Abstract
Objective: Although main components of the venoms from Viperidae snakes are hemotoxins, several studies indicate the presence of neurotoxins in these venoms. We previously found that the venom of pit viper Gloydius saxatilis inhibited the muscle-type nicotinic acetylcholine receptor (nAChR). The objective of present work is to isolate and characterize a neurotoxic protein from this venom. Methods: The protein was isolated by liquid chromatography and characterized using high resolution mass-spectrometry. Results and Discussion: The isolated protein called glosaxin inhibited the binding of the α-bungarotoxin to the nAChR of muscle type from Torpedo californica. Investigation of the amino acid sequence of the isolated protein by high resolution mass spectrometry and the subsequent bioinformatic analysis showed that it is homologous to the amino acid sequences of disintegrin-like proteins, consisting of non-catalytic domains of class PIII metalloproteinases from the venom of pit vipers of genus Gloydius. Glosaxin was shown to inhibit the binding of α-bungarotoxin to T. californica nAChR with IC50 = 51 μM. It also inhibited ACh-induced functional responses of the human neuronal nAChR of α3β2 subtype. Conclusions: This is the first evidence for the ability of proteins consisting of non-catalytic domains of snake venom class PIII metalloproteinase to inhibit the nAChR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Snake venoms, which are used as a means of defense and/or a means of attack/hunting, most effectively disrupt the functions of the nervous or cardiovascular systems. The venoms of some species of snakes, mostly from Elapidae family, primarily affect the nervous system and are considered neurotoxic; venoms of other species, mainly those belonging to the Viperidae family, disrupt the function of the cardiovascular system and have a hemotoxic effect. However, this division is quite arbitrary; for example, hemotoxic venoms contain neurotoxic compounds [1]. Nevertheless, the main components of hemotoxic venoms are proteins and peptides that affect the cardiovascular system. These may be enzymes, such as serine proteinases and metalloproteinases, or proteins that do not have enzymatic activity, such as disintegrins. As a rule, enzymes are the predominant components of hemotoxic venoms and are responsible for their coagulopathy. The most abundant toxins in the venoms of snakes from Viperidae family are metalloproteinases, the content of which in viper venoms averages 27% [2], reaching almost 43% in the venom of Vipera anatolica senliki [3].
Snake venom metalloproteinases are a large group of multidomain proteins exhibiting diverse biological activity [4]. In particular, they have the ability to proteolytically cleave fibrinogen and fibrin and inhibit platelet aggregation, which manifests itself in blood clotting disorders in case of the bite by a snake of the Viperidae family. Depending on the composition of the domains that form metalloproteinases they are divided into three classes: PI, PII, and PIII. Class PI proteins contain the catalytic metalloproteinase domain only. Class PII enzymes contain a disintegrin domain along with a metalloproteinase domain. Class PIII metalloproteinases include metalloproteinase, disintegrin-like, and cysteinerich domains. It should be noted that class PII and PIII proteins undergo post-translational proteolysis, and the venoms contain both disintegrins and proteins that include both noncatalytic domains. The latter are also called disintegrin-like proteins. The post-translational cleavage of PI proteins is also possible. Disintegrins selectively bind to integrins, i.e. heterodimeric receptors involved in intercellular and cell–matrix interactions, which are considered as therapeutic targets [5]. The functions of disintegrin-like proteins comprising two noncatalytic domains are not so well understood, and the number of such proteins identified in snake venoms is much less than the number of disintegrins. However, such proteins have the ability to inhibit platelet aggregation [6, 7] and cancer cell adhesion [7].
