Abstract
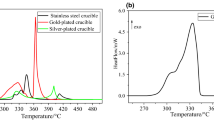
The heat effect of ammonia borane decomposition at 357 K under atmospheric pressure in glass and copper cells has been determined by calorimetry. The enthalpy of BH3NH3 heat-induced decomposition in glass ampoules is of–24.8±2.3 kJ/mol; this value includes the structural reorganization into diammoniate of diborane of–4.0±2.8 kJ/mol and the thermal effect of decomposition into polyamidoborane of–20.8±1.6 kJ/mol. In the case of experiments in copper cells a much higher (–47.6±8.3 kJ/mol) heat evolution is observed. The solidstate 15N and 11B NMR analysis of samples after the calorimetric experiment has shown that partial oxidation of the sample into boric acid occurs in copper cells.
Similar content being viewed by others
References
Jena, P., J. Phys. Chem. Lett., 2011, vol. 2, p. 206. DOI: 10.1021/jz1015372.
Baitalow, F., Baumann, J., Wolf, G., Jaenicke-Roßlerb, K., and Leitner, G., Thermochim. Acta, 2002, vol. 391, nos. 1–2, p. 159. DOI: 10.1016/S0040-6031(02)00173-9.
Baumann, J., Baitalow, F., and Wolf G., Thermochim. Acta, 2005, vol. 430, nos. 1–2, p. 9. DOI: 10.1016/jtca.2004.12.002.
Wolf, G., Baumann, J., Baitalow, F., and Hoffmann, F.P., Thermochim. Acta, 2000, vol. 343, nos. 1–2, p. 19. DOI: 10.1016/S0040-6031 (99 00365-2.
Storozhenko, P.A., Svitsyn, R.A., Ketsko, V.A., Buryak, A.K., and Ul’yanov, A.V., Russ. J. Inorg. Khim., 2005, vol. 50, no. 7, p. 980.
Kalidindi, S.B., Joseph, J., and Jagirdar, B.R., Energy Environ. Sci., 2009, vol. 2, p. 1274. DOI: 10.1039/B909448M.
He, T., Xiong, Z., Wu, G., Chu, H., Wu, C., Zhang, T., and Chen, P., Chem. Mater., 2009, vol. 21, no. 11, p. 2315. DOI: 10.1021/cm900672h.
Wolf, G., van Miltenburgb, J.C., and Wolf, U., Thermochim. Acta, 1998, vol. 317, no. 2, p. 111. DOI: 10.1016/S0040-6031 (98 00381-5.
Stowe, A. C., Shaw, W. J., Linehan, J. C., Schmid, B., and Autrey, T., Phys. Chem. Chem. Phys., 2007, vol. 9, p. 1831. DOI: 10.1039/b617781f.
Bowden, M., Heldebrant, D. J., Karkamkar, A., Proffen, T., Schenter, G.K., and Autrey, T., Chem. Commun., 2010, vol. 46, p. 8564. DOI:10.1039/c0cc03249b.
Gunaydin-Sen, O., Achey, R., Dalal, N. S., Stowe, A., and Autrey, T., J. Phys. Chem. B, 2007, vol. 111, p. 677. DOI: 10.1021/jp0649347.
Gervais, C., Babonneau, F., Maquet, J., Bonhomme, C., Massiot, D., Framery, E., and Vaultier, M., Magn. Res. Chem., 1998, vol. 36, p. 407. DOI: 10.1002/(SICI)1097- 458X (199806)36:6 <407::AID-OMR295>3.0.CO;2-Y.
Shimoda, K., Doi, K., Nakagawa, T., Zhang, Y., Miyaoka, H., Ichikawa, T., Tansho, M., Shimizu, T., Burrell, A.K., and Kojima, Y., J. Phys. Chem. C, 2012, vol. 116, p. 5957. DOI:10.1021/jp212351f.
Staubitz, A., Robertson, A.P., and Manners, I., Chem. Rev., 2010, vol. 110, p. 4079. DOI: 10.1021/cr100088b.
Butlak, A V., Kondrat’ev, Yu V., and Timoshkin, A.Y., Russ. J. Gen. Chem., 2014, vol. 84, no. 12, p. 2455. DOI: 10.1134/S1070363214120184.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Butlak, Yu.V. Kondrat’ev, A.S. Mazur, A.Yu. Timoshkin, 2015, published in Zhurnal Obshchei Khimii, 2015, Vol. 85, No. 11, pp. 1761–1764.
Rights and permissions
About this article
Cite this article
Butlak, A.V., Kondrat’ev, Y.V., Mazur, A.S. et al. Thermal decomposition of ammonia borane at 357 K. Russ J Gen Chem 85, 2505–2508 (2015). https://doi.org/10.1134/S1070363215110018
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363215110018