Abstract
A recombinant analog of the human SLURP-1 protein, rSLURP-1, effectively inhibits the growth of carcinomas by interaction with the α7-type nicotinic acetylcholine receptor. Recently, rSLURP-1 inhibition of glioma growth in vitro was shown by the authors; however, the action of rSLURP-1 was not studied. Here, we showed that rSLURP-1 selectively inhibits the growth of U251 MG glioma cells, but not of normal astrocytes, and controls glioma cell migration. In addition, rSLURP-1 induces cell cycle arrest in the G2/M phase in U251 MG glioma cells, but does not result in apoptosis. Incubation of U251 MG cells with rSLURP-1 causes inhibition of phosphorylation of ERK, p38 MAPK, and AKT kinases, whose activation contributes to the progression of gliomas. At the same time, rSLURP-1 does not affect the activity of JNK kinase. Thus, rSLURP-1 is an endogenous protein promising for the development of drugs based on it for the treatment of not only carcinomas but also gliomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Gliomas account for over 80% of brain tumors and consist of astrocytes, oligodendrocytes, and other cell types [1, 2]. The average survival of patients with gliomas is only 12–15 months [2, 3]. Surgical removal of tumors, as well as chemotherapy, show low efficiency [4], which determines the relevance of the search for new drugs and their molecular targets for the treatment of gliomas.
One of the promising molecular targets for the development of new anticancer drugs is the nicotinic acetylcholine receptor (nAChR) [5, 6]. nAChRs are ligand-gated ion channels that are activated by acetylcholine and are involved in the regulation of many vital processes in the central and peripheral nervous systems [7], epithelial cells, and the immune system [8]. The nAChR subtype consisting of five identical α7-type subunits (α7 nAChR) is involved in nicotine-dependent oncogenic transformation and carcinoma progression [9]. Dysregulation of α7 nAChR can lead to activation of the ERK/MEK and PI3K/AKT/mTOR mitogenic signaling pathways, as well as inhibition of cell aging and apoptosis, which results in stimulation of tumorigenesis [10–12]. In accordance with this, the expression of the CHRNA7 gene, encoding the nAChR α7 subunit, is increased in some carcinomas of patients [13] and in glioma cell lines [14].
Some endogenous proteins of the Ly6/uPAR family [15–17], such as C4.4A, uPAR, Ly6D, Ly6E, Ly6H, Ly6K, Lynx1, SLURP-1, and PSCA, are currently considered as biomarkers of development of various tumors [15, 18–27]. One of these human proteins is the secreted Ly6/uPAR-related protein 1 (SLURP-1), which regulates keratinocyte homeostasis [28]. SLURP1 gene expression is decreased in colon carcinoma cells after nicotine treatment [29], in lung carcinomas of male smokers compared to healthy lung tissues [25], in primary and metastatic melanomas [24], as well as in human keratinocytes as a result of oncogenic transformation with NNK and NNN nitrosamines contained in tobacco [30]. At the same time, an increased concentration of endogenous SLURP-1 in blood of patients with pancreatic cancer correlates with a better prognosis of patient survival after surgical removal of the primary tumor [26].
Recombinant analog of the human SLURP-1protein (rSLURP-1) inhibits acetylcholine-induced currents through α7 nAChR [31] and inhibits the growth of various cancer cells of epithelial origin [32–35]. Moreover, rSLURP-1 decreases the proliferation of A549 lung adenocarcinoma cells treated with nicotine through the interaction with α7 nAChR [34]. The PI3K/AKT/mTOR signaling pathway is involved in the antiproliferative and antimigration activity of rSLURP-1 in A549 cells [34, 35].
We have previously shown that, in addition to carcinomas, rSLURP-1inhibits the growth of U251 MG and A172 glioma cells with nanomolar efficiency. In U251 MG glioma cells, rSLURP-1 activity was due to the interaction with α7 nAChR [14]. In this work, we studied the selectivity of SLURP-1 with respect to U251 MG gliomas and normal primary line astrocytes, revealed the presence of antimigration activity of rSLURP-1, as well as the molecular and cellular actions underlying the SLURP-1 effects in glioma cells.
RESULTS AND DISCUSSION
rSLURP-1 inhibits the growth of U251 MG glioma but does not affect normal astrocyte cells. Recombinant SLURP-1 effectively inhibits the growth of U251 MG and A172 glioma cells by interacting with α7 nAChR [14]. Comparative analysis revealed an increased expression of the CHRNA7 gene, which encodes α7 nAChR, in U251 MG cells compared to normal astrocytes [14]. Since α7 nAChR is the main target of rSLURP-1 action [31], we assumed that overexpression of α7 nAChR in gliomas may determine the specificity of the rSLURP-1 effect on tumor cells. In view of this, we compared the effect of rSLURP-1 on U251MG glioma cells and on normal astrocytes isolated from the hippocampus of newborn rats. The analysis of cell viability using the MTT test showed that, unlike U251 MG cells, incubation of astrocytes with 1 µM rSLURP-1 for 72 h did not significantly suppress their growth (Fig. 1). Thus, rSLURP-1 selectively affects various glioma cells and does not affect the growth of normal astrocytes.
