 cells per well according to the groups designated above and 3 dosages of β-E2 (10-5, 10-7, 10-9 mol/L) was added in. After cultured for 48 hours under normoxia condition or hypoxia exposure, solution MTT was added into each well in a 5 mg/mL concentration. Cells were cultured for another 4 hours, and then dimethyl sulfoxide (DMSO) was added in. After vibrating for 10 minutes, the optical density values were detected at 490 nm wavelength by using a spectrophotometer (PowerWave XS, BioTek Inc, Vermont, USA).
Western blotting analysis
Total lysates were obtained from harvested lung tissue and cultured PASMCs. Lung homogenates were prepared in RIPA lysis buffer, containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 2 mM NaF, 5 mM EDTA (pH 8.0), 1 mM sodium orthovanadate (Beyotime Inc, Jiangsu, China). The protease inhibitor of phenylmethylsulfonyl fluoride (PMSF, 1 mM) was added to the RIPA buffer in advance. Equivalent amounts of protein (30 μg) from each sample were separated on 12% SDS-polyacrylamide gels, and then transferred onto 0.22 μM nitrocellulose filter membranes (Millipore, Bedford, USA). The primary antibodies were p27kip1 antibody (1:1000; Millipore, Bedford, USA), Skp-2 polyclonal antibody (1:50; Abcam, Cambridge, UK) and Akt-P (1:500; Cell Signaling Technology, Inc., Massachusetts, USA). The signals were detected by ECL kit (BestBio Inc, Shanghai, China).
RNA extraction and reverse-transcription polymerase chain reaction (RT-PCR) investigation
Total RNA of lung tissue and cultured PASMCs were extracted by using Trizol agent (Invitrogen, Carlsbad, CA, USA). M-MLV reverse transcripase kits (BestBio Inc, Shanghai, China) were used to synthesize first-strand cDNA from 2.5 μg per sample of total RNA according to the manufacturer's instructions. The primer pairs were designed by primer premier 5 (PREMIER Biosoft International, Palo Alto CA, USA), and original information of cDNA were aligned in the GeneBank. The primers were checked and synthesized by Genescript Company (Nangjing, China). The primer pairs for p27kip1 PCR (319 bp) were (forward chain) 5'-CTTGGAGAAGCACTGCCGAGAT-3' and (reverse chain) 5'-CCCTGGACACTGCTCCGCTA-3', for Skp-2 (396 bp) were (forward) 5'-TAAGCGTTAGGTCTTTGGAA-3' and (reverse) 5'-TGGTTGTGTGTGTCTGTGTC3', and for the housekeeping gene β-actin (270 bp) were (forward) 5'-ATCATGTTTGAGACCTTCAACA-3' and (reverse) 5'-CATCTCTTGCTCGAAGTCCA-3' respectively. PCR program for p27kip1 was started by a 5 min denaturation procedure at 95°C, followed by 35 cycles of 95°C for 30 s, 51°C for 30 s and 72°C for 1 min, and a final extension at 72°C for 10 min; for Skp-2 was initiated by a 5 min denaturation step at 95°C, followed by 35 cycles of 95°C for 30 s, 59°C for 30 s and 72°C for 1 min, and a final extension at 72°C for 10 min; and for β-actin was began with a 5 min denaturation step at 95°C, followed by 35 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 1 min, and a final extension at 72°C for 10 min. After amplification was done, the products were separated by 1% agarose gel (containing 0.5 μg/ml ethidium bromide) electrophoresis. The gels were visualized by a gel visualizing system (BioSens SC 810, Shanghai Bio-Tech Inc, Shanghai, China) and densitometry was calculated using the imaging software (BioSens Digital Imaging 5, Shanghai Bio-Tech Inc, Shanghai, China).
Statistical analyses
All values were expressed as mean ± SD. Statistical analysis was processed by using one-way ANOVA, followed by LSD test for post hoc multiple comparisons (SPSS for Windows version 16.0, Chicago, USA). Significant difference was accepted at P < 0.05.
