Abstract
Light-dependent transcription factors GLKs of Arabidopsis thaliana are involved in the anterograde regulation of chloroplast biogenesis during deetiolation: they regulate the expression of photosynthetic nuclear-encoded genes and also mediate the transcription of plastid genes. Chloroplast biogenesis is determined at the same time by light and by endogenous factors (phytohormones), among which cytokinins significantly accelerate the formation of photosynthetically active chloroplasts. In this work, it was shown that trans-factors GLKs function as cytokinin-dependent regulators, mediating the positive cytokinin effect on the plastome expression through the activation of transcription of the SCA3 nuclear gene encoding the plastid RNA polymerase RPOTp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Despite the key role of chloroplasts in plant life, the molecular mechanisms of their biogenesis remain poorly understood. Chloroplasts are usually formed either from proplastids in meristematic tissues or from etioplasts during plant deetiolation.
The main exogenous factor that determines the biogenesis of photosynthetically active chloroplasts from etioplasts is light [1]. Light of different quality is perceived by one or another group of receptors (primarily in the cytoplasm). Then, the signal enters the nucleus, which leads to a large-scale change in the expression of plant nuclear genes, many of which encode structural and regulatory proteins of chloroplasts. The control of the biogenesis of photosynthetically active plastids by nuclear coding proteins is called anterograde regulation, which probably plays the key role at all stages of chloroplast biogenesis.
One of the most striking examples of anterograde control of chloroplast formation is the reprogramming of plastid gene expression as a result of light- and hormone-dependent changes in the activity of the plastid transcription apparatus during deetiolation. In etioplasts, transcription is performed mostly by RNA polymerases of the NEP type (Nuclear-Encoded RNA Polymerase)—RPOTp and, to a lesser extent, RPOTmp, which largely transcribe housekeeping genes. The second multisubunit RNA polymerase PEP (Plastid-Encoded RNA Polymerase) consists of core subunits α, β, β', and β" and exhibits weak transcriptional activity in non-photosynthetic plastids. During deetiolation, the PEP polymerase undergoes significant structural changes due to the formation of a complex with one of the sigma factors (SIG1-SIG6) and nuclear coding proteins associated with PEP (PAP1-PAP12), after which the PEP polymerase initiates transcription of photosynthetic plastome genes [2]. Thus, the nuclear genome controls the transcriptional activity of the plastid genome by coordinating the transcription of the genes encoding RNA polymerases RPOTp and RPOTmp, sigma factors, and PAP proteins.
The key regulators of nuclear genome expression are the light-dependent transcription factors. In A. thaliana, two trans-factors—GLK1 and GLK2 (Golden two-LiKe)—were identified, the inactivation of which leads to disturbances in chloroplast biogenesis [3] due to a decrease in the transcription of nuclear genes encoding chlorophyll biosynthesis enzymes and proteins of photosynthetic complexes and thylakoid membranes [4].
In addition to light, the deetiolation program is determined by endogenous factors—phytohormones, among which cytokinins (CKs) can accelerate the formation of chloroplasts [5]. It was convincingly shown that CKs upregulate the expression of genes encoding the chlorophyll biosynthesis enzymes and proteins of the plastome transcription machinery, stimulate the accumulation of photosynthetic pigments, and accelerate the formation of chloroplast ultrastructure [6, 7]. In a number of tests, a synergistic effect in the action of light and CKs is observed, which allows for the possibility of intersection of light and cytokinin signal transduction pathways [6–8]. To date, it has been shown that one of the “crossing points” is the light-dependent trans-factor HY5, the inactivation of which leads to a decrease in the positive effect of CKs during deetiolation [8]. However, the absence of HY5 does not abolish the effect of CKs, which suggests the involvement of other light-dependent trans-factors that mediate the effect of CKs during deetiolation [9].
