Abstract
Flame acceleration (FA) and the deflagration-to-detonation transition (DDT) are among the most interesting and exciting phenomena in the field of combustion and explosion of gases. From both practical and theoretical points of view, it is important to understand the basic laws governing these phenomena as well as the physical and/or chemical mechanisms and features of the process. High-speed flame-front photography during the deflagration of a premixed gas mixture in a long smooth tube with transparent walls was performed. A stoichiometric mixture of acetylene with oxygen diluted with argon by 25% is used. The experiments are carried out in a transparent cylindrical tube with an inner diameter of 60 mm and a length of 6 meters. The evolution of the structure and shape of the flame front from the moment of initiation of deflagration by a weak ignition source to the formation of a detonation wave is determined. Four characteristic phases of the propagation process are distinguished: at the first stage, the flame accelerates, then slows down, followed by flame propagation at an almost constant speed, and finally repeated acceleration, during which detonation is formed. It is shown how the dynamics of the process changes with a change in the initial pressure of the mixture. The most interesting and poorly studied stage of the DDT, the stage of intensive reacceleration, during which the flame abruptly changes shape, is described in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
When a mixture of a gaseous fuel with an oxidizer is ignited, the process of slow subsonic deflagration is most likely, and the propagation mechanism of the subsonic deflagration wave consists of the transfer of heat and active radicals from the reaction zone to the fresh mixture. However, in a number of cases, when flame propogates in a closed volume, the process develops in a nonstationary mode, and the slow deflagration wave can spontaneously accelerate and transform into a detonation wave [1–4]. In addition to the extreme propagation velocity, the detonation wave is characterized by much higher temperature and pressure than the subsonic deflagration wave. The heat release power per unit surface area in the detonation front is thousands of times higher than in the reaction front during deflagration, and the velocity of detonation products is tens of times higher than the velocity of slow deflagration products. It is generally accepted that it is detonation that is the most effective but also the most dangerous deflagration mode, and the modes of unsteady flame acceleration, within which the conditions for the transition to detonation are realized, are regarded as especially destructive [5].
Rapid flame propagation and detonation of gases began to be actively studied in the 1920–1930s. Both contact methods (measurement of pressure and velocity of the head shock wave, detonation wave by piezoelectric pressure sensors, measurement of the reaction front velocity by ionization current sensors, recording the cellular structure of the detonation wave using the technique of obtaining imprints on a surface covered with a layer of soot) and noncontact methods (recording emission and absorption, high-speed photography using shadow and interference methods, etc.) of experimental diagnostics have been widely used. Among the variety of experimental research methods, high-speed photography of the process is one of the most informative methods, giving the most visual and complete information of the process, its details, and features.
The first photographs of the flame front and the dynamics of changes in its shape over time during gas deflagration in a tube were most likely presented in [6]. Using a technique and equipment unique for that time, the authors visualized the propagation of a flame in a glass tube 25 mm in diameter and 2 m long in a mixture of carbon monoxide and oxygen. A detailed and consistent description, as well as shadow photographs of all stages of the flame acceleration process and the onset of detonation, was first presented in [4].
The initial stage of flame propagation and the subsequent formation of its so-called “tulip” shape in closed and semiopen tubes were subsequently studied in detail in [7–17] in most cases using the shadow photography method. The final stage of flame acceleration and the occurrence of conditions for the formation of a detonation wave became the subject of the study in [18–27]. Various modes of detonation formation were described and the corresponding shadow photographs were presented. All the work, as a rule, was carried out in rectangular channels, which was a direct consequence of the diagnostic capabilities using shadow and interference methods of flow visualization.
A common drawback of the experimental work performed earlier was that due to the imperfection of the recording equipment available at that time, it was possible to obtain the desired images of fast processes only for an extremely short period of time. In addition, the dimensions of the optical windows for observation were small compared to the characteristic scale of the process itself, i.e., the deflagration-to-detonation transition (DDT) length. Together, this led to a sequence of images of only individual stages of the flame acceleration process or the formation of a detonation wave. For example, to obtain information about, the earlier or later stages of flame acceleration under the same initial conditions, it was necessary to change the position of the measuring section by moving it along the tube axis. All this imposed significant restrictions on the possibilities of analyzing the entire DDT process as a whole, since information in the form of a sequence of images was received separately from different series of experiments.