Quite an interesting effect is characteristic of the disintegrin-like protein alternagin-C isolated from Bothrops (Rhinocerophis) alternatus snake venom [8]. This protein is capable of inducing the expression of vascular endothelial growth factor (VEGF), proliferation and migration of endothelial cells, as well as enhancing angiogenesis and increasing the viability of myoblasts. An ex vivo study of the alternagin-C effect on the cardiac function of freshwater fishes showed that the protein enhanced cardiac activity, promoting a significant increase in the force of contraction (positive inotropism) and in the rate of contraction and relaxation (positive chronotropism) with a simultaneous decrease in the time-to-peak muscle tension, as well as an improvement in pumping ability [9]. This study showed that alternagin-C improved cardiac function by increasing the efficiency of calcium ion exchange mechanisms [9]. The effect of alternagin-C on hypoxia/reoxygenation in isolated fish heart ventricle strips and on the morphology and density of blood vessels was also studied [10]. Treatment with alternagin-C provided protection of cardiomyocytes from the negative inotropism caused by hypoxia/reoxygenation. This protein also stimulated angiogenesis and improved coupling between excitation and contraction under hypoxic conditions. These results indicate a new therapeutic strategy for the treatment of ischemia-related diseases.
The neurotoxicity of snake venoms is most clearly manifested in blocking neuromuscular transmission, the main element of which is muscle-type nicotinic acetylcholine receptors (nAChRs). Previously, in the search for new compounds that have the ability to block such nAChRs we studied the venoms of Viperidae family snakes [11]. The venom of the Central Asian pit viper Gloydius saxatilis showed the highest inhibitory activity. It should be noted that the name of this snake species is not well established, and a number of authors call it Gloydius intermedius [12].
The aim of this work was the isolation and structural characterization of a toxin manifesting neurotoxicity from the venom of G. saxatilis. As a neurotoxicity assay, we used the inhibition of the nAChR function, which is the main element of neuromuscular transmission.
RESULTS AND DISCUSSION
Isolation from G. saxatilis Venom of a nAChR Inhibitor and Analysis of Its Amino Acid Sequence
Several stages of liquid chromatography were used to isolate the active compound from G. saxatilis venom. At the first stage, gel filtration on a Superdex 75 column was applied (Fig. 1). The eluted fractions were further analyzed by competitive radioligand assay on the nAChR from the electrical organ of the ray Torpedo californica with radioactive α-bungarotoxin (125I-αBgt) as a ligand. The highest molecular weight fraction I, which showed the greatest ability to inhibit α-Bgt binding, was further separated by ion-exchange chromatography (Fig. 2). The most abundant fraction 5 showed the ability to inhibit the binding of α-Bgt to T. californica nAChR and was subjected to further analysis.
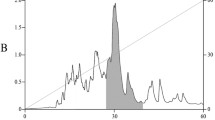
Separation of fraction I (see Fig. 1) by anion-exchange chromatography on a Mono Q column (4.6 × 100 mm) at a NaCl concentration gradient of 0.05–0.55 M for 100 min in 20 μM ethanolamine–HCl buffer (pH 9.5), flow rate 0.5 mL/min. Horizontal lines show the collected fractions.
The molecular mass of the protein from fraction 5 (Fig. 2), as determined by high-resolution mass spectrometry (HRMS), is 23307.64 Da (Fig. 3). Figure 3 shows three sets of signals corresponding to the isotopomers of the protein. The difference between the left and middle, as well as between the middle and third sets of signals is 15.99 Da, and it corresponds, within the measurement error, to the atomic mass of oxygen, i.e. the central and right sets of signals are assignable to the oxidized protein. The methionine residue in proteins undergoes oxidation most easily, and this post-translational modification occurs very often [13]. For example, a similar modification was previously discovered in phospholipase A2 from Crotalus horridus venom [14]. We also observed methionine oxidation in the non-conventional toxin WTX from the cobra Naja kaouthia [15] and the β-bungarotoxin from the krait Bungarus multicinctus [16].
Fragment of high-resolution mass spectrum of the protein isolated from fraction 5 (see Fig. 2). Peaks of the [M + 13H]+13 ions (z = 13) are shown.
To characterize the protein amino acid sequence, it was reduced with tris(2-carboxyethyl)phosphine, carbamidomethylated with 2-chloroacetamide, and hydrolyzed with trypsin; the resulting peptides were analyzed by HPLC-ESI-MS/MS (Fig. 4). The analysis showed that the isolated protein is homologous to proteins comprising disintegrin-like and cysteine-rich domains from class PIII snake venom metalloproteinases. In particular, it has 68% identical amino acid residues with halysetin (VM3H_GLOHA) [17], a protein from the venom of Agkistrodon (Gloydius) halys (Fig. 4). Our isolated protein was given the name glosaxin (an abbreviation derived from GLOydius SAXatlis disintegrIN-like protein).
