Abstract
The production of medical radionuclides is one of the research activities carried out in the framework of the SPES (Selective Production of Exotic Species) project under the completion stage at the Legnaro National Laboratories of the National Institute for Nuclear Physics (INFN-LNL). The heart of SPES is the 70-MeV proton cyclotron having a dual-beam extraction, installed and commissioned in a new building equipped with ancillary laboratories currently under construction. The SPES main goal is the realization of an advanced ISOL (Isotope Separation On-Line) facility to produce re-accelerated exotic ion beams for fundamental nuclear physics studies. The cyclotron double-beam extraction system allows to simultaneously carry out applied research, such as radionuclides production for medicine (SPES-\(\gamma\)). This paper summarizes the results obtained with the interdisciplinary projects LARAMED (LAboratory of RAdionuclides for MEDicine) and ISOLPHARM (ISOL technique for radioPHARMaceuticals). The first one, based upon the direct activation method, is focused on the production of the radionuclides under the spotlight of the international community (e.g., \(^{99m}\)Tc, \(^{67}\)Cu, \(^{52/51}\)Mn, \(^{47}\)Sc and Tb isotopes), from the nuclear cross-section measurements up to the preclinical studies. The other one exploits the ISOL technique for the development and production of radioisotopes with high-specific activity, such as \(^{111}\)Ag, going beyond the state of the art in the field. The most recent SPES-\(\gamma\) research activities and future perspective are here described, characterized by a consolidated network of collaborations with national and international institutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Radionuclides and radiopharmaceuticals are fundamental tools in nuclear medicine, by enabling imaging and treatment in tens of millions of procedures performed worldwide on a yearly basis. In Europe, 9 million patients benefit from nuclear medicine procedures per year, including 1.5 million patients requiring radionuclide therapy against cancer [1]. The production of medical radionuclides is thus a key aspect, and emerging radionuclides are being playing a key role in the development of innovative radiopharmaceuticals. The availability of new research infrastructures dedicated to this goal is thus crucial for Europe. For such reasons, in 2021, the PRISMAP consortium was established as H2020 call, to gather the European research community, first, working in this field [2]. Access to the production and delivery of high-purity grade radionuclides for medical research is offered by PRISMAP consortium, through periodic calls when a selection of the best projects from the applicants is carried out by a panel consisting of experts in the fields of radionuclides production, molecular imaging as well as radionuclide therapy. Moreover, it may also be accessed by the selected biomedical laboratories to perform the associated research and to preclinical research techniques, in self-service or fully performed as a service. The SPES project at the INFN-LNL takes part in the PRISMAP consortium as an emerging facility, including both the direct activation method (LARAMED project, acronym for LAboratory of RAdioisotopes for MEDicine) [3, 4] and the ISOL (Isotope Separation On-Line) technique (ISOLPHARM project, acronym for ISOL technique for radioPHARMaceuticals) [5]. The first PRISMAP workshop on emerging infrastructures was indeed held at the INFN-LNL in November 2022 (additional information can be found in a dedicated report [6]). The research activities on radionuclides production for medicine (e.g., new radiopharmaceuticals development) and applied and interdisciplinary physics (e.g., radiobiology and environmental studies) at the INFN-LNL are coordinated by the Radioisotopes for Medicine and Applied Physics service (RMFA) located under the research division [7]. These activities are carried out in close collaboration with other services, in particular the Target service, which coordinates the production and characterization of different types of targets, for both nuclear physics experiments and applications. There is also a strong connection of these activities with those of European projects such as EUROLABS, focused on transnational access for users in European nuclear physics facilities [8].
The SPES project at the INFN-LNL has an ambitious scientific program, ranging from nuclear physics and astrophysics studies to interdisciplinary and medical applications [9, 10], as sketched in Fig. 1. The heart of SPES is the 70-MeV dual-extraction proton cyclotron (SPES-\(\alpha\)) [11], capable of providing intense beams up to 700 \(\upmu\)A (max 500 \(\upmu\)A allowed at each beam port). The dual-extraction beams systems will allow to perform simultaneously, not only state-of-the-art experiments with Radioactive Ion Beams (RIBs, SPES-\(\beta\)) [12] but also multidisciplinary research activities, such as radionuclides production for medical applications (SPES-\(\gamma\)) [13] and neutron-based research (SPES-\(\delta\)) [14, 15]. SPES-\(\gamma\) includes two approaches to produce medical radionuclides: The first is the direct activation method and is studied within the LARAMED project; the second, it is based on the ISOL technique, and it is exploited by the ISOLPHARM project.
2 The SPES facility
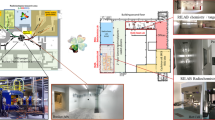
The SPES infrastructure is under completion at the INFN-LNL. Figure 2 shows the layout of the building at the underground level, where the cyclotron as well as all the ISOL and LARAMED bunkers are indicated on the left and right side of the picture, respectively.
The ISOL bunkers are located at the left side of Fig. 2, where the SPES target, called “front end,” is installed. Such a system includes all devices necessary to produce high-intensity and high-purity RIBs, according to the ISOL technique. Briefly, a proton beam with energy up to 40 MeV and intensity up to 200 \(\upmu\)A is extracted from the cyclotron and delivered to one of the ISOL bunkers. The part of the machine dedicated to the transport and focalization of the proton beam is called the protonic front end. A vacuum chamber is placed at the end of the proton beam line, within which a production target is installed. The typical SPES target is composed of seven disks axially spaced, composed of refractory carbides such as uranium carbide. The interaction between the impinging proton beam and the target material generates a characteristic set of radionuclides, most of which are released from the latter thanks to the high operational temperature levels, generally above 2000 \(^{\circ }\)C. The released nuclei migrate toward an ion source, directly connected to the production target. A fraction of such neutral atoms is transformed into singly charged positive ions that can be extracted into a particle beam thanks to intense electric fields. The extracted RIB is further transported and focalized by the so-called radioactive front end that includes all beam optics and diagnostic devices necessary for such task. Subsequently, the beam is injected into a Wien filter, an electromagnetic velocity separator that is used as mass spectrometer. Such device is employed to deflect and dump the beam particles characterized by a different mass respect to the ions of interest. The output of the Wien filter is an ideally isobaric ion beam, composed by the radionuclide of interest together with possible contaminants of other chemical elements with the same mass, that can be further accelerated and purified before being delivered to the experimental users. As one of the applications of the SPES facility, ISOLPHARM will exploit the RIB for providing high-purity unconventional radionuclides for R and D activities on radiopharmaceuticals. Among the producible nuclei, silver-111 (\(^{111}\)Ag) is regarded as one of the most interesting that ISOLPHARM can provide, as it is not easily available, unless nuclear reactors are used, with the drawbacks of high costs and low purity. In the case of ISOLPHARM, high purity is achieved by collecting the isobaric ion beam onto appropriate deposition substrates that are retrieved and dissolved in aqueous media. The desired nuclides are then harvested from the solution thanks to an element selective radiochemical process.