As already mentioned, in A. thaliana, the inactivation of the genes encoding two trans-factors GLK1 and GLK2 leads to disruption of transcription of both nuclear and plastid photosynthetic genes [3, 4]. In addition, Kobayashi et al. [10] showed that proteins GLKs are involved in the realization of the positive effect of CKs on the accumulation of chlorophyll during root deetiolation, and the GLK2 gene expression is activated by the hormone. These results suggest that GLKs may be involved in the regulation of the expression of nuclear genes of the plastid transcription apparatus by light and CKs. In this work, we demonstrated for the first time the involvement of trans-factors GLKs in the cytokinin-dependent regulation of the expression of the nuclear SCA3 gene encoding plastid RNA polymerase, which leads to changes in the expression of plastid genes.
MATERIALS AND METHODS
The objects of the study were wild-type Arabidiopsis thaliana plants of the Columbia-0 ecotype and its knockout mutant for the trans-factor genes glk1glk2 (N9807, NASC, United Kingdom). The presence of the dSpm insertion in the GLK1 and GLK2 genes [3] was confirmed by PCR using primers flanking the insert: for the GKL1 gene: Spm5 (F) 5'-ggatccgacactctttaattaactgacact-3'; (R) 5'-acttcttcaccttttccccgaacta-3'; for the GLK2 gene: Spm1 (F) 5'-cctatttcagtaagagtgtggggttttgg-3'; (R) 5'-aacaatctttacttttcttccctttacg-3'. PCR analysis of the glk1glk2 knockout mutant DNA confirmed the presence of dSpm inserts in the GLK1 and GLK2 genes. Amplification of DNA from the wild-type plants showed the absence of dSpm constructs in the maternal A. thaliana line. Thus, the homozygosity of A. thaliana glk1glk2 knockout lines obtained from the NASC seed bank was confirmed.
To study the involvement of the trans-factors GLK1 (AT2G20570) and/or GLK2 (AT5G44190) in the cytokinin-dependent regulation of the SCA3 gene expression during deetiolation, we used the experimental protocol developed by Chory et al. [11]. Seeds of wild-type A. thaliana and glk1glk2 knockout mutant were sterilized with sodium hypochlorite solution and seeded on Petri dishes with Murashige–Skoog nutrient medium containing 1/2 nutrients (Duchefa, Netherlands) without sucrose and cytokinin or with the addition of trans-zeatin (1 µM). The seeds were stratified for 4 days at 4°C, after which the Petri dishes were incubated at 22°C in complete darkness. Four days after the moment of germination, the plants were fixed in liquid nitrogen under weak green light (5 ± 2 µmol s–1 m–2). The rest of the seedlings were transferred to an MLR-352Н-PE climatic chamber (Sanyo, Japan) illuminated with white light with an intensity of 120 µmol s–1 m–2 and fixed in liquid nitrogen after 6 and 16 h of incubation.
The relative level of transcripts was assessed by real-time PCR after reverse transcription (RT-PCR) using a LigthCyclerR96 thermocycler (Roche, Switzerland). The number of target gene transcripts was normalized relative to the mRNA content of the reference polyubiquitin gene UBQ10 (AT4G05320). The following primer pairs were used for real-time PCR analysis: ARR5: (F) ctactcgcagctaaaacgc, (R) gccgaaagaatcaggaca; GLK1: (F) tcggactaaaaatggatggcttg, (R) ggtagaaggcggaggtaagtgtttg; GLK2: (F) gccaaaacacaagcctaatactccg, (R) tgtggatagagtggttgctgatgc; SCA3: (F) ttgctgctgcttgctattctgc, (R) gcacaatcaccaagccaact; accD; (F) gctaccaatcaatgtttacctc, (R) gattgataatcacataaaaccg; clpP: (F) cattccagatattacccatcca, (R) gccaagaggttgataccgaa; and UBQ10: (F) gcgtcttcgtggtggttctaa, (R) gaaagagataacaggaacggaaaca. The samples were analyzed in three biological replicates, each of which included three or four analytical replicates. Data were statistically processed using Student’s t test (*p < 0.05, **p < 0.01).
RESULTS AND DISCUSSION
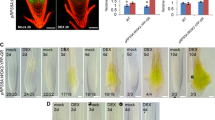
Effect of cytokinin on the morphology of the wild-type seedlings and the glk1glk2 knockout mutant of A. thaliana. The GLKs family of proteins includes two transcription factors, GLK1 and GLK2, whose functions considerably overlap. The first-order knockout mutants have weak phenotypic differences from the wild-type plants, whereas the double mutant glk1glk2, which we used in this study, differs from the maternal line in a smaller rosette size and a reduced chlorophyll content (Fig. 1a) [3].