As for the homologous proteins identified in the Central Asian pit viper G. (intermedius) saxatilis, the amino acid sequences of PII (UniProt KB: VM2SA_GLOSA) and PIII (UniProt KB: A0A0C4ZNF1_GLOIT) metalloproteinases, deduced from the nucleotide sequences, are known (Fig. 5). Hong et al. [18] reported the amino acid sequence of the disintegrin saxatilin (VM2_GLOHA), isolated from G. saxatilis venom. A comparison of the amino acid sequences of halysetin and these proteins is shown in Fig. 5. These are homologous proteins, and the highest degree of similarity is observed in the N-terminal part, up to the active site residues. The active site residues differ significantly. Specifically, these are RGD in VM2SA_GLOSA and VM2_GLOHA as well as ECD in A0A0C4ZNF1_GLOIT and VM3H_GLOHA. Further, toward the C-terminal residue, there is a considerable difference in the amino acid sequences between these pairs of proteins (Fig. 5).
Comparison of the amino acid sequences of noncatalytic domains of metalloproteinases of some species of pit vipers (genus Gloydius): VM3H_GLOHA, halysetin from the venom of Agkistrodon (Gloydius) halys; A0A0C4ZNF1_GLOIT, fragment of PIII Gloydius intermedius metalloproteinase; VM2SA_GLOSA, fragment of PII Gloydius saxatilis metalloproteinase, and VM2_GLOHA, Gloydius saxatilis disintegrin saxatilin. The lines above the sequences indicate the halysetin peptides detected by mass spectrometry in our protein digest. Active site residues are underlined, and identical residues are highlighted in gray.
Interestingly, peptide fragments that inhibit nAChR were previously identified in metalloproteinases [11, 19]. Thus, peptides Pm1 and Pm2, capable of inhibiting human nAChR of the α7 subtype (IC50 ~12 μM) were identified in the propeptide domain of the metalloproteinase of the olive sand snake Psammophis mossambicus [19]. The peptide macoluxin, which can reversibly inhibit muscle-type nAChRs, was isolated from the venom of the cat-eyed snake Madagascarophis colubrinus [11]. This peptide competed with 125I-αBgt for binding to the nAChR from Torpedo californica membranes, IC50 being 46.8 ± 3.9 µM. Macoluxin has a high degree of similarity to a fragment of the catalytic domain of snake venom metalloproteinases and is apparently formed as a result of proteolysis of this enzyme. Considering the problem of the functional diversity of snake toxins at a limited number of their structural types, we can assume the following: since metalloproteinases are the predominant components in the venoms of snakes from Viperidae family, their further processing can lead to the emergence of compounds with other types of biological activity, in particular neurotoxicity.
Interaction of the Isolated Protein with nAChR
The efficiency of the interaction of glosaxin with nAChRs was assessed by its competition with 125I-αBgt for binding to the membranes of the electrical organ of T. californica ray, containing muscle-type receptors (α12β1γδ), as well as to cells of the GH4C1 line expressing human neuronal nAChRs of the α7 subtype. It was found that glosaxin inhibited the binding of 125I-αBgt to T. californica membranes with an IC50 of 50.9 ± 1.83 μM (Fig. 6). At a concentration of 50 μM, it inhibited the binding of 125I-αBgt to α7 nAChR only by 20%.
To find out whether glosaxin is a functional nAChR inhibitor, we performed electrophysiological experiments using the neuronal nAChR of α3β2 subtype heterologously expressed in clawed frog oocytes. Glosaxin itself did not induce ionic currents but inhibited the ACh-induced current (Fig. 7), while at a fairly high concentration. The inhibition was ~20% at a protein concentration of 100 μM. Therefore, glosaxin is a weak antagonist of α3β2 nAChR subtype.