Regarding the LARAMED infrastructure in the SPES building, it includes two bunkers on the underground floor, the first dedicated to irradiation of high-power solid targets (i.e., up to ca. 10 kW), and the second dedicated to nuclear cross-sections measurements with low-intensity proton beams (100 nA); there are also two additional bunkers for direct activation, where two beamlines are foreseen to be installed in further years.
On the second floor of the building, two LARAMED laboratories are under completion: the RILAB Target-preparation laboratory where all the new/alternative cold chemistry technologies devoted to target manufacturing are developed, also in collaboration with the Target service; and the RILAB Radiochemistry laboratory, covering all the radiochemical processing aspects and designed to carry out R and D activities on radioisotope production, separation/purification, up to perform all the requested quality control procedures. The hot cell already installed in the RILAB Radiochemistry laboratory will be soon connected to the bunker with a pneumatic system, to automatically deliver the activated target after the proton irradiation. Additional hot cells are foreseen, and they will also be connected to the LARAMED bunker through the same pneumatic system. A third laboratory, the Radioisotope Factory laboratory for radioisotope/radiopharmaceutical production, is foreseen in further years dedicated to the implementation of a laboratory for Good Manufacturing Practice (GMP) production of radiopharmaceuticals. Figure 3 shows a schematic layout of the upper floor, highlighting the laboratories briefly described.
Since currently the laboratories and infrastructures of the SPES building are not yet operative, the SPES-\(\gamma\) research activities rely on a wide network of collaborations, internal and external the LNL, as outlined in the following paragraphs.
3 State of the art: LARAMED
The LARAMED project was funded by the Italian Ministry of University and Research (MIUR) in 2014 and 2016. It has been conceived to meet a double scope: first, to develop a more efficient production for well-established radionuclides already playing a key role in nuclear medicine (e.g., \(^{99m}\)Tc) [16,17,18,19]; second, to investigate yet unexplored production routes for novel radionuclides having potential interest in medicine, but still unavailable [20, 21]. Nonstandard medical radionuclides production is, indeed, a fundamental step to select new radiopharmaceuticals classes for both diagnostic and therapeutic applications [22]. The LARAMED research activity is envisaged to cover different topics, ranging from basic nuclear physics (experimental measurements of excitation functions) [21, 23,24,25] to target technology (design and manufacturing of high-power targets for proton irradiation to produce important medical radionuclides, such as \(^{99m}\)Tc, \(^{64}\)Cu, \(^{67}\)Cu, \(^{47}\)Sc, \(^{89}\)Zr, etc.) [26,27,28,29,30,31] and radiochemistry (development of highly automated separation/purification techniques and labeling studies of new biological carriers) [32,33,34,35,36,37,38]. The synergy between these skills resulted in high-level research on the cyclotron production of conventional and emerging radionuclides. Innovative target manufacturing techniques have been developed to enlarge the possibility to supply tailored targets for the specific radionuclide, for the nuclear cross-section measurements and for high yield production [26]. In collaboration with some Italian nuclear medicine departments, the targets realized have been tested under the proton beam provided by medical cyclotrons [26, 28]. The technologies and the know-how, available for the Target service of the INFN-LNL and that will be included in the new RILAB Target-preparation laboratory mentioned above, are:
-
An apparatus for the High-Energy Vibrational Powder Plating (HIVIPP) [39] technique able to deposit a thin metallic layer, starting from metal powder, used for the nuclear cross-section measurements [27, 40];
-
An ad hoc spark plasma sintering (SPS) machine, named “TT_Sinter,” developed in collaboration with the University and INFN Unit of Pavia (Italy). The versatility of this technique allows the manufacturing of metal and oxide thick targets for massive radionuclide production [28];
-
The magnetron sputtering (MS) system seems very promising because it allows the deposition of thin and thick film onto several backing plates with a good adhesion [17, 29, 41,42,43];
-
The preliminary target material preparation to achieve the optimal form (e.g., powder of defined size) for the specific target manufacturing technique (e.g., cryomilling) [39].
The wide national and international network of collaborations allowed the LARAMED team to enlarge the research topics to nuclear modeling, imaging and dosimetry [16, 19, 44, 45]. This network includes the GIP ARRONAX facility, the Wisconsin University and the Italian Universities of Ferrara, Milano, Padova and Pavia, the CNR in Milano, the LENA laboratory (Pavia, Italy), the Istituto Oncologico Veneto IOV_IRCCS (Padova, Italy), the Sacro Cuore Don Calabria hospital (Negrar, Verona, Italy) and the Policlinico Sant’Orsola Malpighi hospital (Bologna, Italy). The participation to the IAEA events and meetings organized by the Radioisotope Production and Radiation Technology and the Nuclear Data sections fostered the LARAMED team to share the scientific results with the broad international community. LARAMED research activities started about 10 years ago and were funded mainly by the INFN National Scientific Committee 5 (CSN5) and 3 (CSN3), respectively, focused on technological and interdisciplinary research and nuclear physics. The following paragraphs briefly outline the projects’ aims and outcomes.
3.1 APOTEMA and TECHNOSP (CSN5, 2012–2017)
APOTEMA and TECHNOSP are acronyms for Accelerator-based Production Of TEchnetium/-Molybdenum for medical Applications and TECHNetium direct-production in hOSPital, respectively. The first radioisotope we started working on was indeed the \(^{99m}\)Tc. The purpose of this work was the development of a technology able to produce 1–2 Ci amount of \(^{99m}\)Tc by low-energy cyclotron in a local nuclear medicine radiopharmacy and to prove its feasibility. We investigated the \(^{99m}\)Tc direct cyclotron production route by the (p,2n) reaction on an isotopically enriched \(^{100}\)Mo target. The irradiation parameters were optimized for an in-hospital cyclotron self-production of \(^{99m}\)Tc able to satisfy the needs of a medium-large hospital such as the Policlinico Sant’Orsola Malpighi, to afford its availability in case of shortages. This is the most complete project in which we developed all the technologies necessary to complete the entire production cycle from the target to a solvent extraction-based automatic separation and purification module, including the target recovery and radiopharmaceutical and preclinical studies, as described in Fig. 4 [16, 18, 19, 32, 36, 46,47,48,49,50,51]. With these two projects, the LARAMED group has also contributed to the international IAEA Coordinated Research Project (CRP) No. F22062 focused on Alternative, non HEU-based, \(^{99m}\)Tc/\(^{99}\)Mo supply (2011–2015) [38].
3.2 COME (CSN3, 2016)
COME, acronym for COpper MEasurement, was a 1-year project, dedicated to the first measurements of the \(^{70}\)Zn(p,x)\(^{67,64}\)Cu nuclear reactions above 35 MeV [21], performed in collaboration with the ARRONAX facility (Saint-Herblain, France) [52]. As shown in Fig. 5, these cross-section results were the ground for an INFN patent on a multi-layer target to optimize \(^{67}\)Cu production exploiting a 70-MeV proton beam and enriched \(^{70}\)Zn and \(^{68}\)Zn targets [53].
