Abstract
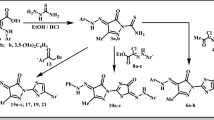
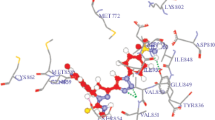
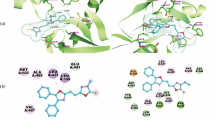
Cancer, which is considered to be the world’s most serious illness cause 8.2 million deaths and this rate may double by 2030. We herein report a new series of 3-(2-(p-substituted)-2-((5-phenyl-1,3,4-thiadiazol-2-yl)imino)-2-(p-substituted)ethylidene)indolin-2-one (15–19) and 5-substituted-5′-substituted phenyl-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one derivatives (20–24) as potent anticancer agents. These compounds were evaluated for in vitro antitumor activity against the National Cancer Institute panel of 60 cancer cell lines. Among all the synthesized compounds, two compounds 15 and 16 showed remarkable antitumor activity with GI50 (MG-MID) values of 0.65 & 0.72 µM, respectively against Non-small cell lung cancer. To gain insight for mode of binding with Epidermal Growth Factor Receptor kinase enzyme, these compounds were further subjected to docking studies.






Similar content being viewed by others
References
Abraham J (2014) Paving the way for biosimilars in oncology, Part 2: Focus on safety and clinical trial considerations. Semin Oncol, 41:S1–S2
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR (1988) Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 48(3):589–601
Altıntop MD, Özdemir A, Turan-Zitouni G, Ilgın S, Atlı Ö, Demirel R, Kaplancıklı ZA (2015) A novel series of thiazolyl–pyrazoline derivatives: synthesis and evaluation of antifungal activity, cytotoxicity and genotoxicity. Eur J Med Chem 92:342–352
Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Varoli L, Calonghi N, Cappadone C (2008) Antitumor activity of new substituted 3-(5-Imidazo [2, 1-b] thiazolylmethylene)-2-indolinones and 3-(5-imidazo [2, 1-b] thiadiazolylmethylene)-2-indolinones: selectivity against colon tumor cells and effect on cell cycle-related events (1). J Med Chem 51(23):7508–7513
Bari S, Mandab S, Ugalec V, Jupallyb VR, Akenab V (2015) Rational design and synthesis of benzothiazolo-isatins for antimicrobial and cytotoxic activities. Indian J Chem B 54(3):418–429
Boyle P, Levin B (2008) World cancer report 2008. IARC Press, International Agency for Research on Cancer, Lyon, France
Bursavich MG, Gilbert AM, Lombardi S, Georgiadis KE, Reifenberg E, Flannery CR, Morris EA (2007) 5′-Phenyl-3′H-spiro[indoline-3,2′-[1,3,4]thiadiazol]-2-one inhibitors of ADAMTS-5 (Aggrecanase-2). Bioorg Med Chem Lett 17(20):5630–5633. doi:10.1016/j.bmcl.2007.07.048
Cane A, Tournaire M-C, Barritault D, Crumeyrolle-Arias M (2000) The endogenous oxindoles 5-hydroxyoxindole and isatin are antiproliferative and proapoptotic. Biochem Biophys Res Commun 276(1):379–384
Cao J, Gao H, Bemis G, Salituro F, Ledeboer M, Harrington E, Wilke S, Taslimi P, Pazhanisamy S, Xie X (2009) Structure-based design and parallel synthesis of N-benzyl isatin oximes as JNK3 MAP kinase inhibitors. Bioorg Med Chem Lett 19(10):2891–2895
Chapman JG, Magee WP, Stukenbrok HA, Beckius GE, Milici AJ, Tracey WR (2002) A novel nonpeptidic caspase-3/7 inhibitor,(S)-(+)-5-[1-(2-methoxymethylpyrrolidinyl) sulfonyl] isatin reduces myocardial ischemic injury. Eur J Pharmacol 456(1):59–68
Chen M, Lin S, Li L, Zhu C, Wang X, Wang Y, Jiang B, Wang S, Li Y, Jiang J (2012) Enantiomers of an indole alkaloid containing unusual dihydrothiopyran and 1, 2, 4-thiadiazole rings from the root of Isatis indigotica. Org Lett 14(22):5668–5671