Glosaxin inhibition of acetylcholine-induced currents in nAChRs of α3β2 subtype. The responses of clawed frog X. laevis oocytes to 50 μM acetylcholine in the absence of the protein (100%) and after 1 min incubation with various protein concentrations (n = 3) were recorded. * p < 0.05 (Student’s t-test).
Thus, we isolated from the venom of the Central Asian pit viper G. (intermedius) saxatilis the protein glosaxin with a molecular weight of 23.3 kDa, which has the ability to interact with different subtypes of nAChRs and exhibits a higher affinity for the muscle-type receptor. An analysis of its amino acid sequence by mass spectrometry showed that this protein is homologous to snake venom disintegrin-like proteins, consists of disintegrin and cysteine-rich domains, and is a fragment of class PIII metalloproteinase, including non-catalytic domains of this enzyme. This work provides the first evidence for the interaction of such proteins with nAChRs.
EXPERIMENTAL
Materials. Torpedo californica membranes were kindly provided by Prof. F. Huho (Free University of Berlin, Germany), and GH4C1 cells transfected with human α7 nAChR cDNA were provided by Eli Lilly (Great Britain). All reagents used in the work were of analytical or higher grade.
Preparation of the venom. The venom was obtained from G. saxatilis specimens housed at the serpentarium of the Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences. The venom sample was dried over anhydrous CaCl2 and stored at –20°C.
Isolation of the protein. The venom was dissolved in 0.1 M ammonium acetate (pH 6.2) and applied to a Superdex 75 column (10 × 300 mm; Cytiva, USA) equilibrated with the same buffer. Elution was carried out at a flow rate of 1.0 mL min–1. The optical density of the eluent was recorded at 226 nm. Fraction I (Fig. 1) was lyophilized and further separated by anion exchange chromatography on a Mono Q 4.6/100 PE column (4.6 × 100 mm; Cytiva, USA). Eluents: buffer A—50 μM ethanolamine hydrochloride (pH 9.5) and buffer B—buffer A containing 1 M NaCl; gradient 5–55% buffer B for 100 min, flow rate 0.5 mL min–1, detection of eluting fractions at wavelength of 280 nm (Fig. 2).
High-resolution mass spectrometry. The HR-MS analysis was performed as described previously [20].
Reduction, alkylation, and hydrolysis of proteins with trypsin. A sample of the toxin was dissolved in a reducing and alkylating buffer (pH 8.5) so that the final concentration of the protein, Tris, sodium deoxycholate, tris(2-carboxyethyl)phosphine, and 2-chloroacetamide was 1 mg/mL, 100 μM, 1%, and 10 and 20 μM, respectively. The protein solution was heated at 95°C for 10 min, cooled to room temperature, and an equal volume of trypsin (Promega, USA) in 100 μM Tris (pH 8.5) was added in a weight ratio of 1 : 100. After incubation overnight at 37°C, the solution of tryptic peptides was acidified with TFA to a final concentration of 1%, the precipitate of deoxycholic acid that formed was extracted into an equal volume of ethyl acetate with vigorous stirring, the ethyl acetate and aqueous phases were separated by centrifugation (15000 g, 2 min), and ethyl acetate was collected and discarded. The extraction procedure was repeated three times. Peptides were desalted on StageTips microcolumns by the procedure in [21, 22] with minor modifications. The microcolumns for peptide desalting were made from automatic pipette tips (200 μL) and an Empore SDB-RPS membrane (3M). To desalt 20 μg of tryptic hydrolyzate, one microcolumn with two membrane pieces cut out with a needle of a 14 G diameter was used. Peptides were applied to the microcolumn by centrifugation at 200 g for ~6 min, successively washed with a mixture of 100 μL of 1% TFA and 100 μL of ethyl acetate, 100 μL of 1% TFA, and 100 μL of 0.2% TFA, and eluted with 60 μL of a solution containing 5% ammonium hydroxide and 40% acetonitrile. The eluate was dried on a centrifugal vacuum evaporator to dryness and stored until LC–MS analysis at –85°C.