3.3 PASTA (CSN5, 2017–2018)
PASTA project, acronym for Production with Accelerator of Sc-47 for Theranostic Applications, was dedicated to nuclear cross-section measurements on \(^{\textrm{nat}}\)V and \(^{48}\)Ti targets for \(^{47}\)Sc production [24, 25, 44], whose results have been also presented in the IAEA CRP no. F22053 focused on Radiopharmaceuticals labeled with new emerging radionuclides (\(^{67}\)Cu, \(^{186}\)Re, \(^{47}\)Sc) [22]. The enriched metallic \(^{48}\)Ti targets have been manufactured and characterized within the E_PLATE project.
3.4 E_PLATE (CSN5, 2018–2019)
E_PLATE, acronym for Electrostatic Powders pLating for Accelerator TargEt, was a technological project, dedicated to thin target manufacturing exploiting the HIVIPP technique and the development of the apparatus already mentioned, as shown in Fig. 6. This technology is also part of the Target service of the INFN-LNL.
3.5 METRICS (CSN5, 2018–2021)
METRICS project, acronym for Multimodal pET/mRi Imaging with Cyclotron-produced \(^{52/51}\)Mn iSotopes, was dedicated to \(^{52}\)Mn accelerator-based production and to the development of innovative radiopharmaceuticals for PET/MRI dual-imaging purposes. To establish the potential of radioactive manganese chloride as a safe clinical PET imaging agent, the radiation effective dose of both \(^{51}\)Mn and \(^{52}\)Mn chloride has been assessed with OLINDA [54]. Simulations and models were also carried out to compare the amount of activity and radionuclidic purity of \(^{52g}\)Mn produced by different reactions as well as the dose increase to the patient caused by the radioactive contaminants co-produced by each reaction [55]. Moreover, different Cr target configurations have been studied using the SPS technique starting from \(^{\textrm{nat}}\)Cr and \(^{52}\)Cr powder (Fig. 7a). After the sintering of the Cr pellet, its adhesion to the backing support (Au/Cu, Nb or Au/Nb) was carried out by SPS process. The obtained targets have been successfully tested at 50 \(\upmu\)A (energy 19 MeV and 16 MeV), the maximum current available in the medical cyclotron of the Sacro Cuore Don Calabria hospital. A dedicated dissolution reactor [37] was developed to dissolve the solid targets after irradiation to process the produced radioisotope with automatic modules (Fig. 7b). Within this project, we recently investigated a new class of Mn(II) complexes to be potentially used as MRI contrast media and in particular the synthesis, characterization and evaluation of magnetic properties by phantom image studies of the new [Mn(II)(1.4-dioxa-8-azaspiro[4.5]decane-8-carbodithioate)\(_{2}\)]\(\cdot\)2 H\(_{2}\)O compound potentially useful as MRI contrast agent [16, 34, 35].
3.6 INTEFF_TOTEM (National Committee Technological Transfer, CNTT, 2021-2022)
INTEFF\(\_\)TOTEM project, acronym for magneTron sputtering cyclotrOn TargEt Manufacturing, is dedicated to the enhancement of the INFN patent on targetry to produce medical radionuclides [29]. Due to the high losses of the material during the sputtering deposition, a material saving approach and a new magnetron sputtering configuration have started to be implemented to use this technique also with the enriched materials [41,42,43]. In addition, the first feasibility study of producing \(^{\text{nat}}\)ZnO pellets using the alternative SPS technique has been investigated as a first technological step for the realization of the multi-layer target described in the INFN patent [30] to produce \(^{67}\)Cu by proton beam irradiation.
3.7 REMIX (CSN5, 2021–2023)
REMIX project, acronym for Research on Emerging Medical radIonuclides from the X-sections, is dedicated to the production of \(^{47}\)Sc and Tb radionuclides (\(^{149}\)Tb, \(^{152}\)Tb, \(^{155}\)Tb and \(^{161}\)Tb), and it is organized in Work Package (WP): WP1 for target realization and characterization by Elastic Backscattering Spectroscopy (EBS); WP2 for the measurements of the \(^{49,50}\)Ti(p,x)\(^{47}\)Sc nuclear cross-sections (exploiting the thin targets realized with the HIVIPP technique); WP3 for the measurements of the \(^{159}\)Tb(p,5n)\(^{155}\)Dy\(\rightarrow {}\) \(^{155}\)Tb and \(^{\text{nat}}\)Dy(p,x)\(^{15y}\)Tb nuclear reactions; WP4 for the nuclear modeling [25]; WP5 for dosimetric calculations; WP6 for the low-energy \(^{155}\)Gd(p,n)\(^{155}\)Tb production route in collaboration with the Sacro Cuore Don Calabria hospital and WP7 for the technology developments, such as a dedicated Target Station (TS) to be installed in the LARAMED beam line dedicated to nuclear cross-section measurements. During 2023, experiments for \(^{155}\)Tb production using a medical cyclotron are foreseen using Gd\(_{2}\)O\(_{3}\) pellets, obtained by SPS, using an aluminum capsule realized in the framework of the INTEFF_TOTEM project. Dosimetric calculations using the OLINDA code were performed for the \(^{47}\)Sc-DOTA folate [45]; results on \(^{155}\)Tb-labeled radiopharmaceuticals are foreseen for 2023.
4 State of the art: ISOLPHARM
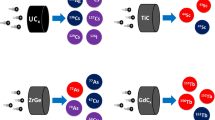
The idea behind the ISOLPHARM method [5, 56] is reported in Fig. 8: as in other ISOL facilities, a beam of protons coming from the cyclotron bombards a production target, activating different nuclear reactions depending on the used material. The generated radioisotopes, mainly neutron-rich \(\beta\) \(^{-}\) emitters, escape from the target, kept at a high temperature, toward an ion source, where they are ionized (1\(^{+}\)) by means of surface, laser or electron bombardment ionization techniques. The choice of the proper ion source depends on the element of interest and on the desired purity. After ionization, the charged radioisotopes are accelerated to form a RIB by means of a 40-kV potential. After some stages of beam focusing and steering, the presence of a mass separator is a crucial aspect for the purposes of ISOLPHARM. Considering a particular radioisotope of interest for medical purposes, mass separation allows to obtain an isobaric beam, removing all other isotopes of the desired element, which would otherwise not be chemically removable. This guarantees the obtainment of radioisotopes of high specific activity, being there not any stable contaminant of the same element. Besides the production of RIBs, already at the state of the art in ISOL facilities, the new approach introduced by ISOLPHARM is the collection of radioisotopes on a secondary target or collector in which the beam is stopped (Fig. 9). This collector can be subsequently dissolved and chemically purified to eliminate isobaric contaminants of other elements. Several materials were considered as deposition substrates, but the most promising results were achieved when 13-mm diameter cellulose-based disks were used. The obtained product can be then used for radiolabeling of specific molecules, which, in turn, can be delivered to universities or external research centers for preclinical studies.
Some key aspects characterize the ISOLPHARM project:
-
A strong collaboration between several INFN laboratories and sections and university departments all over Italy;
-
The production of radionuclides with high specific activity thanks to mass separation;
-
The presence of an INFN patent which proves the innovation and excellence of the project [57];
-
The non-necessity of using nuclear reactors to produce therapeutic radionuclides.
In the framework of ISOLPHARM, two experiments have been carried out in recent years and are briefly outlined hereafter.