Morphology of A. thaliana wild-type and glk1glk2 knockout mutant plants. (a) Three-week-old plants, wild type (WT) on the left, glk1glk2 knockout-mutant on the right; (b) 4-day-old etiolated wild-type and glk1glk2 seedlings grown on nutrient medium without hormone (0 μM trans-zeatin) or in the presence of CK (1 μM trans-zeatin) after 16 h of illumination.
One of the characteristic effects of CKs is the shortening of the seedling hypocotyl in the dark [11]. Under our experimental conditions, seedlings of the wild type and the glk1glk2 double knockout mutant grown on a nutrient medium without CKs did not differ phenotypically (Fig. 1b). The addition of trans-zeatin (1 μM) to the growth medium resulted in the suppression of hypocotyl growth in the wild-type plants by 54% (in the absence of the CK, 12.6 ± 1.5 mm; in the presence of trans-zeatin, 6.88 ± 1.6 mm). Plants of the glk1glk2 knockout mutant in the presence of CK had a hypocotyl length 62% shorter than the seedlings grown without the hormone (in the absence of the CK, 12.9 ± 1.4 mm; in the presence of trans-zeatin, 8.11 ± 1.45 mm). This result confirms the effectiveness of the treatment with the CK under the our experimental conditions and indicates the sensitivity of the mutant to the exogenous hormone.
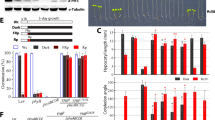
Mutations for trans-factor genes GLKs (glk1glk2) do not disturb the sensitivity of plants to cytokinin. To confirm the sensitivity of the glk1glk2 knockout mutant to the exogenous CK, we studied the dynamics of the content of transcripts of the ARR5 gene belonging to the family of cytokinin-A response regulators. A characteristic feature of this family of genes is their rapid induction in response to CK [12].
The results of real-time PCR showed an increase in the level of ARR5 gene transcripts in the wild-type and glk1glk2 knockout mutant seedlings both in the dark and during cytokinin-dependent deetiolation (Fig. 2). It should be noted that the dynamics of the content of transcripts of the studied gene in the seedlings of the wild type and the glk1glk2 mutant was similar.
Influence of cytokinin on the content of ARR5 gene transcripts in wild-type and glk1glk2 knockout mutant A. thaliana seedlings in the dark and during deetiolation. **Significant differences between the mean values of expression in seedlings grown on a nutrient medium without cytokinin vs. expression in plants treated with trans-zeatin (p ≤ 0.01).
These results led us to conclude that mutations for the GLKs trans-factor genes do not affect the perception and, possibly, transduction of the cytokinin signal and that the glk1glk2 mutant, similarly to the wild-type plants, is sensitive to the exogenous hormone.
Cytokinin regulates the expression of trans-factor genes GLK1 and GLK2 during deetiolation. Previously, Kobayashi et al. [10] demonstrated cytokinin-dependent induction of the GLK2 gene in experiments on the deetiolation of A. thaliana roots. The selection of only the GLK2 gene expression for analysis was determined by the tissue-specific expression of the genes of the GLKs family: GLK1 is expressed in only photosynthetic tissues, whereas the level of GLK1 transcription in roots is below the detection level, and GLK2 is expressed in both green and non-photosynthetic tissues (roots) [3]. However, it remains unknown whether CK induces the expression of the GLK1 gene and whether the cytokinin-dependent regulation of the GLK2 gene is retained during deetiolation of A. thaliana seedlings. Based on the data that both genes of the GLKs family are expressed in cotyledon leaves [3], we assumed that GLKs may be involved in the cytokinin-dependent regulatory cascade of gene expression during deetiolation.
The analysis of the content of GLK1 and GLK2 gene transcripts in the wild-type A. thaliana seedlings showed a light-dependent accumulation of the templates of the studied genes (Fig. 3). This induction of the content of GLK gene transcripts is consistent with the data of Fitter et al. [3].