Despite the large number of theoretical works describing the formation processes of flame acceleration and detonation, to some extent taking into account the influence of various kinds of physicochemical factors (temperature, pressure, density of the medium, its viscosity and thermal conductivity, turbulence, kinetics of chemical reactions, boundary conditions, etc.) [28–70], there is no unified DDT theory. This is due to the limitations of the models themselves, inaccurate knowledge of the values of a large number of different constants necessary to describe the chemical and physical interactions in the system in a wide range of thermodynamic conditions, and the imperfection of numerical methods for solving the equations describing this process. A number of review papers [71–73] generalize the information available as of 2007–2008 on theoretical and experimental studies of flame acceleration and the DDT in gas mixtures. A full-fledged three-dimensional modeling of the DDT process with the required resolution in time and space is hardly possible at present, since it requires serious computing power. However, with the development of computer technology in the near future, such simulations will probably be performed and a comparison of the results obtained with real experimental data will be required.
With the development of modern photographic image recording systems (high-speed digital cameras), it became possible to obtain detailed visual information about the entire process of flame acceleration and the DDT completely along the entire length of the channel, starting from the moment the flame was initiated by a local source and ending with the appearance of a detonation wave. Such information is extremely useful for understanding the dynamics of the acceleration process and the spatial configuration of the flame as a whole, since it makes it possible to compare and verify the existing and developed theoretical models with the real experimental data with a high resolution in time and space. The aim of this study is to obtain detailed visual information with a high spatiotemporal resolution of all the stages of the flame acceleration process and the transition of slow deflagration to detonation, which will make it possible to see the details and understand the physical mechanisms of the phenomenon.
EXPERIMENTAL
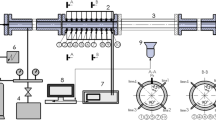
For the experiments, we used a calibrated, transparent-walled plastic tube (Plexiglas) of a cylindrical cross section with an inner diameter of 60 mm and a wall thickness of 5 mm; the length of the tube was 6 m (3 sections of 2 m each). The specified dimensions of the tube length and its diameter made it possible to avoid the effects related to the reflection of compression waves from the far end of the tube and their interaction with the flame front throughout the entire deflagration process up to the transition to detonation. One of the ends of the tube was plugged; a deflagration initiation system was installed in it. The other end of the tube was closed with a thin lavsan diaphragm capable of withstanding a pressure drop of 1 to 1.5 atm. Before the experiment, the tube was vacuumized to a residual pressure of not more than 10–20 Pa, after which it was filled with a working mixture to the required initial pressure. After the initiation of deflagration and as the flame front propagated from the plugged end of the tube to the end lavsan film with a film, due to the pressure increase, the film ruptured and the deflagration products were released into the surrounding space.
The initial pressure of the mixture varied from 8 to 22 kPa at room temperature. To initiate deflagration, a system based on the standard automotive spark plug with a low ignition energy (approximately 0.8 mJ) was used.
The experiments were carried out in a stoichiometric mixture of acetylene and oxygen diluted by 25% with argon. Acetylene was chosen as the fuel for several reasons. From the point of view of technical applications, it is the most demanded among gaseous hydrocarbons. Acetylene can be synthesized artificially, sustains stable deflagration over a wide range of compositions and thermodynamic conditions, and can be used both directly as a fuel and as an additive to the main fuel. The addition of acetylene makes it possible to reduce the emission of nitrogen oxides, thereby ensuring the environmental friendliness of the operating cycle of modern internal deflagration engines. However, the use of acetylene can lead to the development of detonation-like deflagration modes due to its high chemical activity. Acetylene has the highest energy potential and high detonation ability among other hydrocarbons. The mechanism of its ignition and deflagration is considered to be well known and is an important component of the corresponding detailed kinetic mechanisms (DKMs) for more complex hydrocarbons [74–81]. Despite this, in recent studies [82], by comparing experimental data with calculations using DKMs [83, 84], it has been shown that the existing DKMs do not always correctly describe the modes of high-temperature oxidation of acetylene mixtures; and in addition, the issues of reduction and optimization of existing kinetic mechanisms are topical [85].