4.1 ISOLPHARM\(\_\)Ag (CSN5, 2018–2019)
ISOLPHARM\(\_\)Ag project [58] aimed at proving the possibility of producing and using the \(^{111}\)Ag, a promising isotope which is produced in large quantities at SPES using a uranium carbide (UCx) target. This work was carried out with both computational and experimental investigations. As for computational studies, they were focused on the development of Monte Carlo codes able to estimate the production and release of \(^{111}\)Ag from a UCx target. Such codes were executed with the support of a cloud infrastructure in the CloudVeneto environment, being they computing intensive. From the experimental point of view, several studies were carried out: ionization and transport of silver using an electron bombardment (plasma) ion source [59]; chemistry studies to develop and characterize chelators able to form silver complexes [60] and biology studies on the final molecules to evaluate their interaction with a set of cellular targets [61].
4.2 ISOLPHARM\(\_\)EIRA (CSN5, 2020–2022)
In ISOLPHARM\(\_\)EIRA project, acronym for Experiment on Interdisciplinary research on Radioactive Ag, three main goals have been achieved, based on the application of the ISOLPHARM technique to produce \(^{111}\)Ag radiopharmaceuticals:
-
Physics: production of \(^{111}\)Ag at a nuclear reactor, spectroscopy (quality control) studies and laser ionization of Ag (Task 1);
-
Radiochemistry: synthesis and characterization of chelators, linkers, targeting agents, purification of isotopes and synthesis of a radiopharmaceutical (Task 2);
-
Biology: characterization of the produced molecules with tests on cell cultures, scaffold production and 3D cell cultures, in vitro and in vivo studies (Task 3).
As for physics, the main aim has been the production of an appropriate amount of \(^{111}\)Ag to be used for the radiolabeling of the prepared radiopharmaceutical [62]. \(^{111}\)Ag is produced by neutron capture reaction on enriched \(^{110}\)Pd at the TRIGA Mark II nuclear reactor of the LENA, acronym for Laboratorio Energia Nucleare Applicata of the Pavia University (Italy). Several activities have been carried out:
-
Production of \(^{111}\)Ag, including the design and construction of a semi-automatic device for the extraction and handling of the irradiated samples;
-
Spectroscopic studies (quality control) for the isotopic characterization of the irradiated samples;
-
Studies for the laser photoionization of natural Ag, to improve ionization efficiency in the future operation of uranium carbide targets at SPES.
As for chemistry, the final goal of Task 2 is to synthesize and characterize the radiopharmaceutical to be used by Task 3, including the radionuclide produced by Task 1 [63]. Different activities have been carried out:
-
Development of efficient purification methods for Ag from Pd and recovery of the expensive \(^{110}\)Pd target material;
-
Synthesis and characterization of bifunctional chelators for Ag and Cu;
-
Synthesis and characterization of fluorescent targeting vectors, to carry out in vitro studies on 2D and 3D cell cultures grown on scaffolds.
As for biology, the main activities are aimed at the identification of the optimal macromolecules for the subsequent more relevant in vivo tests with radiolabeled compounds [64]. Many activities have been carried out:
-
Selection of proper cell lines expressing relevant targets;
-
In vitro and in vivo studies of targeting vectors;
-
In vitro studies of targeting vectors with fluorescent targeting agents, using stable silver;
-
Production of suitable 3D scaffold for in vitro tissue mimicking;
-
Uptake studies on 3D cell cultures with fluorescent compounds;
-
Biodistribution and in vivo imaging using the most promising developed radiopharmaceutical.
5 New projects
CUPRUM\(\_\)TTD project (\(^{67/64}\)CU PRoduction and Use in Medicine\(\_\)Target Technology Development, funded by CSN5 in 2023-2025) is dedicated to production of copper radionuclides \(^{67/64}\)Cu, currently considered among the best-suited isotopes for radioimmunotherapy and theranostic applications, used as single isotopes, or in pair [22, 65]. The \(^{67}\)Cu half-life (T\(_{1/2}\) 61.9 h) indeed allows for labeling pharmaceuticals having slow pharmacokinetics, while \(\beta\) \(^{-}\)/\(\gamma\) radiation (for \(^{67}\)Cu) and \(\beta\) \(^{-}\)/\(\beta\) \(^{+}\) emission (for \(^{64}\)Cu, T\(_{1/2}\) 12.7 h) being suitable for therapy and SPECT/PET imaging, respectively [66]. Currently, the large-scale production of \(^{67}\)Cu still remains challenging: Only a few sites are producing it worldwide [67], while a stable supplier in Europe is still lacking. The production of \(^{67}\)Cu has been a top priority since the very early days of LARAMED project, as underlined by the COME experiment. The goal of CUPRUM\(\_\)TTD is, therefore, the R and D of the technology necessary for the cyclotron-driven production of \(^{67}\)Cu, i.e., the development of a robust target manufacturing technology and recovery, as well as the related target processing system, aimed at attaining clinical grade batches of \(^{67}\)Cu useful for in vivo/preclinical investigations coupled with new Cu-labeled prototype carriers.
STARDIS project (Solid TARget DIsolution System, funded by National Committee Technological Transfer in 2022-2023) aimed at the design of a commercial solid target dissolution system apt to be implemented in automatic synthesis modules for radiopharmaceuticals production. The project is focused on increasing the technology readiness level of the chemical reactor prototype developed for the METRICS project to obtain a reliable system of interest for research laboratories and the pharmaceutical industry.
As a continuation of the activities carried out in ISOLPHARM\(\_\)EIRA, a new experiment named ADMIRAL (Advanced Dosimetry Methods and In vitro Radiobiology of Ag-111 Labeled radiopharmaceuticals, funded by CSN5 in 2023–2025) was recently financed. This experiment aims at probing the therapeutic and diagnostic properties of \(^{111}\)Ag, enriching ISOLPHARM with a new point of view, which has its roots in the fields of medical physics, radiobiology and radiation detectors.
The SPES research group at LNL has also started a structured program of research and development activities on targets, ion sources and molecular beams, benefiting from prestigious international collaborations (ISOLDE-CERN, ORNL and IPN-ORSAY). Moreover, the INFN Padova Division together with the Department of Industrial Engineering of the University of Padova has recently developed the technology required for the additive manufacturing (AM) of high-temperature materials such as silicon carbide, titanium carbide, tantalum, tungsten and molybdenum. All these materials are widely used to produce ISOL targets, ion sources and numerous auxiliary components. With AM technologies more degrees of freedom are available for the design process, and the geometrical complexity that can be obtained is extremely high. In this context, the HISOL experiment (High-performance ISOL systems for the production of radioactive ion beams, funded by CSN5 in 2023-2024) has been financed with the main aim to create a collaboration network fully focused on a new generation of high-performance ISOL targets and ion sources. Taking as a basis the aforementioned know-how, the objectives of the experiment are “the study and development of innovative recipes and methods to produce ISOL targets,” “the study and development of innovative methods to produce and operate ion sources” and “the characterization of the obtained materials and components by means of advanced techniques.” As a result, we expect to test at high temperature a new high-performance target prototype and a new high-performance ion source prototype, producing stable beams at LNL. This will be the basis for future on-line tests with high-intensity and high-purity RIBs.
