Abstract
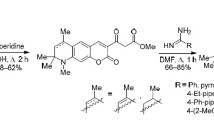
The reaction of pyrrolo[3,2,1-ij]quinoline-1,2-diones with methyl (het)aryl ketones gave the corresponding 1-(het)arylmethylidenepyrroloquinolin-2-ones, and 1,3-dipolar cycloaddition of the latter to azomethine ylide generated from sarcosine and paraformaldehyde afforded 4-acyl-1,4′,4′,6′-tetramethylspiro[pyrrolidine-3,1′-pyrrolo[3,2,1-ij]quinolin]-2′(4′H)-ones. The synthesized compounds were evaluated for inhibitory activity against blood coagulation factors Xa and XIa.



Similar content being viewed by others
REFERENCES
Zbinden, K.G., Anselm, L., Banner, D.W., Benz, J.,Blasco, F, Décoret, J., Himber, J., Kuhn, B., Panday, N., Ricklin, F., Risch, Ph., Schlatter, D., Stahl, M., Thomi, S., Unger, R., and Haap, W., Eur. J. Med. Chem., 2009, vol. 44, p. 2787. https://doi.org/10.1016/j.ejmech.2008.12.025
Anselm, L., Banner, D.W., Benz, J., Zbinden, K.G., Himber, J., Hilpert, H., Huber, W., Kuhn, B., Mary, J.-L., Otteneder, M.B., Panday, N., Ricklin, F., Stahl, M., Thomi, S., and Haap, W., Bioorg. Med. Chem. Lett., 2010, vol. 20, p. 5313. https://doi.org/10.1016/j.bmcl.2010.06.126
Pinto, D.J.P., Smallheer, J.M., Cheney, D.L., Knabb, R.M., and Wexler, R.R., J. Med. Chem., 2010, vol. 53, p. 6243. https://doi.org/10.1021/jm100146h
Trstenjak, U., Ilaš, J., and Kikelj, D., Med. Chem. Commun., 2014, vol. 5, p. 197. https://doi.org/10.1039/c3md00250k
Vavilova, T.V., Kardiologiya, 2019, vol. 59, p. 28. https://doi.org/10.18087/cardio.n951
Podoplelova, N.A., Sulimov, V.B., Ilin, I.S., Tashilova, A.S., Panteleev, M.A., Ledeneva, I.V., and Shikhaliev, Kh.S., Pediatr. Hematol. Oncol. Immunopathol., 2020, vol. 19, p. 139. https://doi.org/10.24287/1726-1708-2020-19-139-157
Santana-Romo, F., Lagos, C.F., Duarte, Y., Castillo, F., Moglie, Y., Maestro, M.A., Charbe, N., and Zacconi, F.C., Molecules, 2020, vol. 25, p. 491. https://doi.org/10.3390/molecules25030491
Kabankin, A.S., Sinauridze, E.I., Lipets, E.N., and Ataullakhanov, F.I., Biochemistry (Moscow), 2019, vol. 84, p. 119. https://doi.org/10.1134/S0006297919020032
Ilin, I., Lipets, E., Sulimov, A., Kutov, D., Shikhaliev, Kh., Potapov, A., Krysin, M., Zubkov, F., Sapronova, L., Ataullakhanov, F., and Sulimov, V., J. Mol. Graphics Modell., 2019, vol. 89, p. 215. https://doi.org/10.1016/j.jmgm.2019.03.017
Sulimov, V.B., Gribkova, I.V., Kochugaeva, M.P., Katkova, E.V., Sulimov, A.V., Kutov, D.C., Shikhaliev, Kh.S., Medvedeva, S.M., Krysin, M.Yu., Sinauridze, E.I., and Ataullakhanov, F.I., BioMed Res. Int., 2015, vol. 2015, article ID 120802. https://doi.org/10.1155/2015/120802
Medvedeva, S.M., Potapov, A.Yu., Gribkova, I.V., Katkova, E.V., Sulimov, V.B., and Shikhaliev, Kh.S., Pharm. Chem. J., 2018, vol. 51, p. 975. https://doi.org/10.1007/s11094-018-1726-4
Novichikhina, N.P., Shestakov, A.S., Potapov, A.Yu., Kosheleva, E.A., Shatalov, G.V., Verezhnikov, V.N., Vandyshev, D.Yu., Ledeneva, I.V., and Shikhaliev, Kh.S., Russ. Chem. Bull., Int. Ed., 2020, vol. 69, p. 787. https://doi.org/10.1007/s11172-020-2834-3
Gao, F., Ye, L., Wang, Y., Kong, F., Zhao, Sh., Xiao, J., and Huang, G., Eur. J. Med. Chem., 2019, vol. 183, article ID 111678. https://doi.org/10.1016/j.ejmech.2019.111678
Leshcheva, E.V., Shikhaliev, Kh.S., Shatalov, G.V., and Ermolova, G.I., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2003, vol. 46, p. 105.
