Abstract
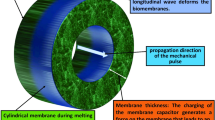
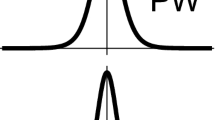
The problem of understanding the neurons function and thereby the brain has been the nexus of research during the last decades, in the fields of medicine and physical neuroscience. In the existing experimental studies, it is shown that the nerve impulse is an electromechanical signal which forces the membrane through the transition while propagating. In this work, we study localized nonlinear excitations in an electromechanical coupled model for biomembranes and nerves. We thus report on the presence of envelope solitons of the nerve impulse in this electromechanical coupled model. More importantly, we reshaped the obtained envelope solitons (breathing pulses) to action potential profile from direct numerical simulation of the coupled model. The numerical results shows a clear concordance with the analytical predictions. The theoretical results obtained in this work shows that the nerve impulse propagating through the proposed model is an electromechanical impulse that propagates along the nerve using spatial and temporal dimensions in the form of localized propagating nonlinear waves as predicted by experimental studies existing in the literature.









Similar content being viewed by others
Data availability
Available on citation.
References
P. Raven, G. Johnson, Biology (The McGraw Hill Companies, Dubuque, 2002)
I. Tasaki, Physiology and Electrochemistry of Nerve Fibers (Elsevier, Amsterdam, 1982)
G. Fongang Achu, S.E. Mkam Tchouobiap, F.M. Moukam Kakmeni, C. Tchawoua, Periodic soliton trains and informational code structures in an improved soliton model for biomembranes and nerves. Phys. Rev. E 98, 022216 (2018)
J. Engelbrecht, T. Peets, K. Tamm, M. Laasmaa, M. Vendelin, On the complexity of signal propagation in nerve fibres. Proc. Est. Acad. Sci. 67, 28 (2018)
P. Grabitz, V.P. Ivanova, T. Heimburg, Relaxation kinetics of lipid membranes and its relation to the heat capacity. Biophys. J. 82, 299–309 (2002)
M.M. Rvachev, On axoplasmic pressure waves and their possible role in nerve impulse propagation. Biophys. Rev. Lett. 5, 73 (2010)
A.M. Dikandé, G.A. Bartholomew, Localized short impulses in a nerve model with self-excitable membrane. Phys. Rev. E 80, 1 (2009)
T. Heimburg, A.D. Jackson, On the action potential as a propagating density pulse and the role of anesthetics. Biophys. Rev. Lett. 2, 57 (2007)
G. Fongang Achu, F.M. Moukam Kakmeni, A.M. Dikandé, Breathing pulses in the damped-soliton model for nerves. Phys. Rev. E 97, 012211 (2018)
R. Appali, U. Van Rienen, T. Heimburg, A comparison of the Hodgkin–Huxley model and the soliton. Theory for the action potential in nerves, in Advances Planar Lipid Bilayers Liposomes, vol. 16. (Academic Press, Cambridge, 2012), pp.275–299
T. Heimburg, A.D. Jackson, On soliton propagation in biomembranes and nerves. Proc. Natl. Acad. Sci. 102, 9790 (2005)
A.L. Hodgkin, A.F. Huxley, A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500 (1952)
J. Nagumo, S. Arimoto, S. Yoshizawa, An active pulse transmission line simulating nerve axon. Proc. IRE 50, 2061 (1962)
J.L. Hindmarsh, R.M. Rose, A model of the nerve impulse using two first-order differential equations. Nature 296, 162 (1982)
C. Morris, H. Lecar, Voltage oscillations in the barnacle giant muscle fiber. Biophys. J. 35, 193 (1981)
I. Tasaki, K. Iwasa, R.C. Gibbons, Mechanical changes in crab nerve fibers during action potentials. Jpn. J. Physiol. 30, 897 (1980)
I. Tasaki, K. Kusano, P.M. Byrne, Rapid mechanical and thermal changes in the garfish olfactory nerve associated with a propagated impulse. Biophys. J. 55, 1033 (1989)
I. Tasaki, P.M. Byrne, Volume expansion of nonmyelinated nerve fibers during impulse conduction. Biophys. J. 57, 633 (1990)
K. Iwasa, I. Tasaki, Mechanical changes in squid giant axons associated with production of action potentials. Biochem. Biophys. Res. Commun. 95, 1328 (1980)
A. El Hady, B.B. Machta, Mechanical surface waves accompany action potential propagation. Nat. Commun. 6, 6697 (2015)
J. Engelbrecht, K. Tamm, T. Peets, Modeling of complex signals in nerve fibers. Med. Hypotheses 120, 90 (2018)