To capture the flame front, two identical high-speed cameras Photron Fastcam SA-Z and the 2100 KM-8GB (Japan) were used. The cameras were positioned perpendicularly to the tube axis one after the other, so that their fields of view overlapped by 10–15% for subsequent alignment of a series of images. The operation frame rate was 180 000 fps at a spatial resolution of 1024 × 88 pixels; the distance from the camera lens to the tube was 850 mm and the field of view of the camera along the tube axis was 650 mm. The exposure time for each frame was 1–1.3 μs. Both cameras were equipped with the Tamron SP 24-70 mm F/2.8 Di VC USD, model A007 lenses, and Edmund Optics (UK) narrow-band optical filters: BP 430 ± 10 nm, OD4, and 50 mm diameter. The bandwidth of the filters corresponds to the emission wavelength of the intermediate CH radical. Thus, it was possible to obtain a high-quality image of the flame front, excluding parasitic illumination from the deflagration products. The settings of both cameras (start time of recording, total recording time, frame resolution, frame rate, exposure time of each frame, etc.), as well as the settings of both lenses and the filters used, were absolutely identical. The cameras were synchronized with each other and controlled using a single computer with the Photron Fastcam Viewer software.
An analysis of the obtained images by the displacement of the glow region from frame-to-frame made it possible to determine the propogation velocity of the flame front along the axis of the tube. Taking into account the insignificant (on the order of several pixels) shifts (especially at low propagation velocities), the velocity values were calculated as an average over 5–20 frames. When determining the speed, the effects related to image distortion when viewed in a direction other than perpendicular were also taken into account. Below, averaged (interpolated) curves are shown on the plots of the flame front velocity versus distance or time.
RESULTS AND DISCUSSION
Within one experimental series, the initial pressure of the mixture was reduced from the reference value of 22 to 8 kPa. This led to the fact that the location of the DDT shifted along the tube from experiment-to-experiment, and the distance and, accordingly, the DDT time increased with a decrease in the initial pressure. In a number of experiments, the moment of the onset of detonation was no longer in the field of view of the cameras, but this gave us the opportunity to study in more detail the earlier stages of the flame acceleration process.
Figures 1–5 show images of the deflagration wave propagation at various initial pressures of the gas mixture. The time interval between the presented frames is chosen so that the evolution of the flame structure from the moment of ignition to the moment of transition to detonation, if possible, consists of the same number of frames (15 or 16).
High-speed camera images of the flame front in an acetylene/oxygen/argon mixture at an initial pressure of 8 kPa (a); the transverse size of each frame (inner diameter of the tube) is 60 mm. Velocity profiles (interpolation based on the results of processing and averaging the array of images) depending on the distance (b) and time (c). The numbers on the graphs indicate the positions in time of the corresponding images of the flame front.
The same as in Fig. 1 at an initial pressure of 12 kPa.
The same as in Fig. 1 at an initial pressure of 14 kPa.
The same as in Fig. 1 at an initial pressure of 16 kPa.
The same as in Fig. 1 at an initial pressure of 20.8 kPa. In frame 15, the red circle shows the onset of local explosion kernel in the region of the boundary layer.
In addition, the graphs of wave motion show dependences of the longitudinal velocity of the leading edge of the flame front on distance or time. The time points and distances along the tube axis, corresponding to the images of the flame front presented in the figures, are shown on the graphs by the numbers of the corresponding images.
Image analysis allows to draw the following conclusions. Before the formation of a detonation wave, four characteristic stages of flame propagation are observed. At the first stage, the flame front accelerates; it then decelerates; this is followed by a propagation stage at an almost constant speed; and, finally, it enters the reacceleration stage, at the end of which detonation is formed.