6 Conclusions and future perspectives
Once the SPES facility will be operative, the 70-MeV proton cyclotron will be exploited to produce radionuclides of medical interest with the direct activation and the ISOL technique. A wide national and international network of collaborations to perform these multidisciplinary research activities, ranging from nuclear physics to radiochemistry, material science, targetry, engineering and radiopharmacy is being built. New projects started in 2023 and are here described.
It is important to mention that the SPES-\(\gamma\) team has full access to the Radiopharmaceuticals and Molecular Imaging Laboratory (LARIM) located at the INFN-LNL. LARIM is one of the few fully equipped facilities in Italy that can be used to develop new diagnostic or therapeutic radiopharmaceuticals labeled with the medical radioisotopes produced within the frame of the previously mentioned SPES projects. In addition, thanks to the convention between INFN-LNL and IOV-IRCCS, the team has the possibility to carry out the preclinical biodistribution and pharmacokinetics studies necessary to evaluate the new developed radiopharmaceuticals, using a state-of-the-art PET/SPECT/CT micro-scanner owned by the IOV-IRCCS (Vector 5, Milabs) and located at LARIM.
As a future perspective, it is worth mentioning the possibility to install a dedicated off-line mass separator devoted to the separation of isotopes produced by direct activation. The use of such a technique must obviously be considered only when the advantages clearly overcome the loss of efficiency due to this additional step in the overall production chain. Although the INFN-LNL is a public research facility, joint ventures with private companies interested in the already available and/or new radionuclides development programs are anyway foreseen.
In conclusion, it is important to underline that a young team of researchers is involved in the SPES-\(\gamma\) research activities, and that the European PRISMAP consortium identifies the INFN-LNL as emerging infrastructure, a unique laboratory where the potential impact of both production techniques can be studied.
Data Availability Statement
No data associated in the manuscript.
References
Nuclear Medicine Europe Medical Uses of Nuclear Technology Role, Challenges Perspectives Annex; Available Online. https://www.nucleareurope.eu/wp-content/uploads/2021/06/2021-06-14-PP-Medical-Uses-of-Nuclear-TechnologyAnnex.pdf
PRISMAP—Building a European Network for Medical Radionuclides; Available online. https://www.prismap.eu/
J. Esposito, D. Bettoni, A. Boschi, M. Calderolla, S. Cisternino, G. Fiorentini, G. Keppel, P. Martini, M. Maggiore, L. Mou, M. Pasquali, L. Pranovi, G. Pupillo, C. Rossi Alvarez, L. Sarchiapone, G. Sciacca, H. Skliarova, P. Favaron, A. Lombardi, P. Antonini, A. Duatti, LARAMED: a laboratory for radioisotopes of medical interest. Molecules 24(1), 20 (2019). https://doi.org/10.3390/molecules24010020
G. Pupillo, P. Antonini, M. Calderolla, A. Calore, D. Bettoni, A. Boschi, S. Cisternino, A. Duatti, F. Evangelisti, P. Favaron, G. Fiorentini, F. Gramegna, G. Keppel, M. Maggiore, P. Martini, L. Mou, M. Pasquali, L. Pranovi, C. Alvarez, J. Esposito, The LARAMED project at LNL: 67Cu and 47Sc production for theranostic applications. AIP Conf. Proc. 2295, 020001 (2020). https://doi.org/10.1063/5.0032898
F. Borgna, M. Ballan, S. Corradetti, E. Vettorato, A. Monetti, M. Rossignoli, M. Manzolaro, D. Scarpa, U. Mazzi, N. Realdon, A. Andrighetto, A preliminary study for the production of high specific activity radionuclides for nuclear medicine obtained with the isotope separation on line technique. Appl. Radiat. Isot. 127, 214–226 (2017). https://doi.org/10.1016/j.apradiso.2017.06.022
M. Manzolaro, S. Corradetti, G. Pupillo, L. Popescu, Prismap deliverable d8.1. In: Proceedings Book Workshop 1 (2023). https://zenodo.org/record/7913190
Research Division—Home INFN Legnaro. https://www.lnl.infn.it/en/reasearch-division
EURO-LABS—EUROpean Laboratories for Accelerator Based Science. https://web.infn.it/EURO-LABS
T. Marchi, G. Prete, F. Gramegna, A. Andrighetto, P. Antonini, M. Ballan, M. Bellato, L. Bellan, D. Benini, G. Bisoffi, J. Bermudez, G. Benzoni, D. Bortolato, F. Borgna, A. Calore, S. Canella, S. Carturan, N. Ciatara, M. Cinausero, P. Cocconi, A. Cogo, D. Conventi, V. Conte, M. Comunian, L. Costa, S. Corradetti, G.D. Angelis, C.D. Martinis, P.D. Ruvo, J. Esposito, E. Fagotti, D. Fabris, P. Favaron, E. Fioretto, A. Galatá, F. Gelain, M. Giacchini, D. Giora, A. Gottardo, M. Gulmini, M. Lollo, A. Lombardi, M. Manzolaro, M. Maggiore, D. Maniero, P.F. Mastinu, A. Monetti, F. Pasquato, R. Pegoraro, A. Pisent, M. Poggi, S. Pavinato, L. Pranovi, D. Pedretti, C. Roncolato, M. Rossignoli, L. Sarchiapone, D. Scarpa, J.J.V. Dobón, V. Volpe, A. Vescovo, D. Zafiropoulos, The SPES facility at Legnaro national laboratories. J. Phys. Conf. Ser. 1643(1), 012036 (2020). https://doi.org/10.1088/1742-6596/1643/1/012036
M. Ballan, et al. (2023) Nuclear physics midterm plan at legnaro national laboratories (lnl). Eur. Phys. J. (EPJ), 138:709. https://doi.org/underpublication
M. Maggiore, D. Campo, P. Antonini, A. Lombardi, M. Manzolaro, A. Andrighetto, A. Monetti, D. Scarpa, J. Esposito, L. Silvestrin, Spes: a new cyclotron-based facility for research and applications with high-intensity beams. Mod. Phys. Lett. A 32(17), 1740010 (2017). https://doi.org/10.1142/S0217732317400107
A. Andrighetto, M. Manzolaro, S. Corradetti, D. Scarpa, A. Monetti, M. Rossignoli, M. Ballan, F. Borgna, F. D’Agostini, F. Gramegna, G. Prete, G. Meneghetti, M. Ferrari, A. Zenoni, SPES: an intense source of neutron-rich radioactive beams at Legnaro. J. Phys. Conf. Ser. 966(1), 012028 (2018). https://doi.org/10.1088/1742-6596/966/1/012028