Light and cytokinin regulation of the content of GLK1 and GLK2 gene transcripts in 4-day-old A. thaliana wild-type seedlings during deetiolation. **Significant differences between the mean values of expression in seedlings grown on a nutrient medium without cytokinin vs. expression in plants treated with trans-zeatin (p ≤ 0.01).
Under the light exposure, CK increased the content of GLK1 and GLK2 gene transcripts both in the dark and after 6 h (GLK2) or 16 h (GLK1) of deetiolation (Fig. 3). This result suggests that the trans-factors GLK1 and GLK2 may be involved in the cytokinin-dependent regulation of gene expression during deetiolation of A. thaliana seedlings.
trans-Factors GLKs regulate the cytokinin-dependent expression of the SCA3 gene encoding plastid RNA polymerase RPOTp. To determine whether GLKs family proteins are involved in the regulation of the expression of the SCA3 gene and RPOTp-dependent plastid genes, the glk1glk2 double mutant was used.
The cytokinin-dependent activation of expression of GLKs trans-factor genes allowed us to assume that these transcription factors are involved in the CK-dependent activation of expression of the RNA polymerase gene SCA3. Previously, we demonstrated the regulation of the level of SCA3 gene transcripts by the cytokinin during A. thaliana deetiolation [7]. However, the components of the molecular cascade underlying this positive effect are still unknown.
The results showed (Fig. 4a) that the illumination of etiolated plants stimulated an increase in the level of SCA3 gene transcripts in the seedlings of both the wild-type plants and the glk1glk2 knockout mutant. The absence of trans-factors GLKs changed neither the profile nor the level of transcripts in the glk1glk2 plants (Fig. 4a). In turn, in contrast to the wild-type seedlings, the treatment of the glk1glk2 seedlings with trans-zeatin did not lead to an increase in the content of SCA3 gene templates. The absence of a response to CK indicates the possible involvement of transcription factors GLK1 and/or GLK2 in the realization of the positive effect of CK on chloroplast formation during deetiolation through the activation of expression of the RNA polymerase gene SCA3.
Influence of light and cytokinin on the transcript level of the SCA3 nuclear gene encoding chloroplast RNA polymerase RPOTp (a) and RPOTp-dependent plastid genes accD (b) and clpP (c) in 4-day-old wild-type and glk1glk2 knockout mutant A. thaliana seedlings in the dark and during deetiolation. *Significant differences between the mean values of expression in the seedlings grown on a nutrient medium without cytokinin vs. expression in the plants treated with trans-zeatin at *p ≤ 0.05 and **p ≤ 0.01.
trans-Factors GLKs mediate the activation of expression of RPOTr-dependent plastid genes accD and clpP by cytokinin during deetiolation of A. thaliana. An additional confirmation of the involvement of trans-factors GLKs in the cytokinin-dependent activation of the SCA3 gene expression was the results of RT-PCR regarding the level of transcripts of RPOTp-dependent genes (namely, accD and clpP) during the cytokinin-dependent deetiolation of the glk1glk2 mutant. Both genes belong to the group of housekeeping genes: accD encodes the β-subunit of acetyl-CoA carboxylase, which is involved in the synthesis of fatty acids, and clpP encodes the catalytic subunit of the Clp protease. Since the accD gene has only the NEP promoter, the transcription of this gene is performed by the NEP polymerase. The clpP promoter contains both NEP and PEP elements, which allows this gene to be transcribed by both NEP and PEP polymerases. However, there are data on a greater contribution of the NEP polymerase to the clpP gene transcription [2].
The analysis showed that the illumination of both the wild-type and knockout mutant seedlings leads to an increase in the content of accD and clpP gene transcripts (Figs. 4b, 4c). Significant differences between the wild-type and glk1glk2 plants were observed in the dynamics of the mRNA level of the accD and clpP genes in response to CK during deetiolation: in the maternal line seedlings, exogenous CK increased the content of transcripts of the studied genes at all time points of the experiment, whereas the inactivation of the genes encoding the transcription factors GLKs in the glk1glk2 mutant led to the absence of cytokinin-dependent regulation (Figs. 4b, 4c).
