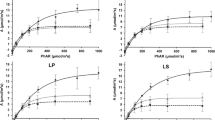
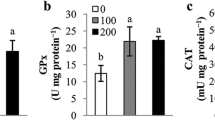
Abstract
The resistance of regenerate Populus alba×P. bolleana no.7, P. alba×P. bolleana no.12, and P. davidiana×P. bolleana cv. Baicheng 1 plants cultured in vitro to osmotic stress is examined. It is found that hybrid no. 7 tolerates d-mannitol in the nutrient medium at concentrations up to 250 mM after a 30-day culturing period, while the Baicheng 1 variety tolerates it only in concentrations up to 50 mM. Hybrid no. 12 remains viable at d-mannitol concentrations up to 150 mM. The application of d-mannitol statistically significantly reduces leaf blade parameters (length, width, and leaf area) only in hybrid no. 7; in the rest of the studied specimens, the differences are statistically insignificant (p < 0.05). The introduction of the osmotic component reduces the fresh and dry weight of all studied specimens by 1.7–2.2 times; the water content in their tissues remains virtually unchanged. In relation to drought tolerance, the results are confirmed by micromorphological parameters (stomatal apparatus and pubescence). The Baicheng 1 poplar variety features the lowest pubescence and the highest stomatal density (155.44 pcs/mm2), while poplar hybrid no. 7 features the highest pubescence and the lowest stomatal density (127.75 pcs/mm2). No direct relationship between the osmotic stress and the number and size of stomata was identified in the studied specimens. Our data indicate that the studied poplar hybrids feature different resistance to osmotic stress when cultured in vitro. Hybrid no. 7 is more drought-resistant and can be recommended for urban landscaping in arid conditions.



Similar content being viewed by others
REFERENCES
Anderegg, W., Kane, J., and Anderegg, L., Consequences of widespread tree mortality triggered by drought and temperature stress, Nat. Clim. Change, 2013, vol. 3, pp. 30–36. https://doi.org/10.1038/nclimate1635
Arzani, K., Ghasemi, M., Yadollahi, A., and Hokmabadi, H., Study of foliar epidermal anatomy of four pistachio rootstocks under water stress, Idesia (Arica), 2013, vol. 31, pp. 101‒107. https://doi.org/10.4067/S0718-34292013000100012
Bakulin, V.T., Topol’ belyi v Zapadnoi Sibiri (White Poplar in Western Siberia), Novosibirsk: Geo, 2012.
Bosabalidis, A.M. and Kofidis, G., Comparative effects of drought stress on leaf anatomy of two olive cultivars, Plant Sci., 2002, vol. 163, pp. 375‒379. https://doi.org/10.1016/S0168-9452(02)00135-8
Claeys, H., Van Landeghem, S., Dubois, M., Maleux, K., and Inzé, D., What is stress? Dose-response effects in commonly used in vitro stress assays, Plant Physiol., 2014, vol. 165, pp. 519–527.
Errabii, T., Gandonou, C.B., Essalmani, H., Abrini, J., I-daomar, M., and Senhaji, N.S., Effects of NaCl and mannitol induced stress on sugarcane (Saccharum sp.) callus cultures, Acta Physiol. Plant., 2007, vol. 29, pp. 95–102. https://doi.org/10.1007/s11738-006-0006-1
Erst, A.A. and Banaev, E.V., Coservation and propagation in culture in vitro of ornamental forms of poplar selected by the Central Siberian Botanical Garden of the Siberian Branch of the Russian Academy of Sciences, Byull. Gl. Bot. Sada, 2021, vol. 1, pp. 61–69. https://doi.org/10.25791/BBGRAN.01.2021.1085
Erst, A.A., Bakulin, V.T, Erst, A.S., Kuznetzov, A.A., and Bayahmetov, E.Zh., In vitro propagation of ornamental hybrids of Populus L., Biosci., Biotechnol., Res. Asia, 2014, vol. 11, pp. 69–77.
Erst, A.A., Shishkin, S.V., and Voronkova, M.S., Evaluation of salinity and osmotic stress resistance of the genus Populus species and hybrids in culture in vitro, Plant Cell Biotechnol. Mol. Biol., 2019, vol. 20, nos. 11–12, pp. 451‒458.