G. Pupillo, A. Andrighetto, A. Boschi, S. Cisternino, S. Corradetti, L. De Dominicis, J. Esposito, E. Fioretto, M. Manzolaro, P. Martini, A. Monetti, L. Morselli, L. Mou, D. Scarpa, G. Sciacca, E. Vettorato, Research activities on the cyclotron-based production of innovative radionuclides: the experience at the Legnaro National Laboratories of INFN (2022). https://conferences.iaea.org/event/264/contributions/26599/attachments/13772/22615/AccConf2022%20Volume-3%20Full-Papers%20FINAL.pdf
D. Bisello, E. Fagotti, J. Esposito, C.-K. Loong, M. Maggiore, P. Mastinu, G. Prete, L. Silvestrin, J. Wyss, LINUS, the integrated LNL neutron source facility. J. Phys. Conf. Ser. (2018). https://doi.org/10.1088/1742-6596/1021/1/012010
D. Bisello, J. Esposito, P. Mastinu, G. Prete, L. Silvestrin, J. Wyss, The phase 0 of the NEPIR project at LNL. Il Nuovo Cimento C 42, 1–4 (2019). https://doi.org/10.1393/ncc/i2019-19072-3
P. Martini, A. Boschi, G. Cicoria, F. Zagni, A. Corazza, L. Uccelli, M. Pasquali, G. Pupillo, M. Marengo, M. Loriggiola, H. Skliarova, L. Mou, S. Cisternino, S. Carturan, L. Melendez-Alafort, N.M. Uzunov, M. Bello, C. Rossi Alvarez, J. Esposito, A. Duatti, In-house cyclotron production of high-purity Tc-99m and Tc-99m radiopharmaceuticals. Appl. Radiat. Isot. 139, 325–331 (2018). https://doi.org/10.1016/j.apradiso.2018.05.033
H. Skliarova, S. Cisternino, G. Cicoria, M. Marengo, V. Palmieri, Innovative target for production of technetium-99m by biomedical cyclotron. Molecules 24(1), 25 (2019). https://doi.org/10.3390/molecules24010025
H. Skliarova, P. Buso, S. Carturan, C. Rossi Alvarez, S. Cisternino, P. Martini, A. Boschi, J. Esposito, Recovery of molybdenum precursor material in the cyclotron-based technetium-99m production cycle. Instruments 3(1), 17 (2019). https://doi.org/10.3390/instruments3010017
N.M. Uzunov, L. Melendez-Alafort, M. Bello, G. Cicoria, F. Zagni, L. De Nardo, A. Selva, L. Mou, C. Rossi-Alvarez, G. Pupillo, G. Di Domenico, L. Uccelli, A. Boschi, F. Groppi, A. Salvini, A. Taibi, A. Duatti, P. Martini, M. Pasquali, M. Loriggiola, M. Marengo, L. Strada, S. Manenti, A. Rosato, J. Esposito, Radioisotopic purity and imaging properties of cyclotron-produced 99mTc using direct 100Mo(p,2n) reaction. Phys. Med. Biol. 63(18), 185021 (2018). https://doi.org/10.1088/1361-6560/aadc88
G. Pupillo, L. Mou, S. Manenti, F. Groppi, J. Esposito, F. Haddad, Nuclear data for light charged particle induced production of emerging medical radionuclides. Radiochim. Acta 110(6–9), 689–706 (2022). https://doi.org/10.1515/ract-2022-0011
G. Pupillo, L. Mou, P. Martini, M. Pasquali, A. Boschi, G. Cicoria, A. Duatti, F. Haddad, J. Esposito, Production of 67Cu by enriched 70Zn targets: first measurements of formation cross sections of 67Cu, 64Cu, 67Ga, 66Ga, 69Zn and 65Zn in interactions of 70Zn with protons above 45 MeV. Radiochim. Acta 108(8), 593–602 (2020). https://doi.org/10.1515/ract-2019-3199
A.R. Jalilian, M.A. Gizawy, C. Alliot, S. Takacs, S. Chakarborty, M.R.A. Rovais, G. Pupillo, K. Nagatsu, J.H. Park, M.U. Khandaker, R. Mikolajczak, A. Bilewicz, S. Okarvi, K. Gagnon, A.H. Al Rayyes, S.E. Lapi, V. Starovoitova, A. Korde, J.A. Osso, IAEA activities on 67Cu, 186Re, 47Sc theranostic radionuclides and radiopharmaceuticals. Curr. Radiopharm. 14(4), 306–314 (2021). https://doi.org/10.2174/1874471013999200928162322
G. Pupillo, T. Sounalet, N. Michel, L. Mou, J. Esposito, F. Haddad, New production cross sections for the theranostic radionuclide 67Cu. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 415, 41–47 (2018). https://doi.org/10.1016/j.nimb.2017.10.022
G. Pupillo, L. Mou, A. Boschi, S. Calzaferri, L. Canton, S. Cisternino, L. De Dominicis, A. Duatti, A. Fontana, F. Haddad, P. Martini, M. Pasquali, H. Skliarova, J. Esposito, Production of 47Sc with natural vanadium targets: results of the PASTA project. J. Radioanal. Nucl. Chem. 322, 1711–1718 (2019). https://doi.org/10.1007/s10967-019-06844-8
F. Barbaro, L. Canton, M.P. Carante, A. Colombi, L. De Dominicis, A. Fontana, F. Haddad, L. Mou, G. Pupillo, New results on proton-induced reactions on vanadium for 47Sc production and the impact of level densities on theoretical cross sections. Phys. Rev. C 104, 044619 (2021). https://doi.org/10.1103/PhysRevC.104.044619
H. Skliarova, S. Cisternino, G. Cicoria, E. Cazzola, G. Gorgoni, M. Marengo, J. Esposito, Cyclotron solid targets preparation for medical radionuclides production in the framework of LARAMED project. J. Phys. Conf. Ser. 1548(1), 012022 (2020). https://doi.org/10.1088/1742-6596/1548/1/012022
H. Skliarova, S. Cisternino, L. Pranovi, L. Mou, G. Pupillo, V. Rigato, C. Rossi Alvarez, HIVIPP deposition and characterization of isotopically enriched 48Ti targets for nuclear cross-section measurements. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 981, 164371 (2020). https://doi.org/10.1016/j.nima.2020.164371
S. Cisternino, E. Cazzola, H. Skliarova, J. Amico, M. Malachini, G. Gorgoni, U. Anselmi-Tamburini, J. Esposito, Target manufacturing by Spark Plasma Sintering for efficient 89Zr production. Nucl. Med. Biol. 104–105, 38–46 (2022). https://doi.org/10.1016/j.nucmedbio.2021.11.004
V. Palmieri, H. Skliarova, S. Cisternino, M. Marengo, G. Cicoria, Method for obtaining a solid target for radiopharmaceuticals production. Istituto Nazionale Di Fisica Nucleare; Patent No. WO/2019/053570; PCT/IB2018/056826, (2019)
L. Mou, G. Pupillo, M. Pasquali, P. Martini, A Method and a Target for the Production of 67Cu. Istituto Nazionale Di Fisica Nucleare; Patent No. WO 2019/220224 A1, (2019)
G. Sciacca, M. Sinico, G. Cogo, D. Bigolaro, A. Pepato, J. Esposito, Experimental and numerical characterization of pure copper heat sinks produced by laser powder bed fusion. Materi. Des. 214, 110415 (2022). https://doi.org/10.1016/j.matdes.2022.110415
