Abstract
The use of heavy actinide targets, including 243Am, 240,242,244Pu, 245,248Cm, 249Bk, and 249Cf, irradiated by intense heavy ion beams of 48Ca has resulted in a significant expansion of the periodic table since 2000, including the discovery of five new heaviest elements and more than 50 new isotopes. These actinide materials can only be produced by intense neutron irradiation in very high flux reactors followed by chemical processing and purification in specialized hot cell facilities available in only a few locations worldwide. This paper reviews the reactor production of heavy actinides, the recovery and chemical separation of actinide materials, and the preparation of actinide targets for superheavy element experiments. The focus is on 248Cm, 249Bk, mixed 249−251Cf, and 254Es, including current availabilities and new production processes. The impacts of new facilities, including the Superheavy Element Factory at Dubna, accelerator and separator upgrades at RIKEN, and proposed upgrades to the High Flux Isotope Reactor at Oak Ridge are also described. Examples of recent superheavy element research are discussed as well as future opportunities for superheavy research using actinide targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The use of actinide targets in combination with intense heavy ion beam irradiation has transformed superheavy element (SHE) research, significantly expanding the periodic table by adding five new heaviest elements since 2012 [1,2,3]. These advances have been accomplished using the “hot fusion” technique [4], where heavy actinide targets, typically 243Am, 240,242,244Pu, 245,248Cm, 249Bk, and 249Cf, are bombarded with 48Ca beams at large accelerator facilities, creating compound nuclei that decay to superheavy nuclei with half-lives ranging up to many hours for lower proton numbers (Z) to hundreds of microseconds for higher Zs. Heavy actinides can only be produced in the required quantities (i.e., tens of milligrams) using very high flux reactors and large-scale radiochemical processing facilities available at only a few research institutions worldwide [5]. The most recent five elements, flerovium, moscovium, livermorium, tennessine, and oganesson, with Z = 114–118, were discovered using actinides from the High Flux Isotope Reactor (HFIR) and adjacent Radiochemical Engineering Development Center (REDC) at Oak Ridge National Laboratory (ORNL) and the SM-3 Reactor at the Research Institute of Advanced Reactors (RIAR) in Dmitrovgrad, Russia. These elements were originally produced [6,7,8,9] using the U400 cyclotron at the Flerov Laboratory at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, with later confirmations at accelerator facilities at Lawrence Berkeley National Laboratory (LBNL) [10, 11]; the Gesellschaft für Schwerionenforschung (GSI), Darmstadt, Germany [12,13,14,15], and JINR [16]. Most actinide materials for current SHE experiments have been produced and/or processed at HFIR/REDC through the US Department of Energy (DOE) Isotope Program.
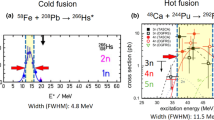
Actinides are radioactive elements with atomic numbers Z = 89–103. They were first described as a new row in the periodic table by Seaborg [17] in the 1940s. Accumulating more than trace amounts of actinides heavier than uranium requires production in nuclear reactors. Heavy actinides are the highest Z target materials available, making them attractive for the synthesis of SHEs (i.e., elements with atomic numbers of 104 or greater). For a given superheavy element, higher Z targets and the neutron-rich 48Ca projectile lead to more asymmetric nuclear reactions with correspondingly lower Coulomb barriers in comparison to the cold fusion reactions with 208Pb and 209Bi targets and beams heavier than 48Ca. Heavy actinides also have higher neutron numbers, and 48Ca projectiles provide additional excess neutrons. This results in compound nuclei closer to the shell closure at N = 184 with corresponding increases in survivability against fission further increasing production cross sections for neutron-rich nuclei. Cold fusion techniques led to the discovery of elements 107–113 [18–24], but production rates steadily declined with increasing Z to below one atom per year for element 113 [24], making further progress impractical. The path of hot fusion with actinide targets and 48Ca beams increased production rates by factors of 10 and more [25], enabling expansion of the periodic table beyond Z = 113 to Z = 118. Ongoing and proposed SHE searches for new elements 119 at RIKEN [26] and 120 at JINR [27] and LBNL [28] are also dependent on actinide targets.
In this paper, we review the production, processing, and applications of heavy actinide materials for SHE research and discovery. This includes actinide production in reactors, recovery of actinides from reactor-irradiated materials, chemical processing and purification of actinides, and target preparation and performance. The focus is on 248Cm, 249Bk, mixed 249–251Cf, and 254Es, including current availabilities and new production processes. The impacts of new facilities, including the Superheavy Element Factory at JINR [29], accelerator upgrades at RIKEN [26], new facilities under construction at GANIL [30] and GSI [31, 32], and proposed upgrades to HFIR [33] are also discussed. We also review the status of some current experiments and discuss future opportunities for superheavy research using heavy actinide targets. In addition to new element discovery, these opportunities include nuclear structure studies [34], atomic physics [35], mass number measurements [11], mass measurements [36, 37], and chemistry studies [38] for superheavy nuclei and atoms, but these are not in the focus of this article.
2 Production and availability of actinides
ORNL is the major global supplier of heavy actinides, including 252Cf, 249Bk, and 254Es. The SM-3 reactor in Russia also contributes to global 252Cf supplies. ORNL has unique facilities for the production and processing of heavy actinides, including HFIR and REDC, and has decades of experience synthesizing, separating, purifying, and transporting transuranium radioisotopes [39,40,41]. These ORNL-produced materials play a key role in advancing scientific understanding of both heavy element chemistry (HEC) and fundamental nuclear structure.
ORNL has provided actinide target materials that have been used for the synthesis of all SHEs above element 112 [6,7,8,9, 42], and in some cases has carried out fabrication of the experimental targets [27]. These experiments have been performed at JINR and GSI in collaboration with US laboratories including ORNL. Future discoveries of elements 119 and 120 will require heavier ion beams, and/or heavier target materials, than those used in earlier experiments. Further, the reactions resulting in synthesis of these heavier isotopes are expected to have significantly lower probabilities [43,44,45] than those of earlier work. This in turn requires larger quantities of materials, and targets capable of sustaining a longer beam time, to observe the same number of reactions. Larger quantities of produced target materials can support thicker targets [46] to increase yields using separators with high acceptance angles, allow for increased target damage and material losses associated with higher beam currents and doses, and compensate for decay losses for experiments involving short-lived targets such as 249Bk.
ORNL further provides actinide materials in support of basic research on the fundamental chemistry of actinide elements. Current electronic structure methods are unable to accurately describe the behavior of f-electrons, such as spin–orbit coupling, multiplet complexity, and relativistic effects. Synthetic chemistry, spectroscopy, and structural characterization explore the chemical and physical properties of these elements to determine their bonding and reactivity in solution, at the interface, and in the solid state. Increased supply of heavy actinides increases the number and complexity of experiments that can be performed on the materials and accelerates the rate of progress in Heavy Element Chemistry [25, 46,47,48].
2.1 ORNL’s Californium-252 Program
The Californium-252 Program is the second largest isotope production program at ORNL, next to the Plutonium-238 Supply Program, and the largest program at ORNL that is managed through the DOE Isotope Program. ORNL has been producing 252Cf for nearly 60 years and to date has supplied approximately 1.2 g of 249Bk, 10.2 g of 252Cf, 39 mg 253Es, and 15 pg of 257Fm from 79 campaigns.
These transcurium isotopes are produced by irradiation of targets composed of mixed Pu, Am, and Cm at HFIR, where they are exposed to one of the highest steady-state neutron fluxes in the world. The feed material is transmuted into heavier isotopes by a series of neutron captures and beta decays as shown in Fig. 1.
Transcurium isotopes are currently produced at ORNL on a regular 2-year schedule. The process begins with conversion of the actinide source material to oxide microspheres that are blended with aluminum powder and pressed to form cermet pellets. As shown in Fig. 2, these pellets are loaded into long, thin, finned aluminum tubes (i.e., targets) that are closure welded and then hydrostatically compressed to provide adequate heat transfer between the pellets and target tubes. A final aluminum jacket is wrapped around the target tube to channel the coolant flow around the target during irradiation (Fig. 2). The amount of actinide material in each target is limited both in mass, to reduce heat generation, and volume, to maintain heat transfer through the aluminum matrix and allow for containment of fission gasses.
Following irradiation, the targets cool in the HFIR storage pool for approximately 2–3 months for decay of fission and activation products, particularly 131I, a highly dispersible and radiotoxic fission product. The targets are then transferred to REDC for separation and purification of transcurium actinides. The chemical processing of neutron-irradiated targets to recover Cf and Bk is illustrated in Fig. 3.