Medvedeva, S.M., Sabynin, A.L., and Shikhaliev, Kh.S., Russ. Chem. Bull., Int. Ed., 2014, vol. 63, p. 2693. https://doi.org/10.1007/s11172-014-0801-6
Vahedi, H., Baradarani, M.M., Rashidi, A., and Joule, J.A., J. Heterocycl. Chem., 2015, vol. 52, p. 1208. https://doi.org/10.1002/jhet.2237
Mazaheri, F., Saatluo, B.E., Baradarani, M.M., and Joule, J.A., J. Heterocycl. Chem., 2017, vol. 54, p. 147. https://doi.org/10.1002/jhet.2555
Gupta, A.K., Bharadwaj, M., and Mehrotra, R., J. Heterocycl. Chem., 2019, vol. 56, p. 703. https://doi.org/10.1002/jhet.3424
Santos, M.M.M., Tetrahedron, 2014, vol. 70, p. 9735. https://doi.org/10.1016/j.tet.2014.08.005
Xia, M. and Ma, R.-Zh., J. Heterocycl. Chem., 2014, vol. 51, p. 539. https://doi.org/10.1002/jhet.1114
Gupta, A.K., Bharadwaj, M., Kumar, A., and Mehrotra, R., Top. Curr. Chem., 2017, vol. 375, p. 3. https://doi.org/10.1007/s41061-016-0089-0
Leoni, A., Locatelli, A., Morigi, R., and Rambaldi, M., Expert. Opin. Ther. Pat., 2016, vol. 26, p. 149. https://doi.org/10.1517/13543776.2016.1118059
Prakash, C.R. and Raja, S., Mini-Rev. Med. Chem., 2012, vol. 12, p. 98. https://doi.org/10.2174/138955712798995039
Dandia, A., Jain, A.K., Laxkar, A.K., and Bhati, D.S., Tetrahedron, 2013, vol. 69, p. 2062. https://doi.org/10.1016/j.tet.2012.12.021
Shaabanzadeha, M. and Khabarib, F., J. Heterocycl. Chem., 2010, vol. 47, p. 949. https://doi.org/10.1002/jhet.394
Singh, D., Fatma, Sh., Ankit, P., Mishra, P., Singh, S., Singh, Sh.B., and Singh, J., Synth. Commun., 2013, vol. 43, p. 3072. https://doi.org/10.1080/00397911.2013.769604
Edeson, S.J., Jiang, J., Swanson, S., Procopiou, P.A., Adams, H., Meijer, A.J.H.M., and Harrity, J.P.A., Org. Biomol. Chem., 2014, vol. 12, p. 3201. https://doi.org/10.1039/c4ob00496e
Gangarapu, K., Thumma, G., Manda, S., Jallapally, A., Jarapula, R., and Rekulapally, S., Med. Chem. Res., 2017, vol. 26, p. 819. https://doi.org/10.1007/s00044-017-1781-5
Nikoofar, K. and Peyrovebaghi, S.S., J. Chin. Chem. Soc., 2020, vol. 67, p. 1303. https://doi.org/10.1002/jccs.201900365
Pandey, G., Banerjee, P., and Gadre, S.R., Chem. Rev., 2006, vol. 106, p. 4484. https://doi.org/10.1021/cr050011g
Tsuge, O., Kanemasa, Sh., Ohe, M., and Takenaka, Sh., Bull. Chem. Soc. Jpn., 1987, vol. 60, p. 4079. https://doi.org/10.1246/bcsj.60.4079
Shvets, A.A. and Kurbatov, S.V., Chem. Heterocycl. Compd., 2009, vol. 45, p. 866. https://doi.org/10.1007/s10593-009-0344-1
Huang, Y., Min, W., Wu, Q.-W., Sun, J., Shi, D.-H., and Yan, Ch.-G., New J. Chem., 2018, vol. 42, p. 16211. https://doi.org/10.1039/c8nj03813a
Lescheva, E.V., Medvedeva, S.M., and Shikhaliev, Kh.S., J. Org. Pharm. Chem., 2014, vol. 12, p. 15. https://doi.org/10.24959/ophcj.14.798
Funding
This study was performed under financial support by the Russian Science Foundation (project no. 18-74-10097).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Novichikhina, N.P., Skoptsova, A.A., Shestakov, A.S. et al. Synthesis and Anticoagulant Activity of New Ethylidene and Spiro Derivatives of Pyrrolo[3,2,1-ij]quinolin-2-ones. Russ J Org Chem 56, 1550–1556 (2020). https://doi.org/10.1134/S1070428020090080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020090080