Liquid chromatography–mass spectrometry analysis of peptides. The dry eluate was dissolved in 20 μL of an aqueous solution containing 2% acetonitrile and 0.1% TFA, and 3 μL was applied to a column (diameter 75 μM, length 25 cm) with an Aeris Peptide XB-C18 2.6 μM sorbent (Phenomenex, USA). Separation was carried out on an Ultimate 3000 Nano LC System coupled to a Q Exactive HF mass spectrometer using a nanoelectrospray source (Thermo Fisher Scientific, USA). Peptides were loaded onto a column thermostated at 40°C in buffer A [0.2% formic acid (FA) in water] and eluted for 120 min with a linear gradient of 4–55% buffer B (0.1% FA, 19.9% water, 80% acetonitrile) at a flow rate of 350 nL/min. After every gradient elution, the column was washed with 95% buffer B for 5 min and equilibrated with buffer A for 5 min. The mass spectrometry data were stored by automatically switching between MS1 scans and up to 15 MS/MS scans (topN method). The target value for the MS1 scan was set to 3 × 106 in the m/z range 300−1200 with a maximum ion injection time of 60 ms and a resolution of 60000. Isolation of precursor ions was carried out with a window width of m/z 1.4 and the m/z value fixed at 100. Precursor ions were fragmented by high-energy dissociation in a C-trap with a normalized collision energy of 28 eV. The MS/MS scans were saved at a 15000 resolution at m/z 400 and at 1 × 105 for target ions in the m/z range 200–2000 with a maximum ion injection time of 30 ms.
Analysis of the liquid chromatography–mass spectrometry data. Cataloging venom proteins and analysis of their post-translational modifications was carried out using PEAKS Studio 8.0 build 20160908 software [23]. The primary structures of the peptides generated by this program were analyzed by comparison with an array of protein sequences of the Serpentes taxonomic group (70112 structures) retrieved from the UNIPROT KB database (10.2017), with the following settings: Cys carbamidomethylation as fixed modification; N-terminal protein acetylation, Met oxidation, and Asn/Gln deamidation as variable modifications; and trypsin as protease specificity. The false positive identification rate (FDR) of peptides was set to 0.01 and determined by correlating the MS/MS dataset with the reverse protein sequence database generated by PEAKS Studio. Identification of peptides was carried out with an acceptable initial deviation of the precursor ion mass of up to 10 ppm and an acceptable deviation of the fragment mass of 0.05 Da.
Competitive radioligand assay. For competitive binding experiments, a suspension of T. californica electric organ membranes (at a final concentration of 1.2 nM α-Bgt-binding sites) or GH4C1 cells (0.4 nM α-Bgtbinding sites) in 20 μM Tris-HCl buffer (pH 8.0) , containing BSA at a concentration of 1 mg/mL, was incubated for 90 min with various concentrations of glosaxin. Radioactive [125I]-labeled α-Bgt (500 Ci/mmol) was then added to a final concentration of 0.2–0.4 nM and incubation was continued for another 5 min. The identification of nonspecific binding, sample filtration, and calculation of bound radioactivity were performed as described by Lebedev et al. [23].
Electrophysiological measurements. The measurements on clawed frog Xenopus laevis oocytes were carried out following the procedure of Lebedev et al. [24]. Various concentrations of glosaxin were preincubated with oocytes for 1 min, after which 50 μM acetylcholine was added, and the response was recorded. The maximum current induced by 50 μM acetylcholine in the absence of protein was taken as 100%.