The aluminum components are dissolved in a sodium hydroxide (NaOH) and sodium nitrate (NaNO3) mixture [49] and heated, which results in dissolved aluminum as Al(OH)4− and precipitated metal hydroxides. The actinide and lanthanide solids are filtered and washed several times and then dissolved in HNO3. After dissolution, the desired actinide materials are contained in solution, and any remaining undissolved solids are filtered and discarded. Batch solvent extraction with di-(2-ethylhexyl) phosphoric acid (HDEHP) [50] is used to extract the actinides, lanthanides, and molybdenum from other metals in the solution. A lithium chloride (LiCl)–based anion exchange procedure separates the lanthanides from the actinides [51, 52], with the stripped aqueous phase from the previous step serving as the feed. The lanthanides are eluted first, followed by the bulk of americium and curium, 90–95%, and then the heavier actinides (i.e., berkelium, californium, einsteinium, fermium). During the column run, the elution of heavier actinides is tracked by a neutron profile of the column and by observation of the alpha activity of column eluent. Because berkelium is a pure beta emitter, its location is estimated to be after the curium and before the californium. The americium and curium from both columns are stored in a combined fraction to await conversion into targets for future irradiations, while the transcurium products are further purified. The transcurium products are converted from the chloride to nitrate form by precipitating the actinides using LiOH and then filtering the solids. Finally, the individual actinides are separated from each other using α-hydroxyisobutyric acid (AHIB) and cation exchange [53, 54].
2.2 Berkelium recovery
Additional steps are required to recover a purified berkelium product free from any californium contamination [49]. In the hot cell, the berkelium fraction from the previous AHIB actinide separation step is concentrated by passing through a cation ion exchange column and washed with H2O to remove all of the AHIB reagent, then stripped with HNO3. The berkelium is oxidized from Bk(III) to Bk(IV) using NaBrO3 and extracted into HDEHP. The phases are allowed to separate, and the Bk(IV) is stripped from the organic phase and reduced from Bk(IV) to Bk(III) using H2O2. At this point, the decontamination factor for 252Cf and other radioactive impurities in the berkelium product is ~ 105, and the solution can be safely transferred to a glove box for further processing.
Once in the glove box, the berkelium solution is washed with trichloroethylene to remove any HDEHP/dodecane remaining from the solvent extraction procedure. The berkelium solution is then evaporated, dissolved in dilute HCl, and processed through a small AHIB–cation column. This separates the berkelium from any remaining Cf. The purified berkelium fraction is acidified and processed through an AG50X4 cation exchange resin column to remove mono- and divalent metals, iron, and AHIB reagent from the berkelium. The stripped berkelium is then evaporated, analyzed, and ready for shipment.
2.3 Recovery of additional feedstock material for future transcurium production
To produce measurable quantities of the heavy actinides of berkelium through fermium, a feedstock of curium greater than 50 wt% of 246/248Cm is necessary. The only remaining domestic source of this curium feedstock is the Mark-18A (Mk-18A) targets located at the Savannah River Site (SRS). DOE manages an inventory of materials that contain a range of long-lived radioactive isotopes that were produced from the 1960s through the 1980s by irradiating targets in production reactors at SRS. During the late 1960s, the K reactor was configured to operate in a very-high-flux mode, and > 8 kg of 242Pu contained in 86 Mk-18A targets were irradiated to produce 252Cf for use in neutron source market development activities [55]. Twenty-one of these targets were processed at ORNL in the early 1970s to recover the 252Cf, heavy curium (i.e., curium rich in 246–248Cm isotopes), and 244Pu. The remaining 65 Mk-18A irradiated targets contain the unique supply of heavy curium, which is likely to never to be produced again; the targets are currently in wet storage at the SRS [56]. These materials will be processed to recover the heavy curium [57, 58].
The targets will be retrieved from wet storage at SRS and transferred to shielded cells at Savannah River National Laboratory where they will be processed to recover an americium-curium-lanthanide product from the targets. The recovered materials will then be packaged and shipped to ORNL for storage, processing, and future distribution to end users. This will preserve the world’s supply of irreplaceable heavy curium, as well as provide the DOE Isotope Program with feedstock for heavy isotope production. The Mk-18A materials will provide sufficient curium feedstock to produce 252Cf and other heavy elements for the next several decades.
2.4 Long-lived actinides
The longer-lived lighter actinide target materials with atomic numbers below Z = 97, including 244,245,248Cm, 241,243Am, 240,242,244Pu, 238U, 237Np, and 232Th are used in SHE research that involves connecting the “island of stability”Footnote 1 to the nuclear mainland, to study ground-state fission, alpha emission probabilities, and the structure of ground and excited states.
These target materials can also be used to investigate reaction mechanisms using increasingly available high intensity beams such as 48Ca, 50Ti, 51V, and 54Cr. The heaviest among these isotopes, Z = 96 248Cm, is presently used in a search for Z = 119 (using a 51V beam at RIKEN) and can be used for a new element Z = 120 synthesis (using a 54Cr beam). Experiments using curium, americium, plutonium, and neptunium isotopes, with moderately high cross sections at the level of 1 to 10 picobarn with 48Ca beams are suitable—based on their scientific importance or actual efficiency of the experiments—for beam times of several months to years. The demand for these isotopes is expected to be approximately 15 to 100 mg per target. The lighter actinides, thorium, uranium, americium, radium, and 243−244Cm can also be used for reaction and spectroscopy studies.
ORNL maintains a legacy inventory of these long-lived isotopes in inventory and purifies and dispenses in the desired form for use in SHE and other research.
2.4.1 Long-lived 248 Cm recovery from decayed 252 Cf
Particularly important for SHE research is the long-lived heavy isotope 248Cm (T1/2 = 348,000 years), only one proton below 249Bk. Used for research similar to lighter elements neptunium through americium, the chemical properties of curium make it a very robust target, proven to survive long irradiations with heavy ion beams including doses in excess of 1019cm2 for 48Ca [26, 63]. Long half-life and relatively low radioactivity make high-weight-percent 248Cm a target of choice for many laboratories. A discovery campaign for element 119 is currently underway at RIKEN using ORNL-supplied 248Cm and 51V beams, with additional material required to supplement this target in the coming years. Following an upgrade to the 54Cr beam, a discovery campaign for element 120 can be run with 248Cm target material. This reaction was already tried for the element 120 synthesis at GSI in collaboration with ORNL and the University of Tennessee, Knoxville, resulting in an upper limit single event cross section of 0.58 (+ 1.34, − 0.48) pb [64] for this reaction. Upper limits for element 120 synthesis of 0.4 pb for the 244Pu + 58Fe reaction [65] and of 0.065 pb for the 249Bk + 50Ti reaction [66] have also been established. In addition to its importance as a target for SHE research, 248Cm is the starting point for production of the heavier transcurium isotopes that are also used in SHE research.
During the initial processing and recovery of 252Cf from irradiated targets, Cf storage packages are prepared. As the material is processed for source preparation and dispensing, individual storage packages are stripped with nitric acid to recover the Cf isotopes for further purification and program use, leaving behind 248Cm in the range of 95–96 wt%, with some residual 252Cf [67].
Another source of decay-produced 248Cm is from 20- to 30-year decayed 252Cf sources, which also generate high wt% 251Cf with trace amounts of 252Cf depending upon the age of the source. This level of 248Cm enrichment is low for SHE target material but may be useful for the production of 249Bk or other transcurium isotopes. The process of harvesting decayed 252Cf sources is presented in more detail in the discussion concerning the recovery of 251Cf in Sect. 2.4.2.
2.4.2 Long-lived mixed californium isotopes following 252 Cf decay
Since the beginning of operations at the REDC in 1966, 79 campaigns have been completed to produce the transcurium actinides, with 252Cf being the isotope produced in the highest quantity. During production of 252Cf, the other californium isotopes (i.e., 249Cf, 250Cf, 251Cf), and shorter-lived 253Cf are also produced. A typical distribution for the Cf product is shown in Table 1 with the corresponding half-life for each isotope.
The 252Cf sources were provided to various DOE national laboratories, other government agencies, and universities for research. When a facility no longer needed the sources or needed a replacement source of higher strength, the decayed source was returned to ORNL and placed in the storage pool for potential future use. Many of these sources have been in the storage pool for decades, resulting in decay-enriched material that contains the longer-lived californium isotopes. This unique target material has been recovered twice in recent history for use in mixed californium sources [68]. Recovery involves the following steps:
-
1.
Sources are retrieved from the storage pool and cut open in a shielded hot cell (Fig. 4).
-
2.
The aluminum pellets are dissolved out of the source in a solution of NaOH and NaNO3, leaving behind the stainless-steel capsule and the solid, black californium and curium (Fig. 5).
-
3.
The solid californium and curium are dissolved in HNO3 and processed though several cleanup columns (DGA, LN, TEVA, MP-1) and concentrator columns to remove other actinides and contaminants from the californium fraction.
2.5 Short-lived 249Bk
Berkelium-249, with a half-life of 327 days, is a radioisotope of interest in the nuclear physics and chemistry communities as a target for production of the most neutron-rich and longer-lived isotopes of superheavy elements. In addition to nuclear structure studies, the isotopes produced in the decay chains of Z = 117 isotopes have half-lives long enough to conduct studies of their chemical properties.