J. Engelbrecht, K. Tamm, T. Peets, Electromechanical coupling of waves in nerve fibres. Biomech. Model. Mechanobiol. 17, 1771 (2018)
T. Heimburg, A. Blicher, L.D. Mosgaard, K. Zecchi, Electromechanical properties of biomembranes and nerves. J. Phys. Conf. Ser. 558, 012018 (2014)
T. Peets, K. Tamm, On mechanical aspects of nerve pulse propagation and the Boussinesq paradigm. Proc. Estonian Acad. Sci. 64, 331 (2015)
J. Engelbrecht, K. Tamm, T. Peets, On mathematical modelling of solitary pulses in cylindrical biomembranes. Biomech. Model. Mechanobiol. 14, 159 (2015)
T. Heimburg, The capacitance and electromechanical coupling of lipid membranes close to transitions: the effect of electrostriction. Biophys. J. 103, 918 (2012)
J. Tian, G. Huang, M. Lin, J. Qiu, B. Sha, T.J. Lu, F. Xu, A mechanoelectrical coupling model of neurons under stretching. J. Mech. Behav. Biomed. Mater. 93, 213 (2019)
A.S.F. Kamga, G.F. Achu, F.M.M. Kakmeni, P.G. Ghomsi, F.T. Ndjomatchoua, C. Tchawoua, Continuous signaling pathways instability in an electromechanical coupled model for biomembranes and nerves. Eur. Phys. J. B 95, 12 (2022)
G.F. Achu, F.M.M. Kakmeni, Neuromechanical modulation of transmembrane voltage in a model of a nerve. Phys. Rev. E 105, 014407 (2022)
F.M. Moukam Kakmeni, E.M. Inack, E.M. Yamakou, Localized nonlinear excitations in diffusive Hindmarsh–Rose neural networks. Phys. Rev. E 89, 052919 (2014)
C.S. Drapaca, An electromechanical model of neuronal dynamics using Hamilton’s principle. Front. Cell. Neurosci. 9, 1 (2015)
D. Debanne, E. Campanac, A. Bialowas, E. Carlier, G. Alcaraz, Axon physiology. Physiol. Rev. 91, 555 (2011)
H. Chen, D. Garcia-Gonzalez, A. Jérusalem, Computational model of the mechanoelectrophysiological coupling in axons with application to neuromodulation. Phys. Rev. E 99, 1 (2019)
I. Cinelli, M. Destrade, M. Duffy, P. McHugh, Electro-mechanical response of a 3D nerve bundle model to mechanical loads leading to axonal injury. Int. J. Numer. Methods Biomed. Eng. 34, e2942 (2018)
A. Gonzalez-Perez, L.D. Mosgaard, R. Budvytyte, E. Villagran-Vargas, A.D. Jackson, T. Heimburg, Solitary electromechanical pulses in lobster neurons. Biophys. Chem. 216, 51 (2016)
A. Jérusalem, Garca-Grajales JA. Merchan-Perez, J.M. Pena, A computational model coupling mechanics and electrophysiology in spinal cord injury. Biomech. Model Mechanobiol. 13, 883896 (2014)
L. Holland, H.W. de Regt, B. Drukarch, Thinking about the nerve impulse: the prospects for the development of a comprehensive account of nerve impulse propagation. Front. Cell. Neurosci. 13, 1 (2019)
I. Tasaki, Collision of two nerve impulses in the nerve fibre. Biochim. Biophys. Acta Biomembr. 3, 494 (1949)
A. Gonzalez-Perez, R. Budvytyte, L.D. Mosgaard, S. Nissen, T. Heimburg, Penetration of action potentials during collision in the median and lateral giant axons of invertebrates. Phys. Rev. X 4, 031047 (2014)
J. Engelbrecht, T. Peets, K. Tamm, Electromechanical coupling of waves in nerve fibres. Biomech. Model. Mechanobiol. 17, 1771 (2018)
B.A. Malomed, Solitary pulses in linearly coupled Ginzburg–Landau equations. Chaos Interdiscip. J. Nonlinear Sci. 17, 037117 (2007)
T. Heimburg, Mechanical aspects of membrane thermodynamics. Estimation of the mechanical properties of lipid membranes close to the chain melting transition from calorimetry. Biochim. Biophys. Acta Biomembr. 1415, 147 (1998)
K. Tamm, J. Engelbrecht, T. Peets, Temperature changes accompanying signal propagation in axons. J. Non-Equilibrium Thermodyn. 44, 277 (2019)
J. Engelbrecht, K. Tamm, T. Peets, On solutions of a Boussinesq-type equation with displacement-dependent nonlinearities: the case of biomembranes. Philos. Mag. 97, 967 (2017)
A. Blicher, T. Heimburg, Voltage-gated lipid ion channels. PLoS ONE 8, e65707 (2013)
A. Mondal, S.K. Sharma, R.K. Upadhyay, M.A. AzizAlaoui, P. Kundu, C. Hens, Diffusion dynamics of a conductance-based neuronal population. Phys. Rev. E 99, 042307 (2019)
I.B. Tagne nkounga, F.M. Moukam Kakmeni, R. Yamapi, Birhythmic oscillations and global stability analysis of a conductance-based neuronal model under ion channel fluctuations. Chaos Solitons Fract. 159, 112126 (2022)