Let us consider in more detail the structure and velocity of the flame front at each stage separately. Due to the buoyant action of the deflagration products, whose density is much lower and the pressure is slightly higher than in the initial mixture, the gas in front of the wave begins to move, involving the reaction zone in motion along the tube axis. At the initial moments of time after the ignition of the mixture, while the flame has not yet reached the walls of the tube, its shape is almost an ideal hemisphere (frame 1 in Figs. 1–5). Then, as it reaches the tube walls, the flame begins to stretch in the direction of motion along the axis, occupying the entire cross section of the channel—the so-called finger-like (finger flame) shape of the flame is formed [14] (frames 2–4, Figs. 1–5). By this time (1.5 ms from the moment of ignition for an initial pressure of 8 kPa and about 1 ms for a pressure of 20 kPa), the wave velocity relative to the observer reaches 300 m/s for a pressure of 8 kPa and more than 400 m/s for a pressure of 22 kPa, respectively. By the time the wave velocity reaches its maximum in the first phase of acceleration, the extent of the luminescence region of the reacting mixture, i.e., the zone where the chemical reaction proceeds intensively reaches two channel diameters. The contours of the flame front are smooth and clear, which indicates the laminar nature of deflagration.
At the next stage, the flame front begins to slow down. Near the closed end of the tube, i.e., in the ignition zone, where the deflagration products stay for the longest time, an area of rarefaction arises, which forms a gas flow in the direction opposite to the flame’ propogation. The velocity of the flame front along the tube axis drops from 300 to 200 m/s for a pressure of 8 kPa; i.e., the speed deficit is approximately 1/3 of its maximum value at the first stage of acceleration (Figs. 1b, 1c). With an increase in the initial pressure to 22 kPa, the braking effect is not as pronounced. The deficit of the wave velocity in relation to its amplitude in the first maximum decreases about 10% (Figs. 5b, 5c). The flame front acquires a more flatter shape (frames 4–6, Figs. 1–5), which is especially noticeable at a low initial pressure of the mixture, equal to 8 kPa. Its length along the tube axis decreases significantly: from two or more diameters to half the tube diameter. The surface of the flame front begins to fragment and a cellular or honeycomb structure is formed on it. Before the flame front, there is a flow of unburned gas in the direction of the front movement, caused by the buoyant action of expanding deflagration products and disturbances generated by the flame that propagate downstream at the speed of sound. Behind the front, the flow caused by rarefaction waves in the deflagration products is directed in the opposite direction. The front, as a discontinuity surface, is subject to gas-dynamic (Darrieus–Landau) and thermal diffusion (Rayleigh–Taylor) instabilities, which also significantly affect the change in the shape of its surface and leads to the appearance of cellular structures. The dimensions of the emerging structure strongly depend on the initial pressure of the gas mixture and sharply decrease with its increase. The flame front’s deceleration phase lasts from 1.5 ms for a pressure of 8 kPa to 0.6–0.7 ms for a pressure of 22 kPa. During this time interval, the flame front travels distances of 0.4 and 0.2 m along the tube axis, respectively.
The deceleration phase is followed by a period of time when the flame front propagates at a nearly constant velocity. As can be seen from the images (frames 7–14, Fig. 1; frames 7–12, Figs. 2–4; frames 7–9, Fig. 5), the duration of this stage is from 0.7 ms at an initial pressure of 20.8 kPa to 3 ms at a pressure of 8 kPa. The structure and shape of the flame front during this period barely change. The shape of the deflagration front is close to hemispherical and extended forward by approximately the channel diameter along the tube axis. The surface of the flame front has a pronounced cellular structure, and as the flame moves, the size of the cells begins to grow larger, and their number decreases. They also begin to stretch in the longitudinal direction along the tube axis. This effect is most pronounced at low initial pressures.
Finally, in the fourth stage, the flame front accelerates again. At the same time, its shape and structure undergo significant changes. One of the sections of the flame front begins to move forward sharply in the direction of motion. The authors of [4] found that the upper section of the flame moves forward and explained this as follows: the flame front is the interface between a heavy fresh mixture with a higher density and lighter deflagration products. Under the action of gravity, the heavy fresh mixture spreads along the bottom of the tube, and the lighter products of deflagration tend to settle in the upper part, pushing the flame front forward. Our observations show that the orientation of the head tongue of the flame front relatively to the tube perimeter is probably random (Figs. 1–5). As the flame front stretches along the tube axis, a significant increase in the deflagration area occurs and its velocity begins to increase drastically. The flame takes on a conical shape strongly elongated along the tube axis, which is described in detail in [82]. In frame 15, Fig. 5, at an initial mixture pressure of 20.8 kPa, the circle shows the onset of local explosion kernel in the region of the boundary layer in one of the folds of the flame edge encircling the channel walls. The development of this focus leads to the formation of an overcompressed detonation wave, which propagates up and downstream. In experiments at lower initial pressures (8–16 kPa, Figs. 1–4), the process of detonation initiation was no longer within the field of view of the observation system.