CONCLUSIONS
Using high-performance liquid chromatography, we isolated from the venom of the Central Asian pit viper G. saxatilis the protein glosaxin, which inhibited different subtypes of nAChRs. The mass spectrometry analysis of its amino acid sequence showed that this protein is homologous to the sequences of proteins consisting of noncatalytic domains of class PIII metalloproteinases from the venom of pit vipers (genus Gloydius). The biological activity of glosaxin was studied and it was found that the toxin inhibited the binding of α-Bgt to the Torpedo californica muscle-type receptor with IC50 = 51 μM and only slightly inhibited the binding of α-Bgt to the α7 subtype of nAChR. Glosaxin also inhibited ACh-induced functional responses of human nAChRs of α3β2 subtype. This is the first evidence of the ability of proteins consisting of noncatalytic domains of PIII snake venom metalloproteinase to inhibit nAChRs.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Osipov, A. and Utkin, Y., Int. J. Mol. Sci., 2023, vol. 24,Article ID: 2919. https://doi.org/10.3390/ijms24032919
Tasoulis, T., Pukala, T.L., and Isbister, G.K., Front. Pharmacol., 2022, vol. 12, Article ID: 768015. https://doi.org/10.3389/fphar.2021.768015
Hempel, B.F., Damm, M., Mrinalini, Göçmen, B.,Karış, M., Nalbantsoy, A., Kini, R.M., and Süssmuth, R.D., J. Proteome Res., 2020, vol. 19, pp. 1731–1749. https://doi.org/10.1021/acs.jproteome.9b00869
Olaoba, O.T., Karina Dos Santos, P., Selistre-de-Araujo, H.S., and Ferreira de Souza, D.H., Toxicon X, 2020,vol. 7, Article ID: 100052. https://doi.org/10.1016/j.toxcx.2020.100052
Vasconcelos, A.A., Estrada, J.C., David, V., Wermelinger, L.S., Almeida, F.C.L., and Zingali, R.B., Front. Mol. Biosci., 2021, vol. 8, Article ID: 783301. https://doi.org/10.3389/fmolb.2021.783301
Liu, J.W., Du, X.Y., Liu, P., Chen, X., Xu, J.M., Wu, X.F.,and Zhou, Y.C., Biochem. Biophys. Res. Commun., 2000,vol. 278, p. 112–118. https://doi.org/10.1006/bbrc.2000.3724
Limam, I., Bazaa, A., Srairi-Abid, N., Taboubi, S., Jebali, J.,Zouari-Kessentini, R., Kallech-Ziri, O., Mejdoub, H., Hammami, A., El Ayeb, M., Luis, J., and Marrakchi, N., Matrix Biol., 2010, vol. 29, pp. 117–126. https://doi.org/10.1016/j.matbio.2009.09.009
Souza, D.H., Iemma, M.R., Ferreira, L.L., Faria, J.P., Oliva, M.L., Zingali, R.B, Niewiarowski, S., and Selistre-de-Araujo, H.S., Arch. Biochem. Biophys., 2000,vol. 384, pp. 341–350. https://doi.org/10.1006/abbi.2000.2120
Monteiro, D.A., Kalinin, A.L., Selistre-de-Araujo, H.S., Vasconcelos, E.S., and Rantin, F.T., Toxicon, 2016,vol. 110, pp. 1–11. https://doi.org/10.1016/j.toxicon.2015.11.012
Monteiro, D.A., Kalinin, A.L., Selistre-de-Araújo, H.S., Nogueira, L.A.N., Beletti, M.E., Fernandes, M.N., and Rantin, F.T., Comp. Biochem. Physiol. C Toxicol. Pharmacol., 2019, vol. 215, pp. 67–75. https://doi.org/10.1016/j.cbpc.2018.10.003
Kryukova, E.V., Ivanov, D.A., Kopylova, N.V., Starkov, V.G., Andreeva, T.V., Ivanov, I.A., Tsetlin, V.I., and Utkin, Yu.N., Russ. J. Bioorg. Chem., 2023, vol. 49,pp. 529–537. https://doi.org/10.1134/S1068162023030159
Gloydius Intermedius (STRAUCH, 1868), The ReptileDatabase, 2024. https://reptile-database.reptarium.cz/species?genus=Gloydius&species=intermedius&search_param=%28%28taxon%3D%27Crotalinae%27%29%29