Esmaeilpour, A., Van Labeke, M.-C., Samson, R., Boeckx, P., and Van Damme, P., Variation in biochemical characteristics, water status, stomata features, leaf carbon isotope composition and its relationship to water use efficiency in pistachio (Pistacia vera L.) cultivars under drought stress condition, Sci. Hortic, 2016, vol. 211, pp. 158‒166. https://doi.org/10.1016/j.scienta.2016.08.026
Fomin, E.S. and Fomina, T.I., Changes in the phenology of perennial plants in Western Siberia against the background of global warming, Contemp. Probl. Ecol., 2021, vol. 14, pp. 434–445. https://doi.org/10.1134/S199542552105005X
Galdon-Armero, J., Fullana-Pericas, M., Mulet, P.A., Conesa, M.A., Martin, C., and Galmes, J., The ratio of trichomes to stomata is associated with water use efficiency in Solanum lycopersicum (tomato), Plant J., 2018, vol. 96, no. 3, pp. 607–619. https://doi.org/10.1111/tpj.14055
Hauser, M.T., Molecular basis of natural variation and environmental control of trichome patterning, Front. Plant Sci., 2014, vol. 5, p. 320. https://doi.org/10.3389/fpls.2014.00320
Hetherington, A. and Woodward, F., The role of stomata in sensing and driving environmental change, Nature, 2003, vol. 424, pp. 901–908. https://doi.org/10.1038/nature01843
Kang, J.M., Kojima, K., Ide, Y., and Sasaki, S., Growth response to the stress of low osmotic potential, salinity and high pH in cultured shoot of Chinese poplars, J. For. Res., 1996, vol. 1, pp. 27–29. https://doi.org/10.1007/BF02348336
Kim, T.H., Böhmer, M., Hu, H., Nishimura, N., and Schroeder, J.I., Guard cell signal transduction network: advances in understanding abscisic acid, CO2 and Ca2+ signaling, Annu. Rev. Plant Biol., 2010, vol. 61, pp. 561–591. https://doi.org/10.1146/annurev-arplant-042809-112226
Miller, G., Suzuki, N., Ciftci-Yilmaz, S., and Miller, R., Reactive oxygen species homeostasis and signaling during drought and salinity stresses, Plant Cell Environ., 2010, vol. 33, pp. 453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mo, Y., Yang, R., Liu, L., Gu, X., Yang, X., Wang, Y., Zhang, X., and Li, H., Growth, photosynthesis and adaptive responses of wild and domesticated watermelon genotypes to drought stress and subsequent re-watering, Plant Growth Regul., 2016, vol. 79, pp. 229–241. https://doi.org/10.1007/s10725-015-0128-9
Munir, M., Khan, M.A., Ahmed, M., Bano, A., Ahmed, S.N., Tariq, K., Tabassum, S., Mukhtar, T., Ambreen, M., and Bashir, S., Foliar epidermal anatomy of some ethnobotanically important species of wild edible fruits of northern Pakistan, J. Med. Plants Res., 2011, vol. 5, pp. 5873‒5880.
Muradoğlu, F. and Gündoğdu, M., Stomata size and frequency in some walnut (Juglans regia) cultivars, Int. J. Agric. Biol., 2011, vol. 13, no. 6, pp. 1011–1015.
Murashige, T. and Skoog, F., A revised medium for rapid growth and bioassays with tobacco tissue culture, Physiol. Plant., 1962, vol. 15, no. 2, pp. 473–497.
Ostry, M.E., In vitro screening and selection for disease resistance, in Micropropagation, Genetic Engineering and Molecular Biology of Populus, Klopfenstein, N.B., Chun, Y.W., Kim, M.S., and Ahuja, M.R., Eds., Fort Collins, 1997, pp. 155–160.
Ouyang, S.Q., Liu, Y.F., Liu, P., Lei, G., He, S.J., Ma, B., Zhang, W.K, Zhang, J.S., and Chen, S.Y., Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants, Plant J., 2010, vol. 62, pp. 316–329. https://doi.org/10.1111/j.1365-313X.2010.04146.x
Pant, N.C., Agarrwal, R., and Agrawal, S., Mannitol-induced drought stress on calli of Trigonella foenum-graecum L. Var. RMt-303, Indian J. Exp. Biol., 2014, vol. 52, pp. 1128–1137.
Polle, A., Chen, S.L., Eckert, C., and Harfouche, A., Engineering drought resistance in forest trees, Front. Plant Sci., 2019, vol. 9, p. 1875. https://doi.org/10.3389/fpls.2018.01875
Quan, W., Liu, X., Wang, H., and Chan, Z., Comparative physiological and transcriptional analyses of two contrasting drought tolerant Alfalfa varieties, Front. Plant Sci., 2016, vol. 6, p. 1256. https://doi.org/10.3389/fpls.2015.01256
Razavizadeh, R., Farahzadianpoor, F., Adabavazeh, F., and Komatsu, S., Physiological and morphological analyses of Thymus vulgaris L. in vitro cultures under polyethylene glycol (PEG)-induced osmotic stress, In Vitro Cell. Dev. Biol.-Plant., 2019, vol. 55, pp. 342–357. https://doi.org/10.1007/s11627-019-09979-1
Singh, D., Kaur, S., and Kumar, A., In vitro drought tolerance in selected elite clones of Eucalyptus tereticornis Sm., Acta Physiol. Plant., 2020, vol. 42, p. 17. https://doi.org/10.1007/s11738-019-3009-4
Szyp-Borowska, I., Ukalska, J., Niemczyk, M., Wojda, T., and Thomas, B.R., Effects of water deficit stress on growth parameters of Robinia pseudoacacia L. selected clones under in vitro conditions, Forests, 2022, vol. 13, no. 12, p. 1979. https://doi.org/10.3390/f13121979
Vuksanović, V., Kovačević, B., Orlović, S., Kebert, M., and Kovač, M., The influence of drought on growth and development of white poplar shoots in vitro, Poplar, 2019, vol. 203, pp.13‒18.
Watanabe, S., Kojima, K., Ide, Y., and Sasaki, S., Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro, Plant Cell, Tissue Organ Cult., 2000, vol. 63, pp. 199–206. https://doi.org/10.1023/A:1010619503680
ACKNOWLEDGMENTS
This study was performed using materials from the bioresource scientific collection of the Central Siberian Botanical Garden, Siberian Branch, Russian Academy of Sciences “Collections of Living Plants Cultivated Outdoors and Indoors,” Unique Scientific Facility no. USU 440534.
Funding
This study was performed as part of the State Task of the Central Siberian Botanical Garden, Siberian Branch, Russian Academy of Sciences, projects no. AAAA-A21-121011290027-6 and AAAA-A21-121011290025-2.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest. The authors declare that they have no conflicts of interest.
Additional information
Translated by L. Emeliyanov
Rights and permissions
About this article
Cite this article
Erst, A.A., Karakulov, A.V. Drought Tolerance of Ornamental Poplar Forms Cultured In Vitro. Contemp. Probl. Ecol. 16, 339–345 (2023). https://doi.org/10.1134/S1995425523030058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995425523030058