Results
Beta-estradiol treatment attenuated chronic hypoxia-induced pulmonary artery remodeling and pulmonary hypertension in rats
The right ventricular systolic pressure (RVSP) was measured by catheterization via jugular vein to right ventricle, which substitutes for the pulmonary artery pressure. After 4 weeks hypoxia exposure, the RVSP of hypoxia group was significantly elevated compared with the normoxia group (47.36 ± 3.47 vs. 24.90 ± 1.66 mmHg; Table 1, n = 7, P < 0.01). In the hypoxia+β-E2 group, RVSP was significantly lower than the chronic hypoxia exposed group (35.17 ± 1.67 vs. 47.36 ± 3.47 mmHg; Table 1, n = 7, P < 0.01), even it was a little higher than those of normoxia group. There was no significant difference of the RVSP between the normoxia group and the normoxia treated with β-E2 group (24.90 ± 1.66 vs. 25.17 ± 1.20 mmHg; Table 1, n = 7, P < 0.01). There was no significant difference in the mCAP between each group (as shown in Table 1).
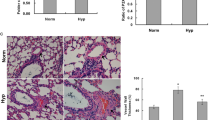
Wall-thickened pulmonary arterioles with medial smooth muscle cell proliferation and hypertrophy, and inflammatory cell infiltration were observed in the chronically hypoxia-exposed rats lungs compared with those in the normoxia group. The adventitial thickening together with extracellular matrix accumulation, intima hyperplasia and endothelial cells proliferation were also observed in those rats' lungs under chronic hypoxia exposure. Destruction of lung alveolar structure can also be seen in the chronic hypoxia treated rats lungs. The vessel changes as described above in the hypoxia+β-E2 group were better than those of the hypoxia group (Figure 1a, Hematoxylin and eosin staining).
Effects of β-E2 on chronic hypoxia-induced pulmonary vascular structure remodeling of rats. (a) Hematoxylin and eosin staining of pulmonary arterioles (original magnification ×20). (b) Medial wall thickness (MT%) of pulmonary arterioles. (c) Medial wall area (MA%) of pulmonary arterioles. Scale bars = 50 μm. *P < 0.01, significant difference from the normoxia group. # P < 0.01, significant difference from the hypoxia group. Arterioles of external diameter ranged from 50-200 μm, 5-6 vessels per animal, total 40 small arteries for each group, were obtained. Values are expressed as mean ± SD (n = 40).
Furthermore, the medial wall thickness (MT)% of the arterioles, the index of pulmonary artery remodeling, was significantly elevated after chronic hypoxia exposure versus the normoxia group (Figure 1b, n = 40, P < 0.01). In the hypoxia+β-E2 group, (MT)% was notably smaller than in the hypoxia group (Figure 1b, n = 40, P < 0.01). There was no significant difference of the (MT)% between the normoxia group and normoxia+β-E2 group (Figure 1b). In accordance with the medial wall thickness, the medial wall area (MA)% in the hypoxia group was also significantly higher than in the normoxia group (Figure 1c, n = 40, P < 0.01). Consistent with the result of (MT)%, there was no significant difference of the (MA)% between the normoxia group and normoxia+β-E2 group (Figure 1c). Beta-estradiol treatment significantly decreased the (MA)% of the arterioles after hypoxia exposure compared with the hypoxia group (Figure 1c, n = 40, P < 0.01). Nevertheless, both (MT)% and (MA)% were still higher than that of the normoxia group (Figure 1b, c).
Cell proliferation analysis of PASMCs
The OD values were representatives of the cells number. As shown in Figure 2, hypoxia exposure dramatically increased the OD values compared with those of the normoxia group (Figure 2, P < 0.01), that is, hypoxia exposure significantly increased the cell proliferation. The hypoxia-induced proliferation of PASMCs were obviously inhibited by three various dosages of β-E2 treatment (Figure 2, P < 0.01).
Protein expression of p27kip1 and Skp-2 in rat lung tissue and cultured PASMCs
Aiming to know whether p27kip1 and Skp-2 were involved in chronic hypoxia-induced pulmonary hypertension and artery remodeling, the protein levels of p27kip1 and Skp-2 in the 4 experimental groups were compared. Western blotting results showed that the relative p27kip1 level in hypoxia group was significant lower than that of the normoxia group. The relative p27kip1 level in β-E2 treatment group was significantly higher than that of the hypoxia group (Figure 3a, P < 0.01). There was no notable difference of the relative p27kip1 level between the normoxia and the normoxia+β-E2 group (Figure 3a). The results also showed that the relative Skp-2 level in hypoxia group was significantly higher than that of the normoxia group (Figure 3a, P < 0.01). The relative Skp-2 level in hypoxia+β-E2 group was significantly lower than that of the hypoxia group (Figure 3a, P < 0.01). In accordance with the results of p27kip1, there was no significant change of the Skp-2 relative level between the normoxia group and the normoxia+β-E2 (Figure 3a).