CONCLUSIONS
The evolution of the structure and shape of the flame front during the DDT of an acetylene-oxygen mixture in a cylindrical tube 60 mm in diameter was studied by high-speed visualization. The high-speed images show and describe the four characteristic stages of the deflagration process of a gas mixture: at the first stage, the flame accelerates; this is followed by the deceleration stage; then there is the stage of propagation at an almost constant velocity; and, finally, repeated acceleration, during which detonation is formed. Images of the flame shape typical for each stage are given and the dependences of the flame front velocity along the tube axis are determined. The most interesting and insufficiently studied, in our opinion, is the stage of repeated acceleration of the flame, when its speed rapidly increases from 300–400 to 1000–1200 m/s and a detonation wave is formed in front of the flame front (or at its edge). At the same time, during the development of this stage, the shape of the flame front undergoes drastic changes: a conical structure strongly elongated along the tube axis is formed, which is described in detail in [82]. The new data presented in our study compare favorably with those published earlier in that the visualization of the process for each initial pressure of the mixture at all stages was obtained in one experimental run with a high spatiotemporal degree of detail of the flame front structure.
REFERENCES
Ya. B. Zel’dovich, Theory of Combustion and Detonation of Gases, Ed. by N. N. Semenov (Akad. Nauk SSSR, Moscow, 1944) [in Russian].
A. Lewis and G. von Elbe, Combustion, Flames and Explosion of Gases (Academic, New York, 1961).
K. I. Shchelkin, Sov. Phys. Usp. 8, 780 (1965).
G. D. Salamandra, T. B. Bazhenova, and I. M. Naboko, Sov. Tech. Phys. 4, 1244 (1959).
H. D. Ng and J. H. Lee, J. Loss Prev. Process Ind. 21, 136 (2008).
O. C. Ellis and R. V. Wheeler, J. Chem. Soc., 3215 (1928). https://doi.org/10.1039/JR280003215
H. Guenoche and M. Jouy, in Proceedings of the 4th International Symposium on Combustion (The Combust. Inst., Pittsburgh, 1953), p. 403.
D. Dunn-Rankin and R. E. Sawyer, in Proceedings of the 10th Workshop of Institute for Dynamics of Explosions and Reactive Systems ICDERS, Berkley, CA, 1985.
R. Starke and P. Roth, Combust. Flame 66, 249 (1986).
D. Dunn-Rankin, P. K. Barr, and R. F. Sawyer, in Proceedings of the 21st International Symposium on Combustion (The Combust. Inst., Pittsburgh, 1986), p. 1291.
D. A. Rotman and A. K. Oppenheim, in Proceedings of the 21st International Symposium on Combustion (The Combust. Inst., Pittsburgh, 1986), p. 1303.
R. Starke and P. Roth, Combust. Flame 75, 111 (1989).
M. Gonzalez, R. Borghi, and A. Saouab, Combust. Flame 88, 201 (1992).
C. Clanet and G. Searby, Combust. Flame 105, 225 (1996).
D. Dunn-Rankin and R. F. Sawyer, Exp. Fluids 24, 130 (1998).
H. H. Xiao, J. H. Sun, D. Makarov, et al., Combust. Flame 159, 1523 (2012).
H. Xiao, X. Shen, S. Guo, et al., Combust. Flame 160, 1725 (2013).
V. S. Babkin and L. S. Kozachenko, Prikl. Mat. Tekh. Fiz., No. 3, 165 (1960).
R. I. Soloukhin, Prikl. Mat. Tekh. Fiz., No. 4, 128 (1961).
R. I. Soloukhin, Shock Waves and Detonation in Gases (Fizmatlit, Moscow, 1963) [in Russian].
P. A. Urtiew and A. K. Oppenheim, Proc. R. Soc. London, Ser. A 295, 13 (1966).
P. A. Urtiew and A. K. Oppenheim, in Proceedings of the 11th International Symposium on Combustion (The Combust. Inst., Pittsburgh, 1967), p. 665.