The absence of a positive effect of CK on the content of transcripts of the SCA3 gene and two RPOTp-dependent genes accD and clpP in the glk1glk2 mutant confirms the involvement of the trans-factors GLK1 and/or GLK2 in the realization of the positive effect of CK on the chloroplast formation by controlling the plastid nuclear-coding RNA polymerase.
It is known that CKs have a wide spectrum of functional activity. The initial cytokine signal transduction pathway includes the cytoplasmic AHKs receptors (Arabidopsis Histidine Kinase), AHPs transmitters (Arabidopsis Histidine phosphotransfer Proteins), and 11 ARR (Arabidopsis Response Regulator) type B trans-factors [13]. Despite the fact that type B trans-factors have 4000 to 8000 direct binding sites for nuclear gene promoters [14], numerous transcriptomic studies indicate a much larger cluster of cytokinin-regulated genes, which suggests the involvement of trans-factors of a higher order in the hormone-dependent expression.
The latter include three families of GATA trans-factors of nuclear localization: GNC (GATA Nitrate-inducible Carbon-metabolism-involved), GNL/CGA1 (GNC-Like/Cytokinin-responsive GATA factor 1), and GLKs (Golden two-Like) [2, 15]. These regulatory proteins mediate the action of CK on chloroplasts; moreover, the expression of their coding genes is positively regulated by CK [3, 16]. In addition to being involved in the CK-dependent regulation of nuclear gene expression, GNCs and GLKs are involved in the control of biogenesis and division of chloroplast; however, the molecular mechanism of this effect is different. The GNC factor suppresses the transcription of the genes for negative photomorphogenesis regulators PIFs, as well as the genes encoding biosynthesis enzymes and trans-factors brassinosteroids, thereby initiating photomorphogenesis. Conversely, GLKs activate the transcription of the genes encoding proteins of light-harvesting complexes and enzymes involved in chlorophyll biosynthesis. Our results for the first time showed the involvement of transcription factors GLKs in the cytokinin-dependent activation of SCA3 gene expression, which significantly deepens the understanding of the mechanisms of regulation of chloroplast biogenesis by the GLKs trans-factors.
Another light- and cytokinin-dependent regulator of chloroplast biogenesis is the HY5 trans-factor. In the studies on the deetiolation of A. thaliana roots, Kobayashi et al. [10] showed the interdependence of the effects of HY5 and GLKs factors. This is confirmed by the fact that the expression of both genes is suppressed by auxin and activated by CK. Overexpression of the GLK1 and GLK2 genes leads to an increase in the level of the HY5 protein and the light harvesting complex protein LHCP. Crossing the hy5-215 mutant with a plant overexpressing GLK1ox or GLK2ox compensates to some extent, but does not completely restores, the pale green hy5-215 phenotype, suggesting that the functionally active HY5 is required for the functioning of GLKs trans-factors. In addition, we have previously shown [17] that the cytokinin-dependent expression of the SCA3 gene during deetiolation is mediated by the HY5 trans-factor, and in this study we showed the dependence of the RPOTp gene expression on GLKs factors. Apparently, GLKs and HY5 proteins are elements of the same transcriptional cascade that ensures the regulation of the expression of the SCA3 gene encoding plastid RNA polymerase RPOTp during the cytokinin-dependent deetiolation.
REFERENCES
Kusnetsov, V.V., Doroshenko, A.S., Kudryakova, N.V., et al., Role of phytohormones and light in de-etiolation, Russ. J. Plant Physiol., 2020, vol. 67, pp. 971–984.
Börner, T., Aleynikova, A., Zubo, Y., et al., Chloroplast RNA polymerases: Role in chloroplast biogenesis, Biochim. Biophys. Acta—Bioenergetics, 2015, vol. 1847, pp. 761–769.
Fitter, D., Martin, D., Copley, M., et al., GLK gene pairs regulate chloroplast development in diverse plant species, Plant J., 2002, vol. 31, pp. 713–727.
Zubo, Y., Blakley, I., Franco-Zorrilla, J., et al., Coordination of chloroplast development through the action of the GNC and GLK transcription factor families, Plant Physiol., 2018, vol. 178, pp. 130–147.