Effects of β-E2 on p27 and Skp-2 expression in rat lungs and cultured rat PASMCs. (a) Representative Western blotting analysis of p27 and Skp-2 protein levels in rat lungs. (b) Representative Western blotting analysis of p27 and Skp-2 protein levels in cultured rat PASMCs. *P < 0.05, significant difference from the hypoxia group. # P < 0.05, significant difference from the normoxia group. $ P < 0.05, significant difference from the hypoxia group. & P < 0.01, significant difference from the normoxia group. Values are expressed as mean ± SD (n = 3).
To further confirm if p27kip1 and Skp-2 participated in the process of chronic hypoxia-induced pulmonary artery remodeling, the lysates of each group of PASMCs were used to do WB assays. As the results showed, compared with the normoxia group, the relative p27kip1 levels was significant lower in hypoxia group. However, the relative p27kip1 levels were markedly higher in all three dosages of β-E2 treatment groups than that of the hypoxia group (Figure 3b, P < 0.05). The relative p27kip1 level in the β-E2 (10-5 Mol/L) treated group was nearly 2 folds higher than in the hypoxia group (Figure 3b, P < 0.01). The data also revealed that hypoxia exposure notably escalated the expression of Skp-2 compared with the normoxia group (Figure 3b, P < 0.01). All three dosages of β-E2 treatment resulted in significant reduction of Skp-2 versus the hypoxia group (Figure 3b, P < 0.05).
Protein expression of Akt-P in rat lung tissue and cultured PASMCs
Our next step focused on phosphorylated Akt which initiates Skp-2 activation. Similar with Skp-2, the relative Akt-P level in hypoxia group was significantly higher than that of the normoxia group in rat lung tissue (Figure 4a, P < 0.01). The relative Akt-P level in hypoxia+β-E2 group was significantly lower than that of the hypoxia group (Figure 4a, P < 0.01).
Effects of β-E2 on Akt-P expression in rat lungs and cultured rat PASMCs. (a) Representative Western blotting analysis of Akt-P protein levels in rat lungs. (b) Representative Western blotting analysis of Akt-P protein levels in cultured rat PASMCs. *P < 0.01, significant difference from the hypoxia group. # P < 0.05, significant difference from the normoxia group. Values are expressed as mean ± SD (n = 3).
In accordance with the expression of Skp-2, in cultured PASMCs, hypoxia exposure resulted in notably elevation of Akt-P versus the normoxia group (Figure 4b, P < 0.01). The expression of Akt-P in the three dosages of β-E2 treatment groups were all significantly lower compared with the hypoxia group (Figure 4b, P < 0.05).
Changes in mRNA levels of p27kip1 and Skp-2 in rat lung tissue and cultured PASMCs
To further investigate whether p27kip1 and Skp-2 were regulated at transcriptional level, the mRNA levels of p27kip1, Skp-2 and β-actin in lung tissue were analyzed by RT-PCR. The results of RT-PCR showed that there were no significant differences between all groups in relative p27kip1 mRNA level, whether in rat lung tissue or in cultured PASMCs (Figure 5a, b).
Effects of β-E2 on mRNA expression in rat lungs and cultured PASMCs. (a) Analysis of p27 and Skp-2 mRNA levels in rat lungs. (b) Analysis of p27 and Skp-2 relative mRNA levels in cultured rat PASMCs. *P < 0.01, significant difference from the hypoxia group. # P < 0.01, significant difference from the normoxia group. $ P < 0.05, significant difference from the hypoxia group. & P < 0.01, significant difference from the normoxia group. Values are expressed as mean ± SD (n = 3).