Berkelium is currently produced as a byproduct of the Californium-252 Program. Yields of 249Bk are not proportional to yields of 252Cf, but rather are proportional to the total amount of irradiated 248Cm in the batch of 252Cf production targets. Californium production campaigns operate on a regular a 2-year schedule and typically produce 10–15 mg of 249Bk. The historical process of purifying 249Bk is described in Sect. 2.2.
2.5.1 Ongoing research and development for production of 249 Bk
Current research aims to establish capabilities for production of 249Bk from high-weight-percent curium feedstock and evaluate the use of thermal neutron filters to reduce the burnup rate of 249Bk in reactor, increasing the saturation activity [39–41]. A diagram of the target to be used for this irradiation is shown in Fig. 6; the target employs a 1.6 mm natural gadolinium sleeve inside a large bore aluminum capsule. Inside the gadolinium sleeve is a curium oxide and aluminum cermet pellet. The gadolinium serves as the thermal neutron filter, which reduces the effective flux of neutrons at energies that burn up the 249Bk at a higher rate than those inducing the desired 248Cm transmutation.
Modeling and simulation of this target design has predicted that 35 mg of 248Cm irradiated for one cycle will produce 1.0 mg of 249Bk and 0.1 mg of fission products, with 248Cm transmutation losses of 5%. Irradiation for two cycles will produce 1.6 mg of 249Bk and 0.3 mg of fission products, with 248Cm transmutation losses of 9%. Irradiation beyond two cycles will likely significantly deplete the Gd sleeve, resulting in loss of 249Bk due to thermal neutron absorption. The projected yield curves for 249Bk for this target, vs. for a pellet in a 252Cf production target, are shown in Fig. 7.
In addition to increased 249Bk yields, the gadolinium filter reduces the production of 252Cf by over 90% such that the irradiated material does not require heavy neutron shielding.
2.5.2 Ongoing research and development for purification of 249 Bk
A dual-column separation procedure has recently been developed to replace the cation exchange (CX) -AHIB purification of 249Bk. The new technique is based on the discovery of a specific feature of Bk(IV) ions [69]: Unlike any other tetravalent actinides, Bk(IV) does not form anion in high HNO3 and is not adsorbed by anion exchange (AX) resin in high HNO3 media. The HNO3 concentration here can be higher than 10 M [69]. This feature allows Bk(IV) to pass through an AX column with no adsorption while any other tetravalent actinides/impurity metals are adsorbed by the AX column. With the knowledge of Bk(IV) being adsorbed by Eichrom LN resin (impregnated with HDEHP), a dual column of MP-1 and LN resins is designed as shown in Fig. 8, where a MP-1 resin column is installed on top of a LN resin column, both preconditioned with HNO3 and NaBrO3.
Bk(III) is oxidized to Bk(IV) by NaBrO3, along with some radioactive impurities such as 141,144Ce(III). Tetravalent metal ions are adsorbed by MP-1 column while 249Bk(IV) and mono-, di-, trivalent metal impurities (including iron) pass through the MP-1 column to the lower LN column. Bk(IV) is adsorbed by the LN column while mono-, di-, trivalent metal impurities (including iron) pass through the LN column into the waste collection bottle. The LN resin column is then detached from the stacked dual column and the purified 249Bk is stripped. This strip solution can be easily evaporated and adjusted to customer-required specifications [69].
Compared to the CX-AHIB process, dual-column operations are faster and require less expertise to execute. The process requires less scrubbing and evaporation, saving several days of process time. There are no high-temperature requirements and no drop counts for a chromatographic separation of impurities before or after the Bk fraction. Based on recent developmental work, the dual-column Bk recovery process results in a purified 249Bk fraction containing < 1 ng of 252Cf, with a 107 or greater decontamination factor for all radionuclides.
2.6 Short-lived 254Es produced via irradiation
Einsteinium-254, with a half-life of 276 days, is another radioisotope of interest in the nuclear physics and chemistry communities. At 5 atomic mass units heavier than 249Bk, 254Es is capable of producing even heavier neutron-rich isotopes of SHEs. However, as there is no quasi-stable precursor to 254Es, as 248Cm is to 249Bk, production quantities are extremely small from multiple neutron-capture chains on heavy curium.
Einsteinium-254 is currently produced as a byproduct of the Californium-252 Program, similar to 249Bk, with approximately 1 µg available from every biennial 252Cf campaign. These quantities have been used for radiochemical determination of cross sections for the heaviest actinides produced in transfer reactions of 16,18O and 22Ne with an 254Es target [70], but are three orders of magnitude less than typically required for SHE production targets. 254Es material produced and separated at ORNL was recently used for the studies of the fission mechanism of 258Md produced in 4He + 254Es reactions at the JAEA tandem accelerator facility and spontaneous fission of Fm isotopes at JAEA-ISOL. Fission studies using multinucleon transfer reactions of 18O + 254Es have also been carried out at JAEA [71].
Einsteinium fractions are obtained from the hot cell AHIB–cation exchange and transferred to a glove box. Product finishing consists of a cation cleanup column, TEVA column with thiocyanate, several iterations of AHIB separation, and a final cation column for AHIB removal.
2.6.1 Ongoing research and development for production of 254 Es
Current research aims to establish capabilities for production of 254Es from high-weight-percent curium feedstock and evaluate the use of interchangeable external neutron filters to reduce the burnup rate of 254Es in reactor, increasing the saturation activity. This work differs from the target design for 249Bk production, in that 254Es production first requires buildup of 253Es and 253Cf through capture of thermal neutrons. Therefore, a thermal neutron filter embedded in the sealed capsule would be ineffective. This research aims to develop a filter that can be located externally to the sealed capsule, being exposed to the HFIR cooling water. This would both allow for removal and replacement of the filter during HFIR maintenance outages and would provide improved heat removal for the highly neutron absorbing material. This work is still in early development but is expected to increase 254Es production per unit 248Cm by roughly 500%, without a commensurate increase in 253Es. The amount of Es that can be shipped to customers in a Type A package is and will be limited by the 253Es activity.
2.7 Short-lived 257Fm produced via neutron irradiation
Fermium-257, with a half-life of 100.5 days, is the heaviest isotope that can feasibly be produced in the HFIR. Fermium is currently produced as a byproduct of the Californium-252 Program, similarly to 249Bk and 254Es, with 0.5–1.0 pg of 257Fm typically available per biennial campaign. Ongoing research and development aimed at increasing yields of 249Bk and 254Es will not produce increased yields of 257Fm. Such an effort would be extremely difficult due to the short half-lives of the precursor isotopes.
3 Fabrication of actinide targets
The history of actinide targets dates to the fabrication of a uranium target prepared by precipitation of ammonium diuranate [72] nearly 100 years ago. The fabrication of actinide targets has since had a pivotal role in numerous scientific endeavors, particularly the nucleosynthesis of new nuclides. Today, the variety and complexity of actinide target fabrication techniques has expanded beyond precipitation to include painting [73], vapor deposition [74], sputter coating [75], polymer assisted deposition (with heavy metal surrogates for the actinides) [76, 77], electrodeposition [74], and inkjet printing [78]. The discovery of several new SHEs, including tennessine (element 117) [9] and oganesson (element 118) [8], involved bombarding actinide targets electrodeposited onto thin metal foils with a high-energy and high-intensity 48Ca beam for several months. The discovery of additional SHEs will require higher beam intensities of several particle microamps and many months of irradiation due to the rapidly decreasing cross sections for the nucleosynthetic pathways of interest [5]. Improvements in separator transmission to around 60% (doubling earlier values of around 30%) and increases in charge particle detection efficiency to about 80% can also contribute to higher rates of observed nuclei [26, 66, 79]. The utilization of thicker targets (up from around 300 μg/cm2) may also improve sensitivity by covering a larger energy window as suggested by others [79, 80]. Additionally, the mass of projectile nuclei must continue to increase as the mass of actinide targets reaches a maximum due to practical considerations including decreasing material availability and half-lives. Recent efforts to synthesize increasingly heavy nuclides have seen increased actinide target failure rates as more intense and longer-duration irradiations have become necessary. The common failure mode has been thermal degradation of the targets leading to delamination of the actinide films from backing materials as well as pinhole formation. The extreme irradiation conditions required for the next generation of SHE studies will necessitate continued advancements in many technical areas with actinide targets being a key focus.
Electrodeposition has continued as the preferred fabrication technique for producing actinide targets used in nucleosynthesis over the last several decades [81,82,83]. Electrodeposition results in deposition yields regularly between 90 and 100%. This is very important given the limited availability of many actinide materials. This is especially true for targets made of the rarer actinides, such as berkelium, californium, and einsteinium, for which the maximum amount available at any given time is single to tens of milligrams or far less [5]. Indeed, berkelium and einsteinium rapidly decay relevant to the timescale of nucleosynthesis experiments searching for the heaviest of nuclei. Actinide thin films produced by electrodeposition are also highly reproducible. Target wheel assemblies are required for many SHE studies to dissipate heat loads from the high-current beams through rotation of targets in and out of the beam [84]. Each individual actinide target, often referred to as a segment, must therefore have similar characteristics, such as homogeneity and morphology. Target wheels often consist of more than 10 times the amount of actinide thin film surface area than in the beam at any given time. Electrodeposition is also relatively straightforward to implement in the remote environments often required for handling actinides. Other techniques that require delicate electronics, vacuum chambers, or other complex systems are more difficult to implement remotely, particularly in environments with radiological contamination controls in place.