P. Martini, A. Boschi, G. Cicoria, L. Uccelli, M. Pasquali, A. Duatti, G. Pupillo, M. Marengo, M. Loriggiola, J. Esposito, A solvent-extraction module for cyclotron production of high-purity technetium-99m. Appl. Radiat. Isot. 118, 302–307 (2016). https://doi.org/10.1016/j.apradiso.2016.10.002
P. Martini, A. Boschi, L. Marvelli, L. Uccelli, S. Carli, G. Cruciani, E. Marzola, A. Fantinati, J. Esposito, A. Duatti, Synthesis and characterization of manganese dithiocarbamate complexes: new evidence of dioxygen activation. Molecules 26(19), 5954 (2021). https://doi.org/10.3390/molecules26195954
M. Sguizzato, P. Martini, L. Marvelli, W. Pula, M. Drechsler, M. Capozza, E. Terreno, L. Del Bianco, F. Spizzo, R. Cortesi, A. Boschi, Synthetic and nanotechnological approaches for a diagnostic use of manganese. Molecules 27(10), 3124 (2022). https://doi.org/10.3390/molecules27103124
G. Reale, F. Calderoni, T. Ghirardi, F. Porto, F. Illuminati, L. Marvelli, P. Martini, L. Uccelli, E. Tonini, L. Del Bianco, F. Spizzo, M. Capozza, E. Cazzola, A. Carnevale, M. Giganti, A. Turra, J. Esposito, A. Boschi, Development and evaluation of the magnetic properties of a new manganese (ii) complex: a potential MRI contrast agent. Int. J. Mol. Sci. 24(4), 3461 (2023). https://doi.org/10.3390/ijms24043461
P. Martini, L. Uccelli, A. Duatti, L. Marvelli, J. Esposito, A. Boschi, Highly efficient micro-scale liquid–liquid in-flow extraction of 99mTc from molybdenum. Molecules 26(18), 5699 (2021). https://doi.org/10.3390/molecules26185699
G. Sciacca, P. Martini, S. Cisternino, L. Mou, J. Amico, J. Esposito, G. Gorgoni, E. Cazzola, A universal cassette-based system for the dissolution of solid targets. Molecules 26(20), 6255 (2021). https://doi.org/10.3390/molecules26206255
J. Esposito, Accelerator-Based Alternatives to Non-HEU Production of Tc-99m. In: IAEA Final Report of the Coordinated Research Project on Accelerator-Based Alternatives to Non-HEU Production of Mo-99 /Tc-99m (2015)
S. Cisternino, L. De Dominicis, L. Mou, J. Esposito, C. Gennari, I. Calliari, G. Pupillo, Cryomilling of isotope-enriched Ti powders for HIVIPP deposition to manufacture targets for nuclear cross section measurement. Materials (2023). https://doi.org/10.3390/ma16113926
S. Cisternino, H. Skliarova, P. Antonini, J. Esposito, L. Mou, L. Pranovi, G. Pupillo, G. Sciacca, Upgrade of the HIVIPP deposition apparatus for nuclear physics thin targets manufacturing. Instruments (2022). https://doi.org/10.3390/instruments6030023
A. Kotliarenko, O. Azzolini, G. Keppel, C. Pira, J. Esposito, Investigation of a possible material-saving approach of sputtering techniques for radiopharmaceutical target production. Appl. Sci. (2021). https://doi.org/10.3390/app11199219
H. Skliarova, S. Cisternino, G. Cicoria, M. Marengo, E. Cazzola, G. Gorgoni, V. Palmieri, Medical cyclotron solid target preparation by ultrathick film magnetron sputtering deposition. Instruments (2019). https://doi.org/10.3390/instruments3010021
A. Kotliarenko, O. Azzolini, S. Cisternino, M. El Idrissi, J. Esposito, G. Keppel, C. Pira, A. Taibi, First results on zinc oxide thick film deposition by inverted magnetron sputtering for cyclotron solid targets production. Materials (2023). https://doi.org/10.3390/ma16103810
A. Boschi, P. Martini, V. Costa, A. Pagnoni, L. Uccelli, Interdisciplinary tasks in the cyclotron production of radiometals for medical applications. The case of 47Sc as example. Molecules (2019). https://doi.org/10.3390/molecules24030444
L. De Nardo, G. Pupillo, L. Mou, D. Furlanetto, A. Rosato, J. Esposito, L. Meléndez-Alafort, Preliminary dosimetric analysis of DOTA-folate radiopharmaceutical radiolabelled with 47Sc produced through natV(p, x)47Sc cyclotron irradiation. Phys. Med. Biol. (2021). https://doi.org/10.1088/1361-6560/abc811
L. Uccelli, A. Boschi, M. Pasquali, A. Duatti, G. Di Domenico, G. Pupillo, J. Esposito, M. Giganti, A. Taibi, M. Gambaccini, Influence of the generator in-growth time on the final radiochemical purity and stability of \(^{99m}\)Tc radiopharmaceuticals. Sci. Technol. Nucl. Install. (2013). https://doi.org/10.1155/2013/379283
J. Esposito, G. Vecchi, G. Pupillo, A. Taibi, L. Uccelli, A. Boschi, M. Gambaccini, Evaluation of \(^{100}\)Mo and \(^{99m}\)Tc productions based on a High-Performance Cyclotron. Sci. Technol. Nucl. Install. (2013). https://doi.org/10.1155/2013/972381
G. Pupillo, J. Esposito, M. Gambaccini, F. Haddad, N. Michel, Experimental cross section evaluation for innovative \(^{99}\)Mo production via the (\(\alpha\), n) reaction on \(^{96}\)Zr target. J. Radioanal. Nucl. Chem. (2014). https://doi.org/10.1007/s10967-014-3321-9
G. Pupillo, J. Esposito, F. Haddad, N. Michel, M. Gambaccini, Accelerator-based production of Mo-99: a comparison between the Mo-100(p, x) and Zr-96(\(\alpha\), n) reactions. J. Radioanal. Nucl. Chem. (2015). https://doi.org/10.1007/s10967-015-4091-8
G. Cicoria, A. Corazza, F. Zagni, S. Vichi, D. Pancaldi, G. Pupillo, A. Boscih, P. Martini, L. Uccelli, M. Pasquali, A. Duatti, G. Di Domenico, M. Loriggiola, M. Bello, J. Esposito, M. Marengo, Preliminary assessment of radionuclidic purity of cyclotron produced \(^{99m}\)Tc. Phys. Med. Eur. J. Med. Phys. (2016). https://doi.org/10.1016/j.ejmp.2016.01.352
L. Meléndez-Alafort, G. Ferro-Flores, L. De Nardo, M. Bello, M. Paiusco, A. Negri, A. Zorz, N. Uzunov, J. Esposito, A. Rosato, Internal radiation dose assessment of radiopharmaceuticals prepared with cyclotron-produced \(^{99m}\)Tc. Med. Phys. 46, 1437–1446 (2019). https://doi.org/10.1002/mp.13393
F. Haddad, L. Ferrer, A. Guertin, T. Carlier, N. Michel, J. Barbet, J.-F. Chatal, ARRONAX, a high-energy and high-intensity cyclotron for nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging (2008). https://doi.org/10.1007/s00259-008-0802-5