On the other hand, the relative Skp-2 mRNA level in the hypoxia group was significantly elevated compared with that of the normoxia group in rat lung tissue (Figure 5a, P < 0.01). In the hypoxia+β-E2 group, the relative mRNA level of Skp-2 was significantly reduced compared with the hypoxia group (Figure 5a, P < 0.01). There was no significant difference between the relative Skp-2 level in rat lung tissue of normoxia group and normoxia+β-E2 (Figure 5a). In cultured PASMCs, the relative Skp-2 mRNA level was notably increased after hypoxia exposure compared with the normoxia group (Figure 5b, P < 0.01). All three different dosages of β-E2 treatment decreased the Skp-2 mRNA level in cultured PASMCs exposed to hypoxia (Figure 5b, P < 0.05).
Taken together, these results suggested that hypoxia exposure resulted in PVSR and pulmonary hypertension. Beta-estradiol treatment exerted benefit effects on hypoxia-induced PVSR and pulmonary hypertension. The elevation of p27kip1 and reduction of Skp-2 may play important roles in β-E2 attenuation effects on PVSR and HPH. The below diagram may elucidate the mechanism we explored in the present study (Figure 6).
Discussion
Pulmonary hypertension is characterized by vasoconstriction and remodeling of small pulmonary arteries [3, 8]. Media hyperplasia is the main pathologic change of the remodeling pulmonary arteries. The nature of the structural alteration and the mechanisms responsible for the pulmonary vasculature remodeling are complex and not yet fully elucidated [32]. Pulmonary hypertension accompanied with pulmonary vascular medial hyperplasia is mainly due to excessive PASMCs proliferation [30, 32].
Charron and colleagues revealed cell cycle as a critical therapeutic target to prevent vascular diseases [34]. As an important CKI and anti-oncogene protein, p27kip1, is now being investigated for its inhibitory effects on cancer and vascular proliferative diseases [49, 50]. Diez-Juan et al elucidated p27kip1 functions as a suppressor in VSMCs proliferation, neovascularization, restenosis and atherosclerosis [51]. On the other hand, Skp-2 also draws lots of attentions from researchers for being an important proto-oncogene protein and the specific degradation ligase for p27kip1. Now, considerable studies focus on the therapeutic potential of p27 kip1 and Skp-2 on cancers or vascular diseases [47, 52]. To date, there are few studies about the role of p27kip1 on hypoxic pulmonary hypertension. More work need to be done to elucidate the beneficial effects of p27kip1 on PVSR and HPH. As mentioned above, estrogen could effectively attenuate pulmonary hypertension and anti-proliferation in proliferative vascular diseases. Therefore, in this study we employed estrogen to explore the roles of p27 kip1 and Skp-2 in the evolution of PVSR and HPH.
It is interesting that p27kip1 and Skp-2 indeed involved in the protective effects of β-E2 on the chronic hypoxia exposed rats. In the present study on the rat models, chronic hypoxia exposure resulted in significantly elevated RVSP, increased RV/LV+S, MT% and MA%, and marked media thickening of pulmonary arterioles. Western blotting data showed that hypoxia diminished the expression of p27kip1 along with the escalation of Skp-2 and Akt-P. Beta-estradiol application reversed the reduction of p27kip1, elevation of Skp-2 and Akt-P, accompanied with the attenuation of pulmonary hypertension and PVSR induced by hypoxia. Consistent with the study in vivo, experiments in vitro also revealed the anti-proliferation effect of β-E2 on PASMCs. Moreover, the study in vitro also demonstrated that hypoxia resulted in marked reduction of p27kip1 and augmentation of Skp-2 and Akt-P, which can be effectively reversed by β-E2 treatment.
As the WB data showed, β-E2 increased the expression of p27kip1 protein in dosage-dependent manner. Moreover, Skp-2 and Akt-P decreased in direct proportion to the dosage of β-E2. On the other hand, RT-PCR data showed that p27kip1 mRNA was not obviously changed by β-E2 application, neither in vivo nor in vitro experiments. The results are consistent with those reported by Hengst L [53]. In contrast with our results, Yu et al found that the p27kip1 mRNA levels were significantly changed in hypoxia-exposed mice models compared with the heparin treated mice [42]. They also demonstrated in their study that p27kip1 protein was up-regulated by heparin. In our opinion, different animal species and varied interventions in the researches may explain the discrepancy. Unlike p27kip1, Skp-2 was found to be both changed at mRNA and protein levels, indicating that β-E2 can decrease Skp-2 expression both through transcriptional and posttranscriptional mechanisms. In accordance with the study of Karin et al [46], the present study also demonstrated that the Akt signal pathway may account for the reduction of Skp-2.