The most frequently employed electrodeposition technique is a form of cathodic electrolytic deposition that is performed in a predominately nonconductive, organic solution and commonly referred to in the target community as “molecular plating” [74, 82, 83].
This process involves the deposition of a molecular species in contrast to electrochemical reduction of the actinide to its metallic form (i.e., electroplating). A review of the molecular plating process was recently published by Artes et al. [83]. The electrochemical potentials necessary for reduction of actinides to their metallic forms are outside the potential range of aqueous electrolytes, thereby resulting in the breakdown of water and other molecules. Although the solutions used for molecular plating are mostly nonconductive, organic solutions (e.g., dimethylformamide or isopropanol-isobutanol), they contain a miscible amount of aqueous solution containing a dissolved actinide salt (e.g., metal nitrate or metal chloride). The electrodeposition therefore contains several orders of magnitude more water molecules than actinide ions. The molecular plating process then theoretically proceeds through electroprecipitation of the actinide as a molecular compound (e.g., metal hydroxide or oxide) onto an electrically conductive backing induced by the electrolytic breakdown of water or other molecules at the electrode–solution interface. The electrolytic breakdown of water and other molecules produces hydroxide ions near the electrode–solution interface, thereby dramatically increasing the local pH of the solution. This is hypothesized to result in the electroprecipitation of insoluble actinide hydroxide, which subsequently decomposes to the metal oxide. Other species may also be produced and contained in the final thin film. The exact decomposition pathway is not fully understood and may involve electrochemical and thermal driving forces during the molecular plating process. Postdeposition thermal treatment of actinide targets is often performed to remove residual solvent and likely further decompose remaining actinide hydroxide to the actinide oxide form.
The final properties of electrodeposited actinide thin films depend on factors that include deposition solution composition (e.g., bulk solvent, supporting electrolyte ions, additives); conditions (e.g., temperature, fluid dynamics); and electrical conditions (e.g., pulsed or constant, current density, voltage, cell geometry). Although numerous studies have been performed over the years with actinides and nonradioactive surrogates [83,84,85,86,87,88,89,90,91,92], there remains a significant amount of the molecular plating process to explore and understand. Dramatic improvements to the properties of actinide thin film targets prepared through molecular plating could be realized through control of the electrodeposition process enabled by a mechanistic understanding of the deposition process. Further processing after the electrodeposition is completed may also be used to improve the actinide target characteristics. Thermal treatment of actinide thin films to remove impurities or control evaporation of residual solvent that remains postdeposition is one example. Addition of protective layers through vapor deposition or other techniques is sometimes used to prevent delamination as well as sputtering of actinide material from the target during irradiation.
Actinide thin film targets with improved performance compared to those in use today will likely be required to continue beyond elements 119 and 120. Continued advancement of technologies for thin film deposition and material synthesis is promising for the SHE community. State-of-the-art thin film fabrication techniques afford control of material structures at the microscale and nanoscale [93,94,95]. Modeling and simulation tools are also increasingly accurate in their prediction of material properties and performance. This unprecedented control can be leveraged to fabricate materials designed to have significantly improved properties for a desired application, such as targets for nucleosynthesis with improved thermal and mechanical properties. Some materials, for example, show strong dependencies of heat transfer and mechanical properties on the orientation of crystallographic planes. Controlled orientation of crystallographic grains within the actinide thin film and/or target backing could improve heat removal and mechanical robustness of actinide targets. Another possibility is the fabrication of alloy or composite materials. Fabrication techniques such as electrodeposition are currently limited to producing actinide oxides on conductive backings. However, electrodeposition methods aside from molecular plating or entirely different fabrication techniques (e.g., inkjet printing) could be developed to produce these types of materials in the future [94].
The materials currently used as backings for actinide targets cannot withstand the irradiation conditions (e.g., doses, particle fluxes) required for future SHE studies. Critical issues are the high thermal loadings and radiation damage. At the same time, current actinide targets benefit from being rotated out of the beam path because there is enough material to build several targets. The development of an actinide target capable of withstanding constant or near-constant irradiation under increasingly intense conditions could enable use of actinide materials with limited availability, such as the transcurium elements californium (certain isotopes), berkelium, and einsteinium. This would likely result in a significant breakthrough for synthesizing new superheavy nuclei. A mechanistic understanding of the processes leading to and causing target failures could enable prediction of ideal target parameters including geometries and compositions, as well as irradiation conditions (e.g., pulse shapes, raster patterns).
Recent SHE target production efforts since 2016 at ORNL have focused on the production of mixed-isotope californium thin films for searches for heavy isotopes of element 118 and new element 120. The californium material, recovered from decades old 252Cf targets, is 36% 251Cf, which would be the heaviest isotope to date for SHE experiments. This material also contains significant amounts of 249Cf (48%) and 250Cf (16%). The amounts of 250Cf and 252Cf present in the target can cause material-handling issues due to its intense neutron and gamma radiation. The fabrication of these “mixed-Cf” targets involves a molecular plating process adapted from Vascon et al. [88] and requires the use of a shielded glove box. In 2014, enough mixed-Cf material was recovered and chemically purified to fabricate 12 target segments with roughly 1 mg of mixed-Cf each. These targets were then shipped to JINR for SHE studies. The final target assemblies produced in 2014 were fabricated using a silicone gasket to maintain a liquid-tight seal during electrodeposition. Unfortunately, a film formed on the targets during irradiation, and the experiments had to be halted after an initial observation of a known isotope of element 118 [27] to investigate the source of the film and fabricate new targets. The mixed-Cf material was returned to ORNL and has since been chemically purified. Analysis of the leached target material identified significant quantities of silicone, leading researchers to hypothesize that the silicone gasket may have contributed to the film. The target fabrication process was altered to accommodate removal of the silicone gasket, and the final target assemblies consist of only metal components and the mixed-Cf thin film electrodeposited on the titanium foil backing. The refurbished targets will be used to continue SHE experiments aimed at producing new heavy isotopes of element 118 and new element 120. The electrodeposited targets are shown in Fig. 9.
4 Recent developments in SHE research
Since the last major review in 2015 [96], SHE research has focused on further exploration of the island of stability, including the discovery of new isotopes of known elements and new elements 119 and 120. These studies have depended on actinide targets 240Pu and mixed 249–251Cf at the Flerov Laboratory of Nuclear Reactions (FLNR) at JINR [27] and 248Cm at the Radioactive Ion Beam Facility at RIKEN in Japan [26], and on 243Am for direct mass number measurements of 288Mc and 284Nh isotopes at Berkeley [11]. These actinides were produced/processed at HFIR/REDC through the DOE Isotope Program and Division of Nuclear Physics. Mass measurements based on cold fusion reactions at GSI [36] and RIKEN [37] have been used to determine masses for 257Rf and 257Db, respectively, but the yields are currently too low by several orders of magnitude to determine masses of the heaviest superheavy nuclei.
FLNR and RIKEN use fast data acquisition systems [27, 80, 97, 98] modeled after digital electronics previously developed at ORNL’s Holifield Radioactive Ion Beam Facility for experiments involving short-lived charged particle emitters [99,100,101,102]. The ORNL-based system was used at GSI in 2011 during the search for element 120 [103, 104], while GSI-developed digitial electronics were used in a confirmation experiment for element 117 [15] and search for elements 119 and 120 [66].
Experiments at JINR with 239Pu and 240Pu targets focused on the identification of new flerovium isotopes produced in the hot fusion reactions with 48Ca beams. Earlier studies using 242Pu and 244Pu target materials with 48Ca beams resulted in the identification of 285Fl to 289Fl in the 3n, 4n, and 5n evaporation channels at JINR [7, 8]; GSI [12, 105]; and Lawrence Berkeley National Laboratory [106]. A long 285Fl decay chain was observed at LBNL in the rare 5n reaction channel of the 242Pu + 48Ca reaction, but the full energy of the alpha particle emitted by 285Fl was not recorded [10, 106].
A joint US–Russia experiment with a 240Pu target was performed at FLNR with a 245 MeV 48Ca beam, corresponding to an excitation energy of the compound nucleus 288Fl* between 35.6 MeV and 41.1 MeV [97]. Three decay chains of 285Fl produced in the 3n evaporation channel were identified. The half-life and emitted alpha energy for 285Fl were determined to be 0.15(+ 0.14, − 0.05) s and 10.41(5) MeV, respectively. The decay properties of 285Fl daughter activities 281Cn, 277Ds, 273Hs, 269Sg, and 265Rf were measured with higher accuracy [97]. Additional irradiations of the 240Pu target at an increased 48Ca beam energy of 250 MeV did not result in additional 285Fl events, suggesting a 3n cross section below 1.3 fb for 5 MeV higher beam energy (40.9–45.4 MeV excitation for 288Fl*). The four instances of evaporation residue (ER) followed by spontaneous fission (SF) events observed with the 250 MeV beam were assigned to the decay of the new isotope 284Fl. This interpretation was supported by the observation of two similar SF events during the irradiation of a 239Pu target (from RIAR) with 48Ca at 245 MeV selected to maximize the 3n reaction channel, with 35.4 MeV–40.0 MeV excitation energies for the compound nucleus 287Fl*.