Q. Xu, L. Huang, N. Wang, H. Bao, H. Wu, Mo Chen, Initial-offset-boosted coexisting hyperchaos in a 2D memristive Chialvo neuron map and its application in image encryption. Nonlinear Dyn., 1–17 (2023)
Q. Xu, X. Chen, X. Yu, W. Fan, Z. Li, N. Wang, Neuromorphic behaviors of a symmetric LAM-based electronic neuron circuit: numerical simulation and experimental measurement. Int. J. Electron. Commun. 162, 154594 (2023)
M. Remoissenet, Waves Called Solitons Concepts and Experiments (Springer, Berlin, 1999)
T. Dauxois, M. Peyrard, Physics of Solitons (Cambridge University Press, Cambridge, 2006)
A. Kamdoum Kuitche, A.B. Togueu Motcheyo, T. Kanaa, C. Tchawoua, Modulational instability in transversely connected nonlinear pendulum pairs. Eur. Phys. J. Plus 123, 1–10 (2023)
E. Tala-Tebue, G. Roger Deffo, S.B. Yamgoue, A. Kenfack-Jiotsa, F.B. Pelap, Monoatomic chain: modulational instability and exact traveling wave solutions. Eur. Phys. J. Plus 135, 715 (2020)
R.Y. Ondoua, J.C. Mimshe Fewu, D. Belobo Belobo, C.B. Tabi, H.P. Ekobena Fouda, Excitons dynamic in a three-stranded a-helix protein chains with diagonal and off-diagonal couplings: effects of strong long-range interactions. Eur. Phys. J. Plus 136, 274 (2021)
N.O. Nfor, P.G. Ghomsi, F.M. Moukam Kakmeni, Dynamics of coupled mode solitons in bursting neural networks. Phys. Rev. E 97, 1 (2018)
T. Heimburg, The thermodynamic soliton theory of the nervous impulse and possible medical implications. Prog. Biophys. Mol. Biol. 173, 24–35 (2022)
J.S. Andrew, W. Winlow, The soliton and the action potential-primary elements underlying sentience. Front. Physiol. 9, 779 (2018)
E. Quak, T. Soomere, Applied Wave Mathematics: Selected Topics in Solids, Fluids, and Mathematical Methods (Springer, Berlin, 2009)
H. Barz, A. Schreiber, U. Barz, Impulses and pressure waves cause excitement and conduction in the nervous system. Med. Hypotheses 81, 768–772 (2013)
H. Barz, U. Barz, Pressure waves in neurons and their relationship to tangled neurons and plaques. Med. Hypotheses 82, 563–566 (2014)
C.N. Takembo, A. Mvogo, H.P.E. Fouda, T.C. Kofané, Localized modulated wave solution of diffusive FitzHugh–Nagumo cardiac networks under magnetic flow effect. Nonlinear Dyn. 95, 1079 (2019)
S.H. White, T.E. Thompson, Capacitance, area, and thickness variations in thin lipid films. Biochim. Biophys. Acta Biomembr. 323, 7 (1973)
M. Peyrard, T. Dauxois, Physique Des Solitons (CNRS EDITION, Paris, 2004)
P. Guemkam Ghomsi, J.T. Tameh Berinyoh, F.M. Moukam Kakmeni, Ionic wave propagation and collision in an excitable circuit model of microtubules. Chaos Interdiscip. J. Nonlinear Sci. 28, 023106 (2018)
A. Mvogo, A. Tambue, G.H. Ben-Bolie, T.C. Kofané, Localized numerical impulse solutions in diffuse neural networks modeled by the complex fractional Ginzburg–Landau equation. Commun. Nonlinear Sci. Numer. Simul. 39, 396 (2016)
A. Karthikeyan, I. Moroz, K. Rajagopal, P. Duraisamy, Effect of temperature sensitive ion channels on the single and multilayer network behavior of an excitable media with electromagnetic induction. Chaos Solitons Fract. 150, 111144 (2021)
E. Villagran, A. Ludu, R. Hustert, P. Gumrich, A.D. Jackson, T. Heimburg, Periodic solutions and refractory periods in the soliton theory for nerves and the locust femoral nerve. Biophys. Chem. 153, 159 (2011)
I.B.T. Nkounga, F.M. Moukam Kakmeni, B.I.C.R. Yamapi, Controlling switching between birhythmic states in a new conductance-based bursting neuronal model. Nonlinear Dyn. (2021)
Author information
Authors and Affiliations
Contributions
ASFK did formal analysis, investigation, writing and editing; GFA and FMMK did concept development, supervision, writing, review and editing; CT did review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Appendices
Appendix A
The following are expressions of parameters used in Eq. (3):
Appendix B
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamga, A.S.F., Achu, G.F., Kakmeni, F.M.M. et al. Reshaping of breathing pulses to action potential profile propagating in an electromechanical coupled model for biomembranes and nerves. Eur. Phys. J. Plus 139, 58 (2024). https://doi.org/10.1140/epjp/s13360-023-04822-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-023-04822-4