Series of studies on estrogen testified that it could effectively alleviate various types of vascular diseases. However, there are many controversies over the protective effects of estrogen, especially over the anti-proliferation effect [54–61]. In this study, we found that β-E2 significantly inhibited PASMCs growth under hypoxia condition, both in vivo and in vitro. In our opinion, the different experimental conditions, different animal models and different treatments may explain the controversies.
The estradiol metabolite, 2-methoxyestradiol (2ME), was found able to abrogate injury-induced neointima formation to decrease proliferating SMCs and to up-regulate the expression of P27kip1 [62]. Other researchers also revealed that 2ME can effectively attenuate bleomycin-induced pulmonary hypertension and fibrosis in rats [63]. Based on these studies, whether 2ME mediate the inhibition effect of β-E2 on PASMCs and elevation of P27kip1 in chronic hypoxia-induced pulmonary hypertension remains to be determined.
Chronic hypoxia exposure increases intimal thickness of arterioles by causing hypertrophy and hyperplasia of the endothelial cells [30, 64]. After being released from endothelium under chronic hypoxia condition, numbers of potent vasoactive substances then promote contraction and proliferation of PASMCs [65, 66]. Investigations revealed that endothelial cells are closely associated with the PASMCs proliferation in the process of HPH. Though studies have shown the effects of estrogen on ECs including repressing adhesion molecules expression and enhancing expression of cytokines [27, 67, 68], more work should be done to explore whether estrogen could elevate the expression of P27kip1 in endothelium and inhibit the proliferation of ECs under chronic hypoxia.
Taken together, our results demonstrated that β-E2 kept p27kip1 from being degraded through decreasing the production of Skp-2. Subsequently, p27kip1 prevented the chronic hypoxia induced PVSR and pulmonary hypertension through its inhibitory effects on PASMCs. Therefore, we concluded that stabilized p27kip1 by β-E2 application indeed participated in the attenuation of PVSR and HPH. The down-regulated Skp-2 through Akt signal pathway may be responsible for the increased expression of p27kip1.
Study limitations
(1) Though our data showed strong evidence that β-E2 could stabilize the expression of p27kip1 and attenuate PVSR and HPH, whether it could have the same beneficial effects on humans with PAH remains unclear. (2) The gender differences were not studied, that is, whether β-E2 has the same protective effects on female rats as males needs to be explored. Since male rat models were employed in the study, whether male hormones involved in β-E2 attenuating HPH should also be furthered. (3) Though the study showed evidence of the inhibitory effects of β-E2 on PASMCs, it still seems somewhat insufficient for not considering the ECs.
Conclusions
In brief, this study reveals that stabilized p27kip1 may be a partial mechanism by which β-E2 exerts its protective effects on PVSR and HPH. Down-regulation of Skp-2 through Akt signal pathway may account for the increased expression of p27kip1 after β-E2 application. So, up-regulating the production of p27kip1 or down-regulating the expression of Skp-2 might be new strategies to treat PVSR and HPH.
Abbreviations
- Akt-P:
-
phosphorylated Akt
- β-E2:
-
beta-estradiol
- CDK:
-
cyclin-dependent kinase
- CKI:
-
cyclin-dependent kinase inhibitor
- ECs:
-
endothelial cells
- HPH:
-
hypoxia-induced pulmonary hypertension
- MA:
-
medial wall area
- mCAP:
-
mean carotid artery pressure
- 2ME:
-
2-Methoxyestradiol
- MT:
-
medial wall thickness
- PASMC:
-
pulmonary artery smooth muscle cell
- PVSR:
-
pulmonary vascular structure remodeling
- RV/LV+S:
-
right ventricle/left ventricle+septum
- RVSP:
-
right ventricular systolic pressure
- Skp-2:
-
S phase kinase associated protein 2
References
Rubin LJ: Primary pulmonary hypertension. N Engl J Med 1997,336(2):111–117.