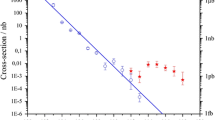
Cross sections for Fl isotopes produced in hot fusion reactions with 239Pu, 240Pu, 242Pu, and 244Pu targets and 48Ca beams show dramatic reductions for the most neutron-deficient flerovium isotopes through 287Fl* compound nucleus, a factor of 50 below the maximum for 290Fl* and 292Fl*. This indicates that the low-N edge of the Island of Stability in Z = 114 isotopes is approximately neutron number N = 170 [97].
Because the correlation times between ER and SF signals during the first experiment were scattered, a second experiment with a 240Pu target was performed [98]. With a 250 MeV 48Ca beam, three new events of 285Fl were detected, improving our understanding of the decay properties of all nuclei appearing in this long alpha decay chain that ends with SF of 265Rf. For example, the half-life and alpha energy associated with 285Fl decay are deduced to be 0.10 (+ 0.06, − 0.03) s and 10.41(5) MeV. The decay of 284Fl was not clarified, and further studies with a higher beam dose are needed for the verification of the origin of detected SF events.
An experiment involving a novel mixed-Cf target was performed at FLNR. The target material, consisting of 51% 249Cf, 13% 250Cf, and 36% 251Cf, was recovered from decayed 252Cf sources at ORNL’s REDC and electrodeposited on titanium backing foils. The mixed Cf was irradiated at FLNR with a 252 MeV 48Ca beam, corresponding to an excitation energy of 35.2 ± 2.2 MeV and 36.4 ± 2.2 MeV for the compound nuclei 297Og* and 299Og* (calculated for the 249Cf and 251Cf target components), respectively. The search for new isotopes of Z = 118 oganesson was the main goal of the investigation. A fifth event of 294Og decay was observed after only 9 days [27]; however, the experiment had to be stopped prematurely due to accumulation of foreign material on the target surface. New target sectors have been electrodeposited at REDC, as discussed in Sect. 3, to continue the search for new heavy isotopes of oganesson and eventually element 120 (with a 50Ti beam) at the new Super Heavy Element Factory at JINR.
Use of digital electronics has also helped in the analysis of fast fission events detected during the 48Ca + mixed-Cf experiment. Some events had ER–SF correlation times within the range corresponding to the half-life of 294Og, now determined to be 0.58 (+ 44, − 18) ms with the new average decay properties of five known events. The SF decay mode of Z = 118 and N = 176 294Og can most likely result in two magic nuclei, 208Pb and 86Kr, which can offer some enhancement of this decay mode. However, no definite conclusion about the origin of fast SF events can be drawn from our data. A setup allowing for some atomic number discrimination such as an ionization chamber in front of the implantation silicon detector may allow future conclusions regarding the properties of implanted nuclei.
Enriched 248Cm material provided by the DOE Isotope Program through ORNL is being used in a joint US–Japan experiment searching for new element 119 at the GARIS-III separator at RIKEN. An intense 51V beam developed at RIKEN and accelerated at the Superconducting RIKEN Linear Accelerator is irradiating a large target wheel with 248Cm sectors electrodeposited at RIKEN. Beam energy was selected by observing elastic and inelastic scattering of heavy ion projectiles on heavy targets including 248Cm [107, 108]. The experiment continues, presently at about 3 particle-microamp beam intensity. This high current is essential to overcome the low-production cross section anticipated for the 51V + 248Cm reaction (e.g., [44, 45]). Distributing the heat associated with these high beam currents requires larger target areas that necessitate more target material for a given thickness. A summary of current and future superheavy isotope searches is shown in Fig. 10.
5 New and upgraded facilities
Continued progress in SHE research depends on the availability of specialized facilities including high flux reactors and related radiochemical processing facilities for actinide production and high-current heavy ion accelerators including mass separators and related technology for superheavy nucleus synthesis and detection. Production cross sections for superheavy nuclei are declining with increasing Z and N, requiring larger targets and higher current ion beams to support new discoveries. This situation is placing increased demand on actinide supplies and accelerator capabilities. New and upgraded facilities are coming online to meet this challenge.
High flux reactors are key to continued availability of heavy actinides. Currently, these reactors exist only in the United States and Russia—HFIR at ORNL and SM-3 in Dmitrograd. The PIK Reactor at the Petersburg Nuclear Physics Institute near St. Petersburg, Russia, may also contribute but is not yet operational. HFIR and SM-3 were constructed in the 1960s, and additional investment will be required to guarantee their operation for the long term. A major refurbishment of the SM-3 core was recently completed [109], and planning is under way for life extension and mission upgrades at HFIR [33].
Significant improvements in the production rates for superheavy nuclei at accelerator facilities will also be required, including significant increases in beam currents. The new Superheavy Element Factory [30] at JINR is demonstrating beam currents of 6 particle-microamps and higher for 48Ca, more than six times higher than previously available beams. Upgrades at RIKEN in Japan are also demonstrating multiparticle microamp ion beams [26]. Together with larger targets, a tenfold increase in discovery potential appears to be within reach.
New facilities for superheavy production and research are also in development in France, Germany, and China. At GANIL in France, the NEWGAIN (injector 2) at Spriral2 projects 48Ca beams of 10 particle-microamps and more by 2030 [30]. At GSI in Germany, early components of the Helmholz Linear Accelerator (HELIAC) [31, 32] are being commissioned. When fully developed, HELIAC offers the potential for an order of magnitude increase in beam intensity compared to UNILAC [31, 32] for new element discovery and related spectroscopy and chemistry studies. The China Facility for Superheavy Elements at the Institute for Modern Physics, Chinese Academy of Sciences (IMP/CAS) together with a new gas-filled separator (SHANS2) are in development for superheavy element studies including synthesis of elements 119 and 120 [110]. In addition, LBNL is developing new capabilities at the 88-inch cyclotron for a proposed search for element 120 using the 50Ti + 249Cf reaction [28].
5.1 Superheavy element factory
JINR’s Superheavy Element Factory [29, 111] was completed in 2020. This facility, shown in Fig. 11, includes a state-of-the-art heavy ion accelerator, experimental hall, facilities for beam transport and superheavy nucleus detection, capabilities for the study of the physical and chemical properties of new elements, and related systems for operations with radioactive materials. The powerful new DC-280 cyclotron has a design beam intensity of up to 10 particle-microamps across a wide range of ion beams including 48Ca. The initial research program will include exploration of the island of stability and searches for new isotopes of element 118 using 48Ca and a search for new element 120 using 50Ti beams with mixed-Cf targets. First experiments with 48Ca on 243Am, 242Pu and 238U targets have been completed [112,113,114].
5.2 High Flux Isotope Reactor
HFIR produces the highest steady-state neutron flux in the world, with a peak thermal neutron flux of 2.5 × 1015 neutrons/cm2-s. This peak flux is matched only by the SM-3 Reactor in Dmitrovgrad, Russia. HFIR provides these very high fluxes for growing missions in isotope production, neutron scattering, materials irradiation, neutron activation analysis, and nuclear physics. The Institut Laue Langevin (ILL) Reactor in France, with a peak thermal neutron flux of 1.5 × 1015 neutrons/cm2-sec, is optimized for neutron scattering with limited capability in isotope production. All other research reactors have neutron fluxes lower than HFIR by a factor of 2.5 or more. The very high neutron fluxes of HFIR and SM-3 are essential to enable the multiple neutron captures required for actinide production.
HFIR was constructed in the 1960s to produce measurable quantities of the heavy actinides californium, berkelium, einsteinium, and fermium for research in the new field of Heavy Element Chemistry [115]. The adjacent REDC was constructed at the same time for the processing and purification of highly radioactive materials, including actinides. The combined capabilities of HFIR and REDC continue to be unique in the world. Figure 12 provides a current photograph of the HFIR/REDC complex.
HFIR has been substantially refurbished over the years. Components that have been replaced/refurbished include primary and backup pumps, the beryllium reflector, beam tubes, heat exchangers, cooling tower, control plates, and electrical and safety systems. The only major system that has not been replaced or refurbished is the pressure vessel. Replacement of the pressure vessel will be required to continue HFIR operation beyond midcentury due to accumulated radiation damage.
HFIR was designed for pressure vessel replacement. Removal of the old vessel and installation of the new vessel can be accomplished using the existing HFIR crane and truck airlock. The vessel can be disconnected and sectioned under water in the existing reactor pool. The vessel is less activated than waste that is routinely disposed at HFIR. Vessel sectioning and replacement has already been demonstrated at the ILL and Petten research reactors in Europe. Figure 13 provides a photo from the original HFIR vessel installation in the mid 1960s.
