Abstract.
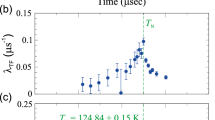
The structural properties of the spin crossover compound [Fe(btr)2(NCS)2](H2O), where btr stands for 4,4’-bis-1,2,4-triazole, are investigated by single crystal X-ray diffraction at different temperatures in the thermal spin transition regime. The 104.0(5) K low spin (LS) crystal structure is compared to the room temperature high spin (HS) crystal structure. The C2/c space group is retained in the LS state with an abrupt anisotropic shortening of the b and c cell parameters and a lengthening of a at the transition temperature. The major structural modifications related to the spin transition are a shortening of the Fe-N bond lengths (\(\Delta d_{{\rm Fe}-NCS} = -0.175\)(4) Å, \(\Delta d_{{\rm Fe-N}(btr)} = -0.213\)(3) Å) and a reorientation of the NCS groups with a more linear Fe-N-C-S geometry on going from HS to LS. Diffraction measurements have also been performed at 124 K on a trapped mixed spin state. The observed diffraction pattern shows the coexistence of two crystal lattices corresponding to ordered LS and HS species, which is a direct evidence of spin-like domain formation during the transition. The corresponding fraction of HS species (\(\gamma_{\it HS}\approx 0.10\)) has been determined by structural refinement using several reference temperature measurements. To investigate dynamical aspects, X-ray data were collected versus time during the spin transition at constant temperature (T = 117.2(2) K). No evidence has been found for any putative presence of an intermediate structural state during the spin transition.
Similar content being viewed by others
References
P. Gütlich, A. Hauser, H. Spiering, Angew. Chem. Int. Ed. Engl. 33, 2024 (1994)
P. Gütlich, Y. Garcia, T. Woike, Coord. Chem. Rev. 219-221, 839 (2001)
P. Gütlich, Struct. Bond. (Berlin) 44, 83 (1981)
E. König, Struct. Bond. (Berlin) 76, 51 (1991)
G.A. Renovitch, W.A. Baker, J. Am. Chem. Soc. 89, 6377 (1967)
E. König, G. Ritter, S.K. Kulshreshtha, S.M. Nelson, Inorg. Chem. 21, 3022 (1982)
E. König, G. Ritter, S.K. Kulshreshtha, J. Waigel, L. Sacconi, Inorg. Chem. 23, 1241 (1984)
D. Chernyshov, M. Hostettler, K.W. Törnroos, H.-B. Bürgi, Angew. Chem. Int. Ed. 42, 3825 (2003)
P. Guionneau, J.-F. Létard, D.S. Yufit, D. Chasseau, G. Bravic, A.E. Goeta, J.A.K. Howard, O. Kahn, J. Mater. Chem. 9, 985 (1999)
M. Marchivie, P. Guionneau, J.-F. Létard, D. Chasseau, Acta Cryst. B 59, 479 (2003)
W. Vreugdenhil, J.H. van Diemen, R.A.G. De Graaff, J.G. Haasnoot, J. Reedijk, A.M. van Der Kraan, O. Kahn, J. Zarembowitch, Polyhedron 9(24), 2971 (1990)
W. Vreugdenhil, J.G. Haasnoot, O. Kahn, P. Thuery, J. Reedijk, J. Am. Chem. Soc. 109, 5272 (1987)
A. Ozarowski, Y. Shunzhong, B.R. McGarvey, A. Mislankar, J.E. Drake, Inorg. Chem. 30, 3167 (1991)
W. Morscheidt, J. Jeftic, E. Codjovi, J. Linares, A. Bousseksou, H. Constant-Machado, F. Varret, Meas. Sci. Technol. 9, 1311 (1998)
J.-P. Martin, J. Zarembowitch, A. Bousseksou, A. Dworkin, J.G. Haasnoot, F. Varret, Inorg. Chem. 33, 6325 (1994)
J.-P. Martin, J. Zarembowitch, A. Dworkin, J.G. Haasnoot, E. Codjovi, Inorg. Chem. 33, 2617 (1994)
H. Constant-Machado, J. Linares, F. Varret, J.G. Haasnoot, J.-P. Martin, J. Zarembowitch, A. Dworkin, A. Bousseksou, J. Phys. I France 6, 1203 (1996)
E. Codjovi, N. Menendez, J. Jeftic, F. Varret, C.R. Acad. Sci. 4, 181 (2001)
Y. Garcia, V. Ksenofontov, G. Levchenko, G. Schmitt, P. Gütlich, J. Phys. Chem. B 104(21), 5045 (2000)
J.G. Haasnoot, W.L. Groeneveld, Z. Naturforsch b 34, 1500 (1979)
Z. Otwinowski, W. Minor, in Methods in Enzymology 276, edited by C.W. Carter Jr, R.M. Sweet (Academic Press, 1996)
R.H. Blessing, J. Appl. Cryst. 22, 396 (1989)
G.M. Sheldrick, SHELX97. Program for structure solution and refinement (University of Gottingen, Germany, 1997)
B.A. Katz, C.E. Strouse, J. Am. Chem. Soc. 101, 6214 (1979)
S. Pillet, C. Lecomte (unpublished)
Y. Garcia, O. Kahn, L. Rabardel, B. Chansou, L. Salmon, J.P. Tuchagues, Inorg. Chem. 38, 4663 (1999)
W. Vreugdenhil, S. Gorter, J.G. Haasnoot, J. Reedijk, Polyhedron 4(10), 1769 (1985)
P. Guionneau, C. Brigouleix, Y. Barrans, A.E. Goeta, J.-F. Létard, J.A.K. Howard, J. Gaultier, D. Chasseau, C.R. Acad. Sci. Paris 4, 161 (2001)
B. Gallois, J.-A. Real, C. Hauw, J. Zarembowitch, Inorg. Chem. 29, 1152 (1990)
P. Domiano, Cryst. Struct. Comm. 6, 503 (1977)
P. Coppens, X-ray Charge Densities and Chemical Bonding (IUCr, Oxford University Press, 1997)
Author information
Authors and Affiliations
Corresponding author
Additional information
Received: 15 January 2004, Published online: 8 June 2004
PACS:
75.30.Wx Spin crossover - 61.50.Ks Crystallographic aspects of phase transformations; pressure effects - 61.10.Nz X-ray diffraction
An erratum to this article is available at http://dx.doi.org/10.1140/epjb/e2006-00054-8.
Electronic Supplementary material
supp.pdf
Supplementary material to S. Pillet, J. Hubsch, and C. Lecomte, Single crystal diffraction analysis of the thermal spin conversion in [Fe(btr)\(\mathsf{_{2}}\)(NCS)\(\mathsf{_{2}}\)](H\(\mathsf{_{2}}\)O): evidence for spin-like domain formation
Rights and permissions
About this article
Cite this article
Pillet, S., Hubsch, J. & Lecomte, C. Single crystal diffraction analysis of the thermal spin conversion in [Fe(btr)\(\mathsf{_{2}}\)(NCS)\(\mathsf{_{2}}\)](H\(\mathsf{_{2}}\)O): evidence for spin-like domain formation. Eur. Phys. J. B 38, 541–552 (2004). https://doi.org/10.1140/epjb/e2004-00150-9
Issue Date:
DOI: https://doi.org/10.1140/epjb/e2004-00150-9