Chazova I, Loyd JE, Zhdanov VS, Newman JH, Belenkov Y, Meyrick B: Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. Am J Pathol 1995,146(2):389–397.
Gaine SP, Rubin LJ: Primary pulmonary hypertension. Lancet 1998,352(9129):719–725.
Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, et al.: Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009,30(20):2493–2537.
Rubin LJ: Pathology and pathophysiology of primary pulmonary hypertension. Am J Cardiol 1995,75(3):51A-54A.
Archer S, Rich S: Primary pulmonary hypertension: a vascular biology and translational research "Work in progress". Circulation 2000,102(22):2781–2791.
Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S: An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007,30(1):104–109.
Runo JR, Loyd JE: Primary pulmonary hypertension. Lancet 2003,361(9368):1533–1544.
Ward JP, McMurtry IF: Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol 2009,9(3):287–296.
Minai OA, Ricaurte B, Kaw R, Hammel J, Mansour M, McCarthy K, Golish JA, Stoller JK: Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol 2009,104(9):1300–1306.
Sunyer J, Anto JM, McFarlane D, Domingo A, Tobias A, Barcelo MA, Munoz A: Sex differences in mortality of people who visited emergency rooms for asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998,158(3):851–856.
Rabinovitch M, Gamble WJ, Miettinen OS, Reid L: Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol 1981,240(1):H62-H72.
Ou LC, Sardella GL, Leiter JC, Brinck-Johnsen T, Smith RP: Role of sex hormones in development of chronic mountain sickness in rats. J Appl Physiol 1994,77(1):427–433.
Galie N, Palazzini M, Leci E, Manes A: Current Therapeutic Approaches to Pulmonary Arterial Hypertension. Rev Esp Cardiol 2010,63(6):708–724.
Toshner M, Tajsic T, Morrell NW: Pulmonary hypertension: advances in pathogenesis and treatment. Br Med Bull 2010, 94:21–32.
Clarkson TB, Appt SE: Controversies about HRT--lessons from monkey models. Maturitas 2005,51(1):64–74.
Lahm T, Patel KM, Crisostomo PR, Markel TA, Wang M, Herring C, Meldrum DR: Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: the effects of sex and menstrual cycle. Am J Physiol Endocrinol Metab 2007,293(3):E865-E871.
Studd J: Ten reasons to be happy about hormone replacement therapy: a guide for patients. Menopause Int 2010,16(1):44–46.
Gardner JD, Murray DB, Voloshenyuk TG, Brower GL, Bradley JM, Janicki JS: Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am J Physiol Heart Circ Physiol 2010,298(2):H497-H504.
Mukundan H, Resta TC, Kanagy NL: 17Beta-estradiol decreases hypoxic induction of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol 2002,283(2):R496-R504.
Earley S, Resta TC: Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J Physiol Lung Cell Mol Physiol 2002,283(1):L86-L93.
Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Tan J, Meldrum DR: Selective estrogen receptor-alpha and estrogen receptor-beta agonists rapidly decrease pulmonary artery vasoconstriction by a nitric oxide-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 2008,295(5):R1486-R1493.
Sherman TS, Chambliss KL, Gibson LL, Pace MC, Mendelsohn ME, Pfister SL, Shaul PW: Estrogen acutely activates prostacyclin synthesis in ovine fetal pulmonary artery endothelium. Am J Respir Cell Mol Biol 2002,26(5):610–616.
Arnal JF, Douin-Echinard V, Tremollieres F, Terrisse AD, Sie P, Payrastre B, Guery JC, Bayard F, Gourdy P: Understanding the controversy about hormonal replacement therapy: insights from estrogen effects on experimental and clinical atherosclerosis. Arch Mal Coeur Vaiss 2007,100(6–7):554–562.
Smith AM, Jones RD, Channer KS: The influence of sex hormones on pulmonary vascular reactivity: possible vasodilator therapies for the treatment of pulmonary hypertension. Curr Vasc Pharmacol 2006,4(1):9–15.
Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Weil B, Meldrum DR: Exogenous estrogen rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction. Shock 2008,30(6):660–667.
Earley S, Resta TC: Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J Physiol Lung Cell Mol Physiol 2002,283(1):L86-L93.
