Abstract
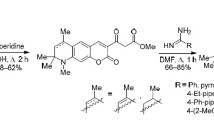
Condensation of 7-hydroxy-1,2,2,4-tetramethylhydroquinoline-6-carbaldehydes with various hetaryl-substituted 3-oxo esters afforded a series of hybrid hydro-6,8,8,9-tetramethyl-2H-pyrano[3,2-g]quinolin-2-ones. A number of these compounds exhibited relatively high inhibitory activity against blood coagulation factors Xa and XIa.
Similar content being viewed by others
References
Y. Abdulsattar, R. Bhambri, A. Nogid, Pharmacy Therapeutics, 2009, 34, 238.
C. Frost, J. Wang, S. Nepal, A. Schuster, Y. C. Barrett, R. Mosqueda-Garcia, R. A. Reeves, F. LaCreta, Br. J. Clin. Pharmacol., 2013, 75, 476; DOI: https://doi.org/10.1111/j.1365-2125.2012.04369.x.
T. Furugohri, K. Isobe, Y. Honda, C. Kamisato-Matsumoto, N. Sugiyama, T. Nagahara, Y. Morishima, T. Shibano, J. Thromb. Haemostasis, 2008, 6, 1542; DOI: https://doi.org/10.1111/j.1538-7836.2008.03064.x.
D. Ostrovsky, M. Udier-Blagovic, W. L. Jorgensen, J. Med. Chem., 2003, 46, 5691; DOI: https://doi.org/10.1021/jm030288d.
A. G. G. Turpie, Arterioscler. Thromb., Vasc. Biol., 2007, 27, 1238; DOI: https://doi.org/10.1161/ATVBAHA.107.139402.
J. M. Walenga, W. P. Jeske, D. Hoppensteadt, J. Fareed, Curr. Opin. Invest. Drugs, 2003, 4, 272.
K. A. Bauer, J. Thromb. Thrombolysis, 2006, 21, 67; DOI: https://doi.org/10.1007/s11239-006-5579-4.
M. L. Quan, P. C. Wong, C. Wang, F. Woerner, J. M. Smallheer, F. A. Barbera, J. M. Bozarth, R. L. Brown, M. R. Harpel, J. M. Luettgen, P. E. Morin, T. Peterson, V. Ramamurthy, A. R. Rendina, K. A. Rossi, C. A. Watson, A. Wei, G. Zhang, D. Seiffert, R. R. Wexler, J. Med. Chem., 2014, 57, 955; DOI: https://doi.org/10.1021/jm401670x.
P. C. Wong, M. L. Quan, C. A. Watson, E. J. Crain, M. R. Harpel, A. R. Rendina, J. M. Luettgen, R. R. Wexler, W. A. Schumacher, D. A. Seiffert, J. Thromb. Thrombolysis, 2015, 40, 416; DOI: https://doi.org/10.1007/s11239-015-1258-7.
D. J. P. Pinto, M. J. Orwat, L. M. Smith, M. L. Quan, P. Y. S. Lam, K. A. Rossi, A. Apedo, J. M. Bozarth, Y. Wu, J. J. Zheng, B. Xin, N. Toussaint, P. Stetsko, O. Gudmundsson, B. Maxwell, E. J. Crain, P. C. Wong, Z. Lou, T. W. Harper, S. A. Chacko, J. E. Myers, Jr., S. Sheriff, H. Zhang, X. Hou, A. Mathur, D. A. Seiffert, R. R. Wexler, J. M. Luettgen, W. R. Ewing, J. Med. Chem., 2017, 60, 9703; DOI: https://doi.org/10.1021/acs.jmedchem.7b01171.
D. J. P. Pinto, J. M. Smallheer, J. R. Corte, E. J. D. Austin, C. Wang, T. Fang, L. M. Smith II, K. A. Rossi, A. R. Rendina, J. M. Bozarth, G. Zhang, A. Wei, V. Ramamurthy, S. Sheriff, J. E. Myers, Jr., P. E. Morin, J. M. Luettgen, D. A. Seiffert, M. L. Quan, R. R. Wexler, Bioorg. Med. Chem. Lett., 2015, 25, 1635; DOI: https://doi.org/10.1016/j.bmcl.2015.01.028.