A new pressure vessel would allow HFIR to return to its original design power level of 100 MWth (from the current operating limit of 85 MWth) with a corresponding 20% increase in neutron flux. It would also allow numerous mission upgrades including improved access for isotope production, improved online insertion/removal of isotope production capsules, and improved facilities for handling irradiated materials. A new vessel would extend HFIR operation into the next century, ensuring continued availability of actinides for research and industrial applications. SM-3 recently completed a major core refurbishment to support continued operation of this reactor beyond 2040 [109].
In 2019, the DOE Office of Science asked its Basic Energy Sciences Advisory Committee to evaluate options for the future of HFIR. The committee recommended [33] that DOE immediately pursue the replacement of the reactor pressure vessel to secure the future operation of HFIR and enable increased capability for isotope production, neutron scattering, and other missions. Preliminary planning is under way to accomplish the vessel replacement and related upgrades. At the same time, DOE has directed ORNL to develop plans for expanded radioisotope processing capabilities to support the rapidly growing DOE isotope production and research program.
6 Future opportunities
The past 20 years have seen significant progress in SHE research from nuclear reactions involving actinide targets with intense 48Ca ion beams. Five new elements have been discovered, extending the periodic table to Z = 118, and more than 50 new isotopes have been added to the nuclear chart in the vicinity of the island of stability. Lifetimes have been consistent with trends expected from nuclear theory as the shell closure at N = 184 is approached. This has been made possible by farsighted investments in high-current accelerators, advanced detectors and mass separators, and actinide production and chemical separation facilities.
Continued progress requires new approaches as production cross sections decline with increasing Z and the limits of 48Ca reactions with available actinides are encountered. Elements 119 and 120 seem accessible, but only with heavier ion beams such as 50Ti, 51V, and 54Cr. Compound nucleus formation using these heavier ions to produce elements 119 an 120 is characterized by lower cross sections by factors of 10 or more [43,44,45] in comparison to 48Ca reactions for elements 117 and 118. Overcoming these lower cross sections requires increased beam currents and larger and more robust actinide targets. New and upgraded accelerator facilities including the Superheavy Element Factory at JINR and expanded facilities at RIKEN are demonstrating increased ion beam currents of factors of 3 and more. New recovery efforts and production approaches offer the potential of increased availability of key actinides such as 248Cm, 249Bk, 249−251Cf, and 254Es. Advances in target technology will also be needed to accommodate the higher thermal loads and increased exposure times of next-generation experiments.
6.1 Exploring the island of stability (Pu, Am, Cm, Bk, and Cf)
Increased beam currents are making 1n and 6n channel experiments with 48Ca beams more attractive, providing potential options for extending the “island of stability” to new lighter and heavier isotopes [112,113,114]. This will provide additional information on the detailed contours of the island, including the dramatic fall-off in production cross sections for low N isotopes and the effect of the shell closure at N = 184 on heavier isotopes. Significant increases in lifetimes are possible, enabling chemistry experiments on some of these new isotopes [38]. These would be primarily 48Ca nuclear reactions with available isotopes of plutonium, americium, curium, berkelium, and californium. As discussed in Sect. 2, many strategies are available for recovering/producing increased amounts of heavy actinide materials for many of these experiments; more than a dozen new isotopes would likely result. For lower N, this could result in decay chains that connect the island to known nuclei on the nuclear mainland, while higher N nuclei will provide additional information on the influence of the shell closure at N = 184.
6.2 Discovering elements 119 and 120 (Cm, Bk, and mixed-Cf)
Ongoing and planned experiments to search for elements 119 and 120 have been enabled by increased beam currents at the Superheavy Element Factory at JINR and at RIKEN. These experiments involve nuclear reactions of 50Ti, 51V, and 54Cr beams on 248Cm, 249Bk, and 249–251mixed-Cf targets. As discussed in Sect. 2, these heavy actinides can be recovered from legacy 252Cf sources and as a byproduct of current 252Cf production. Availability of 249Bk can also be boosted by dedicated production runs in HFIR using special neutron filters and heavy curium targets. A capability to enrich transuranic actinide isotopes through electromagnetic separators or some other technology would enable production of high-isotopic-purity 251Cf targets, the heaviest target material currently available in sufficient quantities for SHE experiments.
6.3 Electron capture and pxn reactions expanding superheavy island closer to N = 184
Moving the existing nuclear chart at the island to higher neutron numbers beyond the reach of present day xn reactions will require new approaches such as pxn reactions followed by electron capture. While pxn channels have previously been observed in cold fusion reactions [116, 117], estimated cross sections for the pxn channels for superheavy nuclei [118,119,120] are two or more orders of magnitude lower than for current xn reactions in SHE studies. If observed, these reactions and the resulting decay schemes could push the nuclear chart at the island several additional neutron numbers to the right. These experiments are low probability but could produce new high-N nuclei that are currently unknown.
6.4 Cold fusion path to higher Z and N = 184 (mixed-Cf)
Rykaczewski et al. [121] have suggested using a 251Cf target with a 58Fe beam to reach N = 184. This would be a cold fusion reaction producing an excited compound nucleus rapidly decaying with one neutron emission to Z = 124, N = 184 (or to Z = 124, N = 183 by emitting two neutrons). The excitation energy of the compound nucleus 309(124)* will likely be around 20–25 MeV lower in comparison to the hot fusion reactions, compare chapter 4. Having experimental data on the reactions between actinide targets and beams heavier than 48Ca would help to predict optimum conditions for the search for new element Z = 124 near the N = 184 neutron number. Although cross sections are expected to be very low (in the femtobarn range [122]), fission barriers should be favorable, and the increased stability at the shell closure may convey additional stabilization [122]. While more theoretical analysis of the expected cross section is needed, the models can be further refined using cross section data following successful searches for element 119 and 120 with beams heavier than 48Ca. An enriched 251Cf target would further increase the probability of success should an actinide separator become available. Reaching the N = 184 region would provide unique information on extreme nuclei at the shell closure and verify our current concept of the Island of Stability.
6.5 Future einsteinium options
If available in sufficient quantities, 254Es would be an ideal target to extend reactions using 48Ca beams to Z = 119 and reactions using 50Ti beams to Z = 121. These reactions should have higher cross sections than the path to Z = 119, 121 using Cf targets with 50Ti and 54Cr beams, respectively. While current production of 254Es in HFIR is limited to 1–2 µg, proposed experiments using specialized neutron filters could demonstrate increased production by as much as a factor of 5. This is approaching the scale that would be required to create a small stationary target of the required thickness for SHE discovery experiments. Significant advances in target technology will be required to manage the heat and radiation loads on such a stationary target.
These SHE research opportunities are dependent on the continued availability of heavy actinide targets. Increased attention to actinide production including new production technologies and recovery from existing high-assay sources will be required to ensure sufficient target material for these increasingly challenging experiments. In many cases, these experiments will also require the development of robust target technologies capable of surviving higher beam currents and heavier ion beams. The continued availability of actinide production and processing facilities such as HFIR and REDC will be essential. There is no apparent substitute for actinide targets as we continue the search for new elements and extreme nuclei with high and low neutron numbers. Expected longer lifetimes at higher N should also enable more sensitive studies of the chemistry of these new elements. Actinide materials have transformed the SHE research field over the past two decades, and the agenda described above offers the potential for decades of progress in the nuclear physics of extreme nuclei and the atomic physics and chemistry of extreme atoms.
Data availability statement
This manuscript has no associated data or the data will not be deposited. [Authors' comment: The focus of this paper is on describing how heavy actinides are used for the synthesis of superheavy nuclei. This paper is not presenting details of those experiments that would have associated data. Nor is this paper presenting details of new processing methods for the supply of actinides. Any such data would be appropriate for inclusion in publications detailing those experiments.]
Notes
The island of stability is a predicted region at the upper limits of the periodic table where shell closures in the vicinity of Z = 114 (or perhaps 120 or 124) and N = 184 are expected to confer increased stability on superheavy nuclei [59,60,61,62], resulting in long lifetimes for nuclei in this region.