Rosano GM, Panina G: Oestrogens and the heart. Therapie 1999,54(3):381–385.
Arnal JF, Laurell H, Lenfant F, Douin-Echinard V, Brouchet L, Gourdy P: Estradiol action in atherosclerosis and reendothelialization. Ernst Schering Found Symp Proc 2006, (1):69–86.
Stenmark KR, Fagan KA, Frid MG: Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 2006,99(7):675–691.
Gordon D, Reidy MA, Benditt EP, Schwartz SM: Cell proliferation in human coronary arteries. Proc Natl Acad Sci USA 1990,87(12):4600–4604.
Prabha M, Jin HF, Tian Y, Tang CS, DU JB: Mechanisms responsible for pulmonary hypertension. Chin Med J (Engl) 2008,121(24):2604–2609.
Braun-Dullaeus RC, Mann MJ, Sedding DG, Sherwood SW, von der Leyen HE, Dzau VJ: Cell cycle-dependent regulation of smooth muscle cell activation. Arterioscler Thromb Vasc Biol 2004,24(5):845–850.
Charron T, Nili N, Strauss BH: The cell cycle: a critical therapeutic target to prevent vascular proliferative disease. Can J Cardiol 2006,22(Suppl B):41B-55B.
Sherr CJ, Roberts JM: CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999,13(12):1501–1512.
Nakayama KI, Hatakeyama S, Nakayama K: Regulation of the cell cycle at the G1-S transition by proteolysis of cyclin E and p27Kip1. Biochem Biophys Res Commun 2001,282(4):853–860.
Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A: p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev 1994,8(1):9–22.
Chiarle R, Pagano M, Inghirami G: The cyclin dependent kinase inhibitor p27 and its prognostic role in breast cancer. Breast Cancer Res 2001,3(2):91–94.
Lapenna S, Giordano A: Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov 2009,8(7):547–566.
Tanner FC, Boehm M, Akyurek LM, San H, Yang ZY, Tashiro J, Nabel GJ, Nabel EG: Differential effects of the cyclin-dependent kinase inhibitors p27(Kip1), p21(Cip1), and p16(Ink4) on vascular smooth muscle cell proliferation. Circulation 2000,101(17):2022–2025.
Fouty BW, Grimison B, Fagan KA, Le CT, Harral JW, Hoedt-Miller M, Sclafani RA, Rodman DM: p27(Kip1) is important in modulating pulmonary artery smooth muscle cell proliferation. Am J Respir Cell Mol Biol 2001,25(5):652–658.
Yu L, Quinn DA, Garg HG, Hales CA: Cyclin-dependent kinase inhibitor p27Kip1, but not p21WAF1/Cip1, is required for inhibition of hypoxia-induced pulmonary hypertension and remodeling by heparin in mice. Circ Res 2005,97(9):937–945.
Zhang H, Kobayashi R, Galaktionov K, Beach D: p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell 1995,82(6):915–925.
Guo HQ, Pu XX, Guo CC, Rao HL, Li HR, Lin TY: The role of Skp2 in extranodal NK/T-cell lymphoma. Chin J Cancer 2010,29(5):567–571.
Chan CH, Lee SW, Wang J, Lin HK: Regulation of Skp2 expression and activity and its role in cancer progression. ScientificWorldJournal 2010, 10:1001–1015.
Ecker K, Hengst L: Skp2: caught in the Akt. Nat Cell Biol 2009,11(4):377–379.
Zhu L: Skp2 knockout reduces cell proliferation and mouse body size: and prevents cancer? Cell Res 2010,20(6):605–607.
Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, et al.: Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature 2010,464(7287):374–379.
Duncan TJ, Al-Attar A, Rolland P, Harper S, Spendlove I, Durrant LG: Cytoplasmic p27 expression is an independent prognostic factor in ovarian cancer. Int J Gynecol Pathol 2010,29(1):8–18.
Zhou CH, Xiang M, He SY, Qian ZY: Crocetin inhibits cell cycle G(1)/S transition through suppressing cyclin D1 and elevating p27(kip1) in vascular smooth muscle cells. Phytother Res 2009.