Dandia A, Saini D, Bhaskaran S, Saini DK (2014) Ultrasound promoted green synthesis of spiro [pyrano [2, 3-c] pyrazoles] as antioxidant agents. Med Chem Res 23(2):725–734
Eldehna WM, Altoukhy A, Mahrous H, Abdel-Aziz HA (2015) Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential anti-proliferative agents. Eur J Med Chem 90:684–694
Fall Y, Barreiro C, Fernández C, Mouriño A (2002) Vitamin D heterocyclic analogues. Part 1: A stereoselective route to CD systems with pyrazole rings in their side chains. Tetrahedron Lett 43(8):1433–1436. doi:10.1016/S0040-4039(02)00031-X
Fares M, Eldehna WM, Abou‐Seri SM, Abdel‐Aziz HA, Aly MH, Tolba MF (2015) Design, synthesis and in vitro antiproliferative activity of novel isatin‐quinazoline hybrids. Arch Pharm (Weinheim) 348(2):144–154
Garcia M, Jemal A, Ward E, Center M, Hao Y, Siegel R, Thun M (2007) Global cancer facts & figures 2007. American cancer society, Atlanta, GA, p 52. 1 (3)
Grever MR, Schepartz SA, Chabner BA (1992) The National Cancer Institute: cancer drug discovery and development program. In: Seminars in oncology, 1992. vol 6. WB SAUNDERS CO INDEPENDENCE SQUARE WEST CURTIS CENTER, STE 300, PHILADELPHIA, PA 19106-3399, pp 622-638
Havrylyuk D, Zimenkovsky B, Lesyk R (2015) Synthesis, biological activity of thiazolidinones bearing indoline moiety and isatin based hybrids. Mini Rev Org Chem 12(1):66–87
Havrylyuk D, Zimenkovsky B, Vasylenko O, Gzella A, Lesyk R (2012) Synthesis of new 4-thiazolidinone-, pyrazoline-, and isatin-based conjugates with promising antitumor activity. J Med Chem 55(20):8630–8641
Ibrahim HS, Abou-Seri SM, Tanc M, Elaasser MM, Abdel-Aziz HA, Supuran CT (2015) Isatin-pyrazole benzenesulfonamide hybrids potently inhibit tumor-associated carbonic anhydrase isoforms IX and XII. Eur J Med Chem 103:583–593
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Karthikeyan C, SH Narayana Moorthy N, Ramasamy S, Vanam U, Manivannan E, Karunagaran D, Trivedi P (2015) Advances in chalcones with anticancer activities. Recent Pat Anticancer Drug Discov 10(1):97–115
Lesyk R, Havrylyuk D, Lelyukh M (2015) Synthesis and anticancer activity of isatin, oxadiazole and 4-thiazolidinone based conjugates, Chem & Chem Technol 9(1):29–36
Liang C, Xia J, Lei D, Li X, Yao Q, Gao J (2014) Synthesis, in vitro and in vivo antitumor activity of symmetrical bis-Schiff base derivatives of isatin. Eur J Med Chem 74:742–750
Medvedev A, Buneeva O, Glover V (2007) Biological targets for isatin and its analogues: implications for therapy. Biol Targets Ther 1(2):151
Mohammadizadeh MR (2006) One-pot rapid and efficient synthesis of new spiro derivatives of 11H-indeno [1, 2-b] quinoxalin-11-one, 6H-indeno [1, 2-b] pyrido [3, 2-e] pyrazin-6-one and isatin-based 2-pyrazolines. Arkivoc 11:47–58
Monteiro Â, Gonçalves LM, Santos MM (2014) Synthesis of novel spiropyrazoline oxindoles and evaluation of cytotoxicity in cancer cell lines. Eur J Med Chem 79:266–272
Nikalje APG, Shaikh SI, Khan FAK, Shaikh S, Sangshetti JN (2015) Molecular sieves promoted, ultrasound-mediated synthesis, biological evaluation and docking study of 3-(5-substituted-1, 3, 4-thiadiazol-2-ylimino) indolin-2-ones as a potential anticonvulsant agents. Med Chem Res 24(12):4058–4069
Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L (2004) EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America 101(36):13306–13311