Levine, R.L., Moskovitz, J., and Stadtman, E.R., IUBMBLife, 2000, vol. 50, pp. 301–307. https://doi.org/10.1080/713803735
Wang, Y.M., Parmelee, J., Guo, Y.W., and Tsai, I.H.,Toxicon, 2010, vol. 56, p. 93–100. https://doi.org/10.1016/j.toxicon.2010.03.015
Starkov, V.G., Polyak, Ya.L., Vulfius, E.A., Kryukova, E.V.,Tsetlin, V.I., and Utkin Yu.N., Russ. J. Bioorg. Chem.,2009, vol. 35, p. 10–18. https://doi.org/10.1134/S1068162009010026
Osipov, A.V., Cheremnykh, E.G., Ziganshin, R.H., Starkov, V.G., Nguyen, T.T.T., Nguyen, K.C., Le, D.T., Hoang, A.N., Tsetlin, V.I., and Utkin, Y.N., Biomedicines, 2023,vol. 11, Article ID: 1115. https://doi.org/10.3390/biomedicines11041115
Liu, J.W., Du, X.Y., Liu, P., Chen, X., Xu, J.M., Wu, X.F., and Zhou, Y.C., Biochem. Biophys. Res. Commun., 2000,vol. 278, pp. 112–118. https://doi.org/10.1006/bbrc.2000.3724
Hong, S.Y., Koh, Y.S., Chung, K.H, and Kim, D.S., Thromb. Res., 2002, vol. 105, pp. 79–86. https://doi.org/10.1016/s0049-3848(01)00416-9
Brust, A., Sunagar, K., Undheim, E.A., Vetter, I.,Yang, D.C., Casewell, N.R., Jackson, T.N., Koludarov, I.,Alewood, P.F., Hodgson, W.C., Lewis, R.J., King, G.F., Antunes, A., Hendrikx, I., and Fry, B.G., Mol. Cell. Proteomics, 2013, vol. 12, pp. 651–663. https://doi.org/10.1074/mcp.M112.023135
Ryabinin, V.V., Ziganshin, R.H., Starkov, V.G., Tsetlin, V.I.,and Utkin, Y.N., Russ. J. Bioorg. Chem., 2019, vol. 45,pp. 107–121. https://doi.org/10.1134/S1068162019020109
Rappsilber, J., Mann, M., and Ishihama, Y., Nat. Protoc.,2007, vol. 2, pp. 1896–1906. https://doi.org/10.1038/nprot.2007.261
Geyer, P.E., Kulak, N.A., Pichler, G., Holdt, L.M., Teupser, D., and Mann, M., Cell Syst., 2016, vol. 2, pp. 185–195. https://doi.org/10.1016/j.cels.2016.02.015
Ma, B., Zhang, K., Hendrie, C., Liang, C., Li, M., DohertyKirby, A., and Lajoie, G., Rapid Commun. Mass Spectrom.,2003, vol. 17, pp. 2337–2342. https://doi.org/10.1002/rcm.1196
Lebedev, D.S., Kryukova, E.V., Ivanov, I.A., Egorova, N.S.,Timofeev, N.D., Spirova, E.N., Tufanova, E.Y., Siniavin, A.E.,Kudryavtsev, D.S., Kasheverov, I.E., Zouridakis, M., Katsarava, R., Zavradashvili, N., Iagorshvili, I., Tzartos, S.J.,and Tsetlin, V.I., Mol. Pharmacol., 2019, vol. 96, pp. 664–673. https://doi.org/10.1124/mol.119.117713
Funding
The work was financially supported by the Russian Science Foundation (project no. 21-14-00316).
Author information
Authors and Affiliations
Contributions
All authors made equal contributions to the writing of the article.
Corresponding author
Ethics declarations
All experiments with snakes were approved by the Commission for the Control and Use of Laboratory Animals of the Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences (application protocol, reg. no. 324/2021 of July 23, 2021).
Informed consent was not required for this article. No conflict of interest was declared by the authors.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations: α-Bgt, α-bungarotoxin; nAChR, nicotinic acetylcholine receptor.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osipov, A.V., Kryukova, E.V., Ojomoko, L.O. et al. A New Protein Glosaxin Composed of Noncatalytic Domains of Class PIII Metalloproteinase from the Pit Viper Gloydius saxatilis Venom Inhibits Nicotinic Acetylcholine Receptor. Russ J Bioorg Chem 50, 706–714 (2024). https://doi.org/10.1134/S106816202403004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816202403004X