Diez-Juan A, Castro C, Edo MD, Andres V: Role of the growth suppressor p27Kip1 during vascular remodeling. Curr Vasc Pharmacol 2003,1(1):99–106.
van Tiel CM, Bonta PI, Rittersma SZ, Beijk MA, Bradley EJ, Klous AM, Koch KT, Baas F, Jukema JW, Pons D, et al.: p27kip1–838C > A single nucleotide polymorphism is associated with restenosis risk after coronary stenting and modulates p27kip1 promoter activity. Circulation 2009,120(8):669–676.
Hengst L, Reed SI: Translational control of p27Kip1 accumulation during the cell cycle. Science 1996,271(5257):1861–1864.
Kolodgie FD, Jacob A, Wilson PS, Carlson GC, Farb A, Verma A, Virmani R: Estradiol attenuates directed migration of vascular smooth muscle cells in vitro. Am J Pathol 1996,148(3):969–976.
Suzuki A, Mizuno K, Ino Y, Okada M, Kikkawa F, Mizutani S, Tomoda Y: Effects of 17 beta-estradiol and progesterone on growth-factor-induced proliferation and migration in human female aortic smooth muscle cells in vitro. Cardiovasc Res 1996,32(3):516–523.
Oparil S: Arthur C. Corcoran Memorial Lecture. Hormones and vasoprotection. Hypertension 1999,33(1 Pt 2):170–176.
Dubey RK, Jackson EK: Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol 2001,280(3):F365-F388.
Parker TA, Ivy DD, Galan HL, Grover TR, Kinsella JP, Abman SH: Estradiol improves pulmonary hemodynamics and vascular remodeling in perinatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2000,278(2):L374-L381.
Lahm T, Crisostomo PR, Markel TA, Wang M, Weil BR, Novotny NM, Meldrum DR: The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: potential new clinical implications for an old hormone. Crit Care Med 2008,36(7):2174–2183.
Sakao S, Tanabe N, Tatsumi K: The estrogen paradox in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2010,299(4):L435-L438.
Tofovic SP: Estrogens and Development of Pulmonary Hypertension - Interaction of Estradiol MeAolism and Pulmonary Vascular Disease. J Cardiovasc Pharmacol 2010.
Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt B, Dubey RK: 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ Res 2006,99(3):266–274.
Tofovic SP, Zhang X, Jackson EK, Zhu H, Petrusevska G: 2-methoxyestradiol attenuates bleomycin-induced pulmonary hypertension and fibrosis in estrogen-deficient rats. Vascul Pharmacol 2009,51(2–3):190–197.
Abidia A: Endothelial cell responses to hypoxic stress. Clin Exp Pharmacol Physiol 2000,27(8):630.
Chen YF, Oparil S: Endothelin and pulmonary hypertension. J Cardiovasc Pharmacol 2000,35(4 Suppl 2):S49-S53.
Aaronson PI, Robertson TP, Ward JP: Endothelium-derived mediators and hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 2002,132(1):107–120.
Caulin-Glaser T, Watson CA, Pardi R, Bender JR: Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest 1996,98(1):36–42.
Chakrabarti S, Lekontseva O, Peters A, Davidge ST: 17beta-Estradiol induces protein S-nitrosylation in the endothelium. Cardiovasc Res 2010,85(4):796–805.
Acknowledgements
This work was supported by the National Basic Research Program of China (No. 2006CB504100); the National Natural Science Foundation of China (No. 30770925, No. 30700965); and Key Laboratory of High altitude Medicine, Third Military Medical University (No.2009GK01, No. 2009GK02).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DQX, YL and Yi L designed the experiment, carried out the data analysis and drafted the manuscript. JW reviewed the manuscript for the first time. DQX, JW and BZ carried out the animal experiment. MX and YXW did the histopathological analysis and cultured the PASMCs. MX and HYD carried out the RT-PCR and Western blotting assays. MQD, PTZ, WN and MLL participated in directing the experimental techniques and coordination of the studies. YQG and ZCL conceived the total study and critically reviewed the manuscript. All authors have read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Xu, DQ., Luo, Y., Liu, Y. et al. Beta-estradiol attenuates hypoxic pulmonary hypertension by stabilizing the expression of p27kip1 in rats. Respir Res 11, 182 (2010). https://doi.org/10.1186/1465-9921-11-182
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1465-9921-11-182