References
R. C. Barber, et al., Pure Appl. Chem. 83, 1485 and 1801 (2011). https://doi.org/10.1351/PAC-REP-10-05-01
P.J. Karol et al., Pure Appl. Chem. 88, 139 (2016)
P.J. Karol et al., Pure Appl. Chem. 88, 155 (2016). https://doi.org/10.1515/pac-2015-0501
Y. Oganessian, J. Phys. G 34, R165 (2007). https://doi.org/10.1088/0954-3899/34/4/R01
J.B. Roberto et al., Nucl. Phys. A 944, 99 (2015). https://doi.org/10.1016/j.nuclphysa.2015.06.009
Yu. Ts. Oganessian et al., Phys. Rev. C 69, 021601(R) (2004). https://doi.org/10.1103/PhysRevC.69.021601
Yu. Ts. Oganessian et al., Phys. Rev. C 69, 054607 (2004). https://doi.org/10.1103/PhysRevC.69.054607
Yu. Ts. Oganessian et al., Phys. Rev. C 74, 044602 (2006). https://doi.org/10.1103/PhysRevC.74.044602
Yu. Ts. Oganessian et al., Phys. Rev. Lett. 104, 142502 (2010). https://doi.org/10.1103/PhysRevLett.104.142502
L. Stavsetra et al., Phys. Rev. Lett. 103, 132502 (2009). https://doi.org/10.1103/PhysRevLett.103.132502
J.M. Gates et al., Phys. Rev. Lett. 121, 222501 (2018)
Ch.E. Düllmann et al., Phys. Rev. Lett. 104, 252701 (2010). https://doi.org/10.1103/PhysRevLett.104.252701
D. Rudolph et al., Phys. Rev. Lett. 111, 112502 (2013). https://doi.org/10.1103/PhysRevLett.111.112502
S. Hofmann et al., Eur. Phys. J. A 48, 62 (2012). https://doi.org/10.1140/epja/i2012-12062-1
J. Khuyagbaatar et al., Phys. Rev. Lett. 112, 172501 (2014). https://doi.org/10.1103/PhysRevLett.112.172501
Yu. Ts. Oganessian et al., Phys. Rev. Lett. 109, 162501 (2012)
G.T. Seaborg, Science 104, 379 (1946). https://doi.org/10.1126/science.104.2704.379
G. Münzenberg et al., Z. Phys. A 300, 107 (1981). https://doi.org/10.1007/BF01412623
G. Münzenberg et al., Z. Phys. A 309, 89 (1982). https://doi.org/10.1007/BF01420157
G. Münzenberg et al., Z. Phys. A 317, 235 (1984). https://doi.org/10.1007/BF01421260
S. Hofmann et al., Z. Phys. A 350, 277 (1995). https://doi.org/10.1007/BF01291181
S. Hofmann et al., Z. Phys. A 350, 281 (1995). https://doi.org/10.1007/BF01291182
S. Hofmann et al., Eur. Phys. J. A 14, 147 (2002). https://doi.org/10.1140/epja/i2001-10119-x
K. Morita et al., J. Phys. Soc. Jpn. 81, 103201 (2012). https://doi.org/10.1143/JPSJ.73.2593
Yu. Ts. Oganessian, V. K. Utyonkov, Nucl. Phys. A 944, 62 (2015). https://doi.org/10.1016/j.nuclphysa.2015.07.003
H. Sakai et al., Eur. Phys. J. A 58, 238 (2022)
N.T. Brewer et al., Phys. Rev. C 98, 024317 (2018). https://doi.org/10.1103/PhysRevC.98.024317
R. Clark, “A US-Led New-Element Search Experiment”, 88-Inch Cyclotron DOE Review, Berkeley, CA, March 4–5 (2020). https://urldefense.us/v2/url?u=https-3A__conferences.lbl.gov_event_301_&d=DwIFaQ&c=v4IIwRuZAmwupIjowmMWUmLasxPEgYsgNI-O7C4ViYc&r=vjmXyXKGbDgCgAjcfSUOoO_20Q-5IrQ334aKR9iHKWU&m=gP3BvKjs2orRtwBGz1jnYXeQht4D_mmI-dutusYhFdcmwi0dHNIuxFOK7sB7ri08&s=tZeX8BtmCiYu25w12AGqAFnr5JHmJvYa4UnjXwlXVP0&e=
A. Karpov, “Superheavy Element Factory”. https://indico.in2p3.fr/event/24078/contributions/95500/attachments/64226/88983/Karpov_SHE-Factory.pdf.
P. Roussel-Chomaz, “GANIL-SPIRAL2 Highlights”, ARIS 2023 Conference, Avignon, France. https://indico.in2p3.fr/event/19688.
Ch.E. Duellmann et al., Radiochim. Acta 110, 417 (2022)
S. Lauber et al., NIMA 1040, 16709 (2022)
Report of the Basic Energy Sciences Advisory Committee on the scientific justification for a U.S. domestic high-performance reactor-based research facility, US Department of Energy/Office of Science/July 2020. https://science.energy.gov/bes/community-resources/reports/ DOI: https://doi.org/10.2172/1647598
D. Ackermann, C. Theisen, Phys. Scripta 92, 083002 (2017). https://doi.org/10.1088/1401-4896/aa7921
O. R. Smits, P. Indelicato, W. Nazarewicz, M. Piibeleht, P. Schwerdtfeger, Pushing the Limits of the Periodic Table – A Review on Atomic Relativistic Electronic Structure Theory and Calculations for the Superheavy Elements, arXiv 2301.02553 (2023), submitted to Phys. Reports.
O. Kaleja et al., Phys. Rev. C 106, 054325 (2022)
P. Schury et al., Phys. Rev. C 104, L021303 (2021)
A. Turler and V. Pershina, Chem. Rev. 113, 1237 (2013).
S.M. Robinson et al., Radiochim. Acta 108(9), 737–746 (2020). https://doi.org/10.1515/ract-2020-0008
Hogle, Susan, "Optimization of Transcurium Isotope Production in the High Flux Isotope Reactor. " PhD diss., University of Tennessee, 2012._https://trace.tennessee.edu/utk_graddiss/1529
S. Hogle, G.I. Maldonado, C. Alexander, Ann. Nucl. Energy 60, 267 (2013) https://doi.org/10.1016/j.anucene.2013.05.018
Yu. Ts. Oganessian et al., Phys. Rev. C 62, 041604(R) (2000). https://doi.org/10.1103/PhysRevC.62.041604
G. Adamian et al., Phys. Particles and Nuclei 47, 387 (2016)
K. Siwek-Wilczyńska, T. Cap, M. Kowal, Phys. Rev. C 99, 054603 (2019). https://doi.org/10.1103/PhysRevC.99.054603
G. G. Adamian and N. V. Antonenko, Eur. Phys. J. A 58, 111 (2022). https://doi.org/10.1140/epja/s10050-022-00764-0
Ch. E.Duellmann et al., J Radioanal. Nucl. Chem. 332, 1505 (2023)
2019 Heavy Element Chemistry Principal Investigators’ Meeting, Gaithersburg, MD, April 15–17 (2019). https://science.osti.gov/-/media/bes/csgb/pdf/docs/2019/HEC_Proceedings_2019.pdf. Accessed 29, July, 2022
K.P. Carter et al., Nature 590, 85–88 (2021). https://doi.org/10.1038/s41586-020-03179-3
I. W. Osborne‐Lee, C. W. Alexander, Oak Ridge National Laboratory Technical Report, ORNL/TM–12706 (1995). https://doi.org/10.2172/205871
R. G. Haire, in The Chemistry of the Actinide and Transactinide Elements 4th edn Vol 3. (Springer 2011), p. 1499
J.E. Bigelow, et al, in Actinide Separations ACS Symposium Series, vol. 117, ed. by J.D. Navratil, W.W. Schulz (American Chemical Society, Washington, DC, 1980), pp.161–171
E.D. Collins, et al, in Transplutonium Elements-Production and Recovery ACS Symposium Series, vol. 161, ed. by J.D. Navratil, W.W. Schulz (American Chemical Society, Washington, DC, 1981), pp.147–160
E.K. Hulet et al., J. Inorg. Nucl. Chem. 17, 350–360 (1961). https://doi.org/10.1016/0022-1902(61)80161-9
D.E. Benker, et al, in Transplutonium Elements-Production and Recovery ACS Symposium Series, vol. 161, ed. by J.D. Navratil, W.W. Schulz (American Chemical Society, Washington, DC, 1981), pp.161–172
G.R. Choppin et al., J. Inorg. Nucl. Chem. 2, 66–68 (1956). https://doi.org/10.1016/0022-1902(56)80105-X
W. Bickford, Westinghouse Savannah River Company Report, OBU-OPD-2003–00043 (2003)
B. D. Patton, et al., Special Actinide Recovery from Mark-18A Target Material. United States. (2017)
S. M. Robinson et al., Oak Ridge National Laboratory Technical Report, ORNL/TM-2014/314 (2014)
A. Sobiczewski, F.A. Gareev, B.N. Kalinkin, Phys. Rev. Lett. 22, 500 (1966)
W.D. Myers, W.J. Swiatecki, Nucl. Phys. 81, 1 (1966)
A. Sobiczewski, K. Pomorski, Prog. Part. Nucl. Phys. 58, 292 (2007)
S. Cwiok, P.-H. Heenen, W. Nazarewicz, Nature (London) 433, 705 (2005)
Yu. Ts. Oganessian, J. Phys. G: Nucl. Part. Phys. R165 (2007)
S. Hofmann et al., Eur. Phys. J. A 52, 180 (2016). https://doi.org/10.1140/epja/i2016-16180-4
Yu. Ts. Oganessian et al., PRC 79, 024603 (2009).