Z. Hu, C. Wang, W. Han, K. A. Rossi, J. M. Bozarth, Y. Wu, S. Sheriff, J. E. Myers, Jr., J. M. Luettgen, D. A. Seiffert, R. R. Wexler, M. L. Quan, Bioorg. Med. Chem. Lett., 2018, 28, 987; DOI: https://doi.org/10.1016/j.bmcl.2018.02.049.
J. R. Corte, T. Fang, D. J. P. Pinto, M. J. Orwat, A. R. Rendina, J. M. Luettgen, K. A. Rossi, A. Wei, V. Ramamurthy, J. E. Myers, Jr., S. Sheriff, R. Narayanan, T. W. Harper, J. J. Zheng, Y.-X. Li, D. A. Seiffert, R. R. Wexler, M. L. Quan, Bioorg. Med. Chem., 2016, 24, 2257; DOI: https://doi.org/10.1016/j.bmc.2016.03.062.
A. J. Obaidullah, R. A. Al-Horani, Cardiovasc. Hematol. Agents Med. Chem., 2017, 15, 40; DOI: https://doi.org/10.2174/1871525715666170529093938.
M. L. Quan, D. J. P. Pinto, J. M. Smallheer, W. R. Ewing, K. A. Rossi, J. M. Luettgen, D. A. Seiffert, R. R. Wexler, J. Med. Chem., 2018; 61, 7425; DOI: https://doi.org/10.1021/acs.jmedchem.8b00173.
S. Maignan, V. Mikol, Curr. Top. Med. Chem., 2001, 1, 161; DOI: https://doi.org/10.2174/1568026013395461.
S. Roehrig, A. Straub, J. Pohlmann, T. Lampe, J. Pernerstorfer, K. H. Schlemmer, P. Reinemer, E. Perzborn, J. Med. Chem., 2005, 48, 5900; DOI: https://doi.org/10.1021/jm050101d.
K. M. Amin, N. M. A. Gawad, D. E. A. Rahman, M. K. E. Ashry, Bioorg. Chem., 2014, 52, 31; DOI: https://doi.org/10.1016/j.bioorg.2013.11.002.
S. Verespy III, A. Y. Mehta, D. Afosah, R. A. Al-Horani, U. R. Desai, Sci. Rep., 2016, 6, 24043, DOI: https://doi.org/10.1038/srep24043.
M. S. Buchanan, A. R. Carroll, D. Wessling, M. Jobling, V. M. Avery, R. A. Davis, Y. Feng, Y. Xue, L. Öster, T. Fex, J. Deinum, J. N. A. Hooper, R. J. Quinn, J. Med. Chem., 2008, 51, 3583; DOI: https://doi.org/10.1021/jm800314b.
O. Fjellström, S. Akkaya, H. G. Beisel, P. O. Eriksson, K. Erixon, D. Gustafsson, U. Jurva, D. Kang, D. Karis, W. Knecht, V. Nerme, I. Nilsson, T. Olsson, A. Redzic, R. Roth, J. Sandmark, A. Tigerstrom, L. Oster, PLOS One, 2015, 10, e0113705; DOI: https://doi.org/10.1371/journal.pone.0113705.
F. Santana-Romo, C. F. Lagos, Y. Duarte, F. Castillo, Y. Moglie, M. A. Maestro, N. Charbe, F. C. Zacconi, Molecules, 2020, 25, 491; DOI: https://doi.org/10.3390/molecules25030491.
G. Wissel, P. Kudryavtsev, L. Ghemtio, P. Tammela, P. Wipf, M. Yliperttula, M. Finel, A. Urtti, H. Kidron, H. Xhaard, Bioorg. Med. Chem., 2015, 23, 3513; DOI: https://doi.org/10.1016/j.bmc.2015.04.029.
F. Gao, L. Ye, Y. Wang, F. Kong, Sh. Zhao, J. Xiao, G. Huang, Eur. J. Med. Chem., 2019, 183, 111678; DOI: https://doi.org/10.1016/j.ejmech.2019.111678.