P. A. Urtiew and A. K. Oppenheim, Proc. R. Soc. London, Ser. A 304, 379 (1968).
N. N. Smirnov and M. Tyurnikov, Combust. Flame 100, 661 (1995).
M. Kuznetsov, V. Alekseev, I. Matsukov, et al., Shock Waves 14, 205 (2005).
M. A. Liberman, M. F. Ivanov, A. D. Kiverin, et al., Acta Astronaut. 67, 688 (2010).
Y. Ballossier, F. Virot, and J. Melguizo-Gavilanes, J. Loss Prev. Process Ind. 72, 104535 (2021).
A. K. Oppenheim and R. I. Soloukhin, Ann. Rev. Fluid Mech. 5, 31 (1973).
Ya. B. Zeldovich, V. B. Librovich, G. M. Makhviladze, et al., Acta Astronaut. 15, 313 (1970).
Ya. B. Zeldovich, Combust. Flame 39, 211 (1980).
Ya. B. Zel’dovich, Zh. Tekh. Fiz. 7 (1), 3 (1947).
Ya. Troshin, in Proceedings of the 7th International Symposium on Combustion (The Combust. Inst., Pittsburgh, 1958), p. 789.
G. K. Adams and D. C. Pack, in Proceedings of the 7th International Symposium on Combustion (The Combust. Inst., Pittsburgh, 1958), p. 812.
S. Taki and T. Fujiwara, in Proceedings of the 13th International Symposium on Combustion (The Combust. Inst., Pittsburgh, 1971), p. 1119.
J. H. S. Lee, R. Knystautas, and N. Yoshikawa, Acta Astronaut. 5, 971 (1978).
N. Yoshikawa, PhD Thesis (McGill Univ., Montreal, Canada, 1980).
J. Kurylo, H. A. Dwyer, and A. K. Oppenheim, AIAA J. 18, 302 (1980).
Ya. B. Zeldovich, B. E. Gelfand, S. A. Tsyganov, et al., Prog. Astronaut. Aeronaut. 114, 99 (1988).
B. Deshaies and G. Joulin, Combust. Flame 77, 201 (1989).
J. E. Shepherd and J. H. S. Lee, Major Research Topics in Combustion, Ed. by M. Y. Hussaini, A. Kumar, and R. G. Voigt (Springer, New York, 1992).