J. Khuyagbaatar et al., Phys. Rev. C 102, 064602 (2020)
L. K. Felker, L. H. Delmau, “Recovery of 248Cm from 252Cf decay,” in The 8th International Conference on Isotopes Proceedings (American Nuclear Society 2014)
Boll, Rose Ann. Californium Electrodepositions at Oak Ridge National Laboratory. United States. https://doi.org/10.1007/s10967-015-4148-8
M. Du, et al., Oak Ridge National Laboratory Technical Report, ORNL/TM- 2019/1138 (2019).
M. Schaedel et al., PRC 33, 1547 (1986)
K. Nishio et al., “Competition between mass-symmetric and asymmetric fission modes in 258Md produced in the 4He + 254Es reaction”, submitted to Phys. Rev. Lett. (2023), and private communication 2023.
G. Herrmann, Nucl. Instrum. Methods A 282, 301 (1989). https://doi.org/10.1016/0168-9002(89)90157-5
L.V. Drapchinsky et al., Nucl. Instrum. Methods Phys. Res. A 438, 116 (1999). https://doi.org/10.1016/S0168-9002(99)00948-1
K.M. Glover, Nucl. Instrum. Methods Phys. Res. A 236, 435 (1985). https://doi.org/10.1016/0168-9002(85)90939-8
J. Kwinta, Nucl. Instrum. Methods 167, 65 (1979). https://doi.org/10.1016/0029-554X(79)90478-6
M.A. Garcia et al., Thin Solid Films 516, 6261 (2008). https://doi.org/10.1016/j.tsf.2007.11.127
M.A. Garcia et al., Nucl. Instrum. Methods Phys. Res. A 613, 396 (2010). https://doi.org/10.1016/j.nima.2009.09.084
R. Haas et al., Nucl. Instrum. Methods Phys. Res. A 874, 43 (2017). https://doi.org/10.1016/j.nima.2017.08.027
Yu. Ts. Oganessian et al., Nucl. Instr. Meth A 1033, 166640 (2022)
P. Brionnet et al., Nucl. Instr. Meth A 1049, 168068 (2023). https://doi.org/10.1016/j.nima.2023.168068
J.D. Burns et al., Nucl. Instrum. Methods Phys. Res. A 830, 95–101 (2016). https://doi.org/10.1016/j.nima.2016.05.062
W. Parker, R. Falk, Nucl. Instrum. Meth. 16, 355–357 (1962). https://doi.org/10.1016/0029-554X(62)90142-8
E. Artes et al., EPJ Web of Conferences 285, 03001 (2023). https://doi.org/10.1051/epjconf/202328503001
S. Antalic et al., Nucl. Instrum. Methods Phys. Res. A 530, 185–193 (2004). https://doi.org/10.1016/j.nima.2004.04.217
J. Runke et al., J. Radioanal. Nucl. Chem. 299, 1081–1084 (2014). https://doi.org/10.1007/s10967-013-2616-6
A. Vascon. Molecular plating of thin lanthanide layers with improved material properties for nuclear applications. Ph.D. Thesis, Johannes Gutenberg-Universität (2013). Available from INIS: http://inis.iaea.org/search/search.aspx?orig_q=RN:46021465
A. Vascon et al., J. Radioanal. Nucl. Chem. 299(2), 1085–1091 (2014). https://doi.org/10.1007/s10967-013-2631-7
A. Vascon et al., Nucl. Instrum. Meth. A 721, 35–44 (2013). https://doi.org/10.1016/j.nima.2013.04.050
K. Myhre et al., Surf. Sci. Spectra 23, 70 (2016). https://doi.org/10.1116/1.4954390
M.N. Torrico et al., Nucl. Instrum. Meth. A 790, 64–69 (2015). https://doi.org/10.1016/j.nima.2015.03.056
K.G. Myhre et al., Surf. Sci. Spectra 25, 024003 (2018). https://doi.org/10.1116/1.5052011
R.A. Boll et al., J. Radioanal. Nucl. Chem. 305(3), 921–926 (2015). https://doi.org/10.1007/s10967-015-4148-8
O.O. Abegunde et al., AIMS Mater. Sci. 6(2), 174–199 (2019). https://doi.org/10.3934/matersci.2019.2.174
C. Androulidakis, et al., 2D Mater. 5(3), 032005 (2018). https://doi.org/10.1088/2053-1583/aac764
Z. Aksamija. ECS Meet. Abstr. MA2020–01(50), 2767 (2020). http:// https://doi.org/10.1149/MA2020-01502767mtgabs
Special Issue on Superheavy Elements, Nucl. Phys. A 944 (2015), edited by Ch. E. Duellmann, R.-D. Herzberg, W. Nazarewicz, and Y. Oganessian
V.K. Utyonkov et al., Phys. Rev. C 92, 034609 (2015). https://doi.org/10.1103/PhysRevC.92.034609
V.K. Utyonkov et al., Phys. Rev. C 97, 014320 (2018). https://doi.org/10.1103/PhysRevC.97.014320
K. Rykaczewski et al., Nucl. Phys. A 701, 179 (2002). https://doi.org/10.1016/S0375-9474(01)01569-X
R. Grzywacz, Nucl. Instrum. Meth. B 204, 649 (2003). https://doi.org/10.1016/S0168-583X(02)02146-8
R. Grzywacz et al., Nucl. Instrum. Meth. B 261, 1103 (2007). https://doi.org/10.1016/j.nimb.2007.04.234
I.G. Darby et al., Phys. Rev. Lett. 105, 162502 (2010). https://doi.org/10.1103/PhysRevLett.105.162502
D. Miller, et al., GSI Scientific Report 2011, (GSI Report 2012–1, pp 220, 2012). http://www.gsi.de/library/GSI-Report-2012-1/
S. Hofmann, S. Heinz, R. Mann et al., Review of even element super-heavy nuclei and search for element 120. Eur. Phys. J. A 52, 180 (2016). https://doi.org/10.1140/epja/i2016-16180-4
J.M. Gates et al., Phys. Rev. C 83, 054618 (2011). https://doi.org/10.1103/PhysRevC.83.054618
P.A. Ellison et al., Phys. Rev. Lett. 105, 182701 (2010). https://doi.org/10.1103/PhysRevLett.105.182701
T. Tanaka et al., Phys. Rev. Lett. 124, 052502 (2020). https://doi.org/10.1103/PhysRevLett.124.052502
M. Tanaka et al., J. Phys. Soc. Jpn. 91, 084201 (2022). https://doi.org/10.7566/JPSJ.91.084201
A. A. Tuzov, et al., SM-3 Core Refurbishment Project. (International Group on Research Reactors, 2021), https://www.igorr.com/Documents/2021/07.1%20TM-%20RIAR-SM-3%20CORE%20REFURBISHMENT%20PROJECT.pdf. Accessed 29 July 2022
Z.G. Gan et al., Eur. Phys. J. A 58, 158 (2022)
S. Dmitriev, et al., EPJ Web Conf. 131, 08001 (2016), Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements. https://doi.org/10.1051/epjconf/201613108001
Yu. Ts. Oganessian et al., Phys. Rev. C 106, L031301 (2022)
Yu. Ts. Oganessian et al., Phys. Rev. C 106, 064306 (2022)
Yu. Ts. Oganessian et al., Phys. Rev. C 106, 024612 (2022)
A. Weinberg, Phys. Today 20(6), 23 (1967). https://doi.org/10.1063/1.3034353
A. Lopez-Martens et al., Phys. Lett. B 795, 271 (2019)
F.P. Hessberger et al., Eur. J. Phys. A 55, 208 (2019)
J. Hong et al., Phys. Lett. B 809, 135760 (2020). https://doi.org/10.1016/j.physletb.2020.135760
J. Hong et al., Phys. Rev. C 103, L041601 (2021). https://doi.org/10.1103/PhysRevC.103.L041601
J. Hong et al., Phys. Lett. B 805, 135438 (2020). https://doi.org/10.1016/j.physletb.2020.135438
K.P. Rykaczewski et al., EPJ Web Conf. 131, 05005 (2016). https://doi.org/10.1051/epjconf/201613105005
K. Wilczynska, private comm. (2016)
Acknowledgements
This research was supported by the U.S. Department of Energy (DOE) Office of Isotope R&D and Production and the DOE Office of Nuclear Physics under contract DE-AC05- 00OR22725 with UT-Battelle, LLC. We are grateful to the staffs of the ORNL Radiochemical Engineering Development Center and High Flux Isotope Reactor, a DOE Office of Science User Facility, for their support in the production and chemical separation of the actinide materials. We also thank our many collaborators at the Flerov Laboratory of Nuclear Reactions (JINR, Dubna, Russia), GSI (Darmstadt, Germany), University of Mainz (Mainz, Germany), Lawrence Livermore National Laboratory, Vanderbilt University, and the University of Tennessee–Knoxville, without whom this research would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Klaus Blaum
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roberto, J.B., Du, M., Ezold, J.G. et al. Actinide targets for the synthesis of superheavy nuclei. Eur. Phys. J. A 59, 304 (2023). https://doi.org/10.1140/epja/s10050-023-01144-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epja/s10050-023-01144-y