Reddy CN, Nayak VL, Mani GS, Kapure JS, Adiyala PR, Maurya RA, Kamal A (2015) Synthesis and biological evaluation of spiro [cyclopropane-1, 3′-indolin]-2′-ones as potential anticancer agents. Bioorg Med Chem Lett 25(20):4580–4586
Rekulapally S, Jarapula R, Gangarapu K, Manda S, Vaidya JR (2015) In silico and in vitro studies of novel 7-azaindole and 7-azaisatin derivatives as potent anticancer agents. Med Chem Res 24(9):3412–3422
Ribeiro CJ, Amaral JD, Rodrigues CM, Moreira R, Santos MM (2016) Spirooxadiazoline oxindoles with promising in vitro antitumor activities. Med Chem Comm 7(3):420–425
Schmidt A, Dreger A (2011) Recent advances in the chemistry of pyrazoles. Properties, biological activities, and syntheses. Curr Org Chem 15(9):1423–1463
Shaaban MR, Mayhoub AS, Farag AM (2012) Recent advances in the therapeutic applications of pyrazolines. Expert Opin Ther Pat 22(3):253–291
Shih M-H, Xu Y-Y, Yang Y-S, Lin G-L (2015) A facile synthesis and antimicrobial activity evaluation of sydnonyl-substituted thiazolidine derivatives. Molecules 20(4):6520–6532
Shoemaker RH (2006) The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6(10):813–823
Singh S, Bharti N, Mohapatra PP (2009) Chemistry and biology of synthetic and naturally occurring antiamoebic agents†. Chem Rev 109(5):1900–1947
Socca EAR, Luiz-Ferreira A, de Faria FM, de Almeida AC, Dunder RJ, Manzo LP, Brito ARMS (2014) Inhibition of tumor necrosis factor-alpha and cyclooxigenase-2 by isatin: a molecular mechanism of protection against TNBS-induced colitis in rats. Chem Biol Interact 209:48–55
Thadhaney B, Sain D, Pemawat G, Talesara G (2010) Synthesis and antimicrobial evaluation of ethoxyphthalimide derivatized spiro [indole-3, 5’-(1, 3) thiazolo (4, 5-c) isoxazol]-2 (1H)-ones via ring closure metathesis. Indian J Chem B 49(3):368
Thi Lan Huong T, Thi Mai Dung D, Thi Kim Oanh D, Thi Bich Lan T, Thi Phuong Dung P, Loi VD, Woo Han B, Yun J, Soon Kang J, Kim Y (2015) 5-Aryl-1, 3, 4-thiadiazole-based hydroxamic acids as histone deacetylase inhibitors and antitumor agents: synthesis, bioevaluation and docking study. Med Chem 11(3):296–304
Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM (2010) The global burden of cancer: priorities for prevention. Carcinogenesis 31(1):100–110
Varma RS, Khan IA (2014) Isatins as potential biologically active agents. Def Sci J 28(4):191–202
Vineis P, Wild CP (2014) Global cancer patterns: causes and prevention. Lancet 383(9916):549–557
Yusuf M, Jain P (2014) Synthesis and biological significances of 1, 3, 4-thiadiazolines and related heterocyclic compounds. Arab J Chem 7(5):525–552
Zhou L, Liu Y, Zhang W, Wei P, Huang C, Pei J, Yuan Y, Lai L (2006) Isatin compounds as noncovalent SARS coronavirus 3C-like protease inhibitors. J Med Chem 49(12):3440–3443
Ziarani GM, Moradi R, Lashgarib N (2016) Synthesis of spiro-fused heterocyclic scaffolds through multicomponent reactions involving isatin. ARKIVOC 1:1–81
Acknowledgements
The authors would like to thank the National Cancer Institute (NCI), Bethesda, MD, USA for performing the antitumor testing of the synthesized compounds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Gangarapu, K., Thumma, G., Manda, S. et al. Design, synthesis and molecular docking of novel structural hybrids of substituted isatin based pyrazoline and thiadiazoline as antitumor agents. Med Chem Res 26, 819–829 (2017). https://doi.org/10.1007/s00044-017-1781-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1781-5