N. N. Smirnov and I. I. Panfilov, Combust. Flame 101, 91 (1995).
I. Brailovsky and G. Sivashinsky, Combust. Flame 122, 492 (2000).
V. N. Gamezo, A. M. Khokhlov, and E. S. Oran, Combust. Flame 126, 1810 (2001).
L. Kagan and G. Sivashinsky, Combust. Flame 134, 389 (2003).
V. Bychkov and V. Akkerman, Phys. Rev. E 73, 066305 (2006).
V. Akkerman, V. Bychkov, A. Petchenko, et al., Combust. Flame 145, 206 (2006).
L. Kagan, Math. Model. Nat. Phenom. 2 (2), 40 (2007).
M. Silvestrini, B. Genova, G. Parisi, et al., J. Loss Prev. Process Ind. 21, 555 (2008).
D. Valiev, V. Bychkov, V. Akkerman, et al., Phys. Lett. A 372, 4850 (2008).
L. Kagan and G. Sivashinsky, Combust. Flame 154, 186 (2008).
D. M. Valiev, V. Bychkov, V. Akkerman, et al., Phys. Rev. E 80, 036317 (2009).
M. F. Ivanov, A. D. Kiverin, and V. A. Gal’burt, Russ. J. Phys. Chem. B 3, 363 (2009).
M. A. Liberman, M. Kuznetsov, A. Ivanov, et al., Phys. Lett. A 373, 501 (2009).
M. F. Ivanov, A. D. Kiverin, M. A. Liberman, et al., Dokl. Phys. 55, 480 (2010).
M. F. Ivanov and A. D. Kiverin, Russ. J. Phys. Chem. B 4, 110 (2010).
M. F. Ivanov, A. D. Kiverin, and M. A. Liberman, Phys. Rev. E 83, 056313 (2011).
M. Ivanov, A. Kiverin, and M. Liberman, Int. J. Hydrogen Energy 36 (2011).
E. Dzieminska and A. Hayashi, Int. J. Hydrogen Energy 38, 4185 (2013).
M. Fukuda, E. Dzieminska, A. Hayashi, et al., Shock Waves 23, 191 (2013).
A. Kiverin, I. Yakovenko, and M. Ivanov, Int. J. Hydrogen Energy 41, 22465 (2016).
L. Kagan and G. Sivashinsky, Proc. Combust. Inst. 36, 2709 (2017).
W. Han, Y. Gao, and C. K. Law, Combust. Flame 176, 285 (2017).
V. B. Akkerman, in Modeling and Simulation of Turbulent Combustion, Ed. by S. De, A. Agarwal, S. Chaudhuri, (Springer, Singapore, 2018).
A. Kiverin and I. Yakovenko, Math. Model. Nat. Phenom. 13 (6), 54 (2018).
B. H. Bang, C. S. Ahn, Y. T. Kim, et al., Appl. Math. Model. 66, 332 (2019).
O. Dounia, O. Vermorel, A. Misdariis, et al., Combust. Flame 200, 1 (2019).
A. D. Kiverin, A. E. Smygalina, and I. S. Yakovenko, Russ. J. Phys. Chem. B 14, 607 (2020).
A. D. Kiverin, A. V. Tyurnin, and I. S. Yakovenko, Russ. J. Phys. Chem. B 15, 984 (2021).
C. Wieland, F. Scharf, H. P. Schildberg, et al., J. Loss Prev. Process Ind. 71, 104504 (2021).
X. Tang, E. Dziemińska, A. K. Hayashi, et al., Int. J. Hydrogen Energy 46, 37487 (2021).
B. Gelfand, S. Frolov, and M. Nettleton, Prog. Energy Combust. Sci. 17, 327 (1991).
E. S. Oran and V. N. Gamezo, Combust. Flame 148, 4 (2007).
G. Ciccarelli and S. Dorofeev, Prog. Energy Combust. Sci. 34, 499 (2008).
C. W. Zhou, Y. Li, U. Burke, et al., Combust. Flame 197, 423 (2018).
E. Ranzi, A. Frassoldati, R. Grana, et al., Prog. Energy Combust. Sci. 38, 468 (2012).
D. Fernandez-Galisteo, A. L. Sanchez, A. Linan, et al., Combust. Theor. Model. 3 (4), 74 (2009).
Y. Tao, G. P. Smith, and H. Wang, Combust. Flame 195, 18 (2018).
A. M. Tereza, S. P. Medvedev, and V. N. Smirnov, Acta Astronaut. 163, 18 (2019).
N. Slavinskaya, A. Mirzayeva, R. Whitside, et al., Combust. Flame 210, 25 (2019).
A. M. Tereza, S. P. Medvedev, and V. N. Smirnov, Acta Astronaut. 181, 612 (2021).
A. M. Tereza, V. G. Slutskii, and E. S. Severin, Russ. J. Phys. Chem. B 3, 99 (2009).
P. Krivosheyev, O. Penyazkov, and A. Sakalou, Combust. Flame 216, 146 (2020).
F. H. V. Coppens, J. de Ruyck, and A. A. Konnov, Combust. Flame 149 (4), 409 (2007).
G. P. Smith, D. M. Golden, M. Frenklach, et al. http://combustion.berkeley.edu/gri-mech/.
A. M. Tereza, G. L. Agafonov, A. S. Betev, and S. P. Medvedev, Russ. J. Phys. Chem. B 14, 951 (2020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krivosheyev, P.N., Novitski, A.O. & Penyazkov, O.G. Evolution of the Reaction Front Shape and Structure on Flame Acceleration and Deflagration-to-Detonation Transition. Russ. J. Phys. Chem. B 16, 661–669 (2022). https://doi.org/10.1134/S1990793122040248
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793122040248