L. Mou, P. Martini, G. Pupillo, I. Cieszykowska, C.S. Cutler, R. Mikołajczak, \(^{67}\)Cu production capabilities: a mini review. Molecules (2022). https://doi.org/10.3390/molecules27051501
F. Barbaro, L. Canton, M.P. Carante, A. Colombi, L. De Nardo, A. Fontana, L. Meléndez-Alafort, The innovative \(^{52g}\)Mn for positron emission tomography (PET) imaging: production cross section modeling and dosimetric evaluation. Med. Phys. 50, 1843–1854 (2023). https://doi.org/10.1002/mp.16130
L. De Nardo, G. Ferro-Flores, C. Bolzati, J. Esposito, L. Meléndez-Alafort, Radiation effective dose assessment of [\(^{51}\)Mn]- and [\(^{52}\)Mn]-chloride. Appl. Radiat. Isot. 153, 108805 (2019). https://doi.org/10.1016/j.apradiso.2019.108805
F. Borgna, M. Ballan, C. Favaretto, M. Verona, M. Tosato, M. Caeran, S. Corradetti, A. Andrighetto, V. Di Marco, G. Marzaro, N. Realdon, Early evaluation of copper radioisotope production at ISOLPHARM. Molecules 23(10), 2437 (2018). https://doi.org/10.3390/molecules23102437
A. Andrighetto, Method for Producing Beta Emitting Radiopharmaceuticals, and Beta Emitting Radiopharmaceuticals Thus Obtained. Istituto Nazionale Di Fisica Nucleare; Patent No. WO/2015/114424, (2015)
A. Andrighetto, M. Tosato, M. Ballan, S. Corradetti, F. Borgna, V. Di Marco, G. Marzaro, N. Realdon, The ISOLPHARM project: ISOL-based production of radionuclides for medical applications. J. Radioanal. Nucl. Chem. (2019). https://doi.org/10.1007/s10967-019-06698-0
M. Ballan, M. Tosato, M. Verona, M. Caeran, F. Borgna, E. Vettorato, S. Corradetti, L. Zangrando, M. Sgaravatto, M. Verlato, M. Asti, G. Marzaro, F. Mastrotto, V. Di Marco, D. Maniglio, A. Bisio, A. Motta, A. Quaranta, A. Zenoni, P. Pastore, N. Realdon, A. Andrighetto, Preliminary evaluation of the production of non-carrier added \(^{111}\)Ag as core of a therapeutic radiopharmaceutical in the framework of ISOLPHARM_Ag experiment. Appl. Radiat. Isot. 164, 109258 (2020). https://doi.org/10.1016/j.apradiso.2020.109258
M. Tosato, M. Asti, M. Dalla Tiezza, L. Orian, D. Häussinger, R. Vogel, U. Köster, M. Jensen, A. Andrighetto, P. Pastore, V. Di Marco, Highly stable silver(i) complexes with cyclen-based ligands bearing sulfide arms: a step toward Silver-111 labeled radiopharmaceuticals. Inorg. Chem. (2020). https://doi.org/10.1021/acs.inorgchem.0c01405
M. Verona, S. Rubagotti, S. Croci, S. Sarpaki, F. Borgna, M. Tosato, E. Vettorato, G. Marzaro, F. Mastrotto, M. Asti, Preliminary study of a 1,5-benzodiazepine-derivative labelled with Indium-111 for cck-2 receptor targeting. Molecules (2021). https://doi.org/10.3390/molecules26040918
M. Ballan, E. Vettorato, L. Morselli, M. Tosato, S. Nardella, F. Borgna, S. Corradetti, A. Monetti, M. Lunardon, A. Zenoni, V. Di Marco, N. Realdon, A. Andrighetto, Development of implantation substrates for the collection of radionuclides of medical interest produced via ISOL technique at INFN-LNL. Appl. Radiat. Isot. 175, 109795 (2021). https://doi.org/10.1016/j.apradiso.2021.109795
M. Tosato, M. Dalla Tiezza, N.V. May, A. Ahmed Isse, S. Nardella, L. Orian, M. Verona, C. Vaccarin, A. Alker, H. Mäcke, P. Pastore, V. Di Marco, Copper coordination chemistry of sulfur pendant cyclen derivatives: an attempt to hinder the reductive-induced demetalation in \(^{64/67}\)Cu radiopharmaceuticals. Inorg. Chem. (2021). https://doi.org/10.1021/acs.inorgchem.1c01550
V. Benfante, A. Stefano, A. Comelli, P. Giaccone, F.P. Cammarata, S. Richiusa, F. Scopelliti, M. Pometti, M. Ficarra, S. Cosentino, M. Lunardon, F. Mastrotto, A. Andrighetto, A. Tuttolomondo, R. Parenti, M. Ippolito, G. Russo, A new preclinical decision support system based on PET radiomics: a preliminary study on the evaluation of an innovative \(^{64}\)Cu-labeled chelator in mouse models. J. Imaging (2022). https://doi.org/10.3390/jimaging8040092
IAEA Contribution to the Development of \(^{64}\)Cu Radiopharmaceuticals for Theranostic Applications - The Quarterly Journal of Nuclear Medicine and Molecular Imaging;- 2020 December;64(4):338-45; Available Online. https://www.minervamedica.it/en/journals/nuclear-med-molecular-imaging/article.php?cod=R39Y2020N04A0338
L. De Nardo, G. Pupillo, L. Mou, J. Esposito, A. Rosato, L. Meléndez-Alafort, A feasibility study of the therapeutic application of a mixture of \(^{67/64}\)Cu radioisotopes produced by cyclotrons with proton irradiation. Med. Phys. 49, 2709–2724 (2022). https://doi.org/10.1002/mp.15524
Product Catalog—NIDC: National Isotope Development Center Available online; Available Online. https://www.isotopes.gov/catalog
Acknowledgements
Authors would like to thank the LNL for the administrative and technical support and all the national and international collaborators for the fruitful partnership.
Funding
This research received the funding described in the text. Ministero dell’Istruzione, dell’Università e della Ricerca, Grant no. (SPES, LARAMED, TERABIO). Commissione Scientifica Nazionale 5, Instituto Nazionale di Fisica Nucleare, Grant no. (APOTEMA, TECHNOSP, PASTA, REMIX, METRICS, CUPRUM_TTD, E_PLATE, ISOLPHARM_Ag, ISOLPHARM_EIRA, ADMIRAL, HISOL). Instituto Nazionale di Fisica Nucleare, Grant no. (COME, INTEFF_TOTEM, STARDIS).
Author information
Authors and Affiliations
Contributions
GP and PM helped in conceptualization; GP, LM, PM, MB, CS and MM contributed to writing—original draft preparation; AAn, AAr, SC, LDD, JE, EF, TG, AM, EM, DS, GS, DS, BM, AB and LMA contributed to writing—review and editing and JE and AAn worked in project administration. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pupillo, G., Andrighetto, A., Arzenton, A. et al. Cyclotron-based production of innovative medical radionuclides at the INFN-LNL: state of the art and perspective. Eur. Phys. J. Plus 138, 1095 (2023). https://doi.org/10.1140/epjp/s13360-023-04564-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-023-04564-3