Cortleven, A. and Schmülling, Th., Regulation of chloroplast development and function by cytokinin, J. Exp. Bot., 2015, vol. 66, pp. 4999–5013.
Cortleven, A., Marg, I., Yamburenko, M., et al., Cytokinin regulates the etioplast-chloroplast transition through the two-component signaling system and activation of chloroplast-related genes, Plant Physiol., 2016, vol. 172, pp. 464–478.
Danilova, M., Doroshenko, A., Kudryakova, N., et al., Plastome transcription machinery and peculiarities of the expression of its genes during cytokinin-dependent deetiolation of Arabidopsis thaliana, Russ. J. Plant Physiol., 2018, vol. 65, pp. 801–812.
Vandenbussche, F., Habricot, Y., Condiff, A., et al., HY5 is a point of convergence between cryptochrome and cytokinin signaling pathways in Arabidopsis thaliana, Plant J., 2007, vol. 49, pp. 428–441.
Doroshenko, A., Danilova, M., Medvedeva, A., et al., Influence of blue-light signaling components on the regulation of cytokinin-dependent Arabidopsis thaliana seedlings’ greening, Russ. J. Plant Physiol., 2019, vol. 66, pp. 864–871.
Kobayashi, K., Baba, S., Obayashi, T., et al., Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis, Plant Cell, 2012, vol. 24, pp. 1081–1095.
Chory, J., Reinecke, D., Sim, S., et al., A role for cytokinins in de-etiolation in Arabidopsis, Plant Physiol., 1994, vol. 104, pp. 339–347. https://doi.org/10.1104/pp.104.2.339
D’Agostino, I., Deruere, J., and Kieber, J., Characterization of the response of the Arabidopsis response regulator gene family to cytokinin, Plant Physiol., 2000, vol. 124, pp. 1706–1717.
Kieber, J.J. and Schaller, G.E., Cytokinin signaling in plant development, Development, 2018, vol. 145, p. dev149344.
Xie, M., Chen, H., Huang, L., et al., A B-ARR-mediated cytokinin transcriptional network directs hormone cross-regulation and shoot development, Nat. Commun., 2018, vol. 9, p. 1604.
Waters, M.T., Wang, P., Korkaric, M., et al., GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis, Plant Cell, 2009, vol. 21, pp. 1109–1128.
Chiang, Y., Zubo, Y., Tapken, W., et al., Functional characterization of the GATA transcription factors GNC and CGA1 reveals their key role in chloroplast development, growth, and division in Arabidopsis, Plant Physiol., 2012, vol. 160, pp. 332–348.
Doroshenko, A.S. and Danilova, M.N., Involvement of blue light signaling components in the regulation of gene expression of the plastome transcription apparatus in cytokinin-dependent deetiolation of A. thaliana, Sbornik materialov Godichnogo sobraniya Obshchestva fiziologov rastenii Rossii “Mekhanizmy ustoichivosti rastenii i mikroorganizmov k neblagopriyatnym usloviyam sredy,” Irkutsk, 10–15 iyulya 2018 g. (Proceedings of Annual Meeting of the Society of Plant Physiologists of Russia “Mechanisms of Resistance of Plants and Microorganisms to Adverse Environmental Conditions,” Irkutsk, July 10–15, 2018), Irkutsk: Inst. Geogr. im. V.B. Sochavy Sib. Otd. Ross. Akad. Nauk, 2018, part 2, pp. 908–912.
Funding
This work was financially supported by the Russian Foundation for Basic Research (project nos. 19-34-90183 and 20-04-00294) and the Ministry of Science and Higher Education of the Russian Federation (topic no. 121040800153-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doroshenko, A.S., Malyukova, A.M., Danilova, M.N. et al. Transcription Factors of the GLRs Family Are Involved in Cytokinin-Dependent Regulation of Plastid RNA Polymerase SCA3 Gene Expression during Deetiolation of Arabidopsis thaliana. Dokl Biochem Biophys 506, 195–201 (2022). https://doi.org/10.1134/S1607672922050040
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1607672922050040