V. Kartsev, Kh. S. Shikhaliev, A. Geronikaki, S. M. Medvedeva, I. V. Ledenyova, M. Yu. Krysin, A. Petrou, A. Ciric, J. Glamoclija, M. Sokovic, Eur. J. Med. Chem., 2019, 175, 201; DOI: https://doi.org/10.1016/j.ejmech.2019.04.046.
J. Gao, Z. Zhanga, B. Zhanga, Q. Maoa, X. Daia, Q. Zoub, Y. Leia, Y. Fenga, S. Wang, Bioorg. Chem., 2020, 95, 103564; DOI: https://doi.org/10.1016/j.bioorg.2019.103564.
N. Novichikhina, I. Ilin, A. Tashchilova, A. Sulimov, D. Kutov, I. Ledenyova, M. Krysin, Kh. Shikhaliev, A. Gantseva, E. Gantseva, N. Podoplelova, V. Sulimov, Molecules, 2020, 25, 1889; DOI: https://doi.org/10.3390/molecules25081889.
A. Djemoui, A. Naouri, M. R. Ouahrani, D. Djemoui, S. Lahcene, M. B. Lahrech, L. Boukenna, H. M. T. Albuquerque, L. Saher, D. H. A. Rocha, F. L. Monteiro, L. A. Helguero, K. Bachari, O. Talhi, A. M. S. Silva, J. Mol. Struct., 2020, 1204, 127487; DOI: https://doi.org/10.1016/j.molstruc.2019.127487.
N. P. Novichikhina, A. S. Shestakov, A. Yu. Potapov, E. A. Kosheleva, G. V. Shatalov, V. N. Verezhnikov, D. Yu. Vandyshev, I. V. Ledeneva, Kh. S. Shikhaliev, Russ. Chem. Bull., 2020, 69, 787; DOI: https://doi.org/10.1007/s11172-020-2834-3.
J. Shi, N. Luo, M. Ding, X. Bao, Chin. Chem. Lett., 2020, 31, 434; DOI: https://doi.org/10.1016/j.cclet.2019.06.037.
S. Li, D. Cao, Z. Hu, Z. Li, X. Meng, X. Han, W. Ma, Chem. Heterocycl. Compd., 2020, 56, 219; DOI: https://doi.org/10.1007/s10593-020-02647-y.
G. Sakaine, G. Smits, P. Arsenyan, Chem. Heterocycl. Compd., 2020, 56, 572; DOI: https://doi.org/10.1007/s10593-020-02702-8.
D. Khan, S. Mukhtar, M. A. Alsharif, M. I. Alahmdi, N. Ahmed, Tetrahedron Lett., 2017, 58, 3183; DOI: https://doi.org/10.1016/j.tetlet.2017.07.018.
C. Ranjith, N. Paul, K. K. Vijayan, Asian J. Chem., 2011, 23, 235.
P. Verdia, F. Santamarta, E. Tojo, Molecules, 2011, 16, 4379; DOI: https://doi.org/10.3390/molecules16064379.
X. He, Y. Shang, Y. Zhou, Zh. Yu, G. Han, W. Jin, J. Chen, Tetrahedron, 2015, 71, 863; DOI: https://doi.org/10.1016/j.tet.2014.12.042.
A. Yu. Potapov, D. Yu. Vandyshev, Y. Refki, I. V. Ledenyova, O. V. Ovchinnikov, M. S. Smirnov, Kh. S. Shikhaliev, Russ. J. Gen. Chem., 2020, 90, 1216; DOI: https://doi.org/10.1134/S1070363220070075.
G. M. Manahelohe, A. Yu. Potapov, Kh. S. Shikhaliev, Russ. Chem. Bull., 2016, 65, 1145; DOI: https://doi.org/10.1007/s11172-016-1427-7.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the Russian Science Foundation (Project No. 18-74-10097).
This work does not involve human participants and animal subjects.
The authors declare no competing interests.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 492–497, March, 2021.
Rights and permissions
About this article
Cite this article
Potapov, A.Y., Paponov, B.V., Podoplelova, N.A. et al. Synthesis and study of new 2H-pyranoquinolin-2-one-based inhibitors of blood coagulation factors Xa and XIa. Russ Chem Bull 70, 492–497 (2021). https://doi.org/10.1007/s11172-021-3114-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3114-6