Antiproliferative effect of recombinant rSLURP-1 protein on U251 MG glioma cells and normal rat hippocampal astrocytes. Cells were incubated with 1 μM rSLURP-1 for 72 h. Data are presented as percentage of control (untreated cells, dotted line) ± standard error of the mean (n = 3 for astrocytes, n = 4 for U251 MG cells). ####p < 0.0001 (significant difference from control according to one-sample t test).
This result is consistent with the studies showing that normal lung fibroblasts with a reduced α7 nAChR expression compared to A549 lung adenocarcinoma [34] are less sensitive to rSLURP-1 than A549 cells [32, 33]. Thus, rSLURP-1 can be considered a universal growth regulator of cancer cells of various origins, and the increased level of α7 nAChR expression may serve as an indicator of a greater sensitivity of tumor cells to rSLURP-1.
However, the rSLURP-1 selectivity may be associated not only with the expression level of the prooncogenic α7 nAChR receptor but also with the probable formation of complexes of α7 nAChRs with other prooncogenic receptors in cancer cells, such as receptors of various growth factors [36]. The possibility of the effect of ligands of α7 nAChR through its associated receptors has long been discussed. In particular, we showed that the target of rSLURP-1 in A549 lung adenocarcinoma cells is the α7 nAChR heterocomplex with the epidermal growth factor receptor (EGFR) and the platelet-derived growth factor receptor (PDGFR) [35].
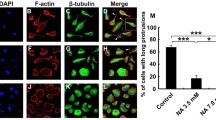
rSLURP-1 causes a decrease in the migration of U251 MG glioma cells. SLURP-1 inhibits the migration of pancreatic cancer cells [26], A549 lung adenocarcinoma cells [35], and metastatic melanoma cells [37]. In this work, we used the migration test (scratch assay) to study the effect of rSLURP-1 on the migration of U251 MG glioma cells (Fig. 2). Incubation of U251 MG cells with 1 μM rSLURP-1 for 24 h resulted in a significant decrease in the area occupied by the cells (~34% compared to the untreated cells). Thus, rSLURP-1 inhibits the migration of cancer cells of not only epithelial origin, but also of gliomas. It was previously shown that the activation of α7 nAChRs by nicotine does not increase the migration of U251 MG cells but increases the activity of the MMP9 metalloprotease [38]. Thus, the inhibition of α7 nAChR by rSLURP-1 possibly also leads to the suppression of cancer cell migration with the involvement of MMP-9. Since migration is a process tightly associated with tumor invasiveness and metastasizing, the inhibition of migration by rSLURP-1 additionally demonstrates the promise of developing drugs for glioma therapy based on this endogenous protein.
Effect of the recombinant rSLURP-1 protein on the migration of U251 MG glioma cells: (a) representative images of the wound healing test for U251 MG cells incubated with 1 μM rSLURP-1 for 72 h; (b) area occupied by migrating U251 MG cells. Data are presented as a percentage of the original surface occupied by migrating cells ± standard error of the mean (n = 7). *** p < 0.001 (significant difference between groups according to two-tailed t test).
rSLURP-1 inhibits cell cycle progression in U251 MG glioma cells. We analyzed the effect of rSLURP-1 on the cell cycle progression in U251 MG glioma cells. Flow cytometric analysis showed that, in U251 MG cells, rSLURP-1 reduced the number of cells in the G1 phase (from ~67 to ~57%) and increased the number of cells in the G2/M phase (from ~17 to ~27%) (Figs. 3a, 3b), which indicates the cell cycle inhibition in the G2/M phase. We found no increase in the population of cells in the sub-G1 phase, characteristic of apoptosis [39]. The analysis of the nuclear morphology also showed no signs of apoptosis in U251 MG glioma cells (Fig. 3c). Thus, in U251 MG glioma cells, rSLURP-1 inhibits the cell cycle passage but does not induce apoptosis. This is consistent with the previous data that rSLURP-1 causes cell cycle arrest in A549 lung adenocarcinoma cells without inducing apoptosis [35]. Moreover, the data obtained are consistent with the effect of another protein of the Ly6/uPAR family, Lynx1, on the cell cycle in A549 cells, which also modulates the functioning of α7 nAChR in epithelial cells [40]. This may indicate a common molecular action for the inhibition of cancer cell growth as a result of α7 nAChR inhibition.
Effect of recombinant rSLURP-1 protein on the cell cycle and morphology of the nuclei of U251 MG glioma cells: (a) representative distribution of the nuclear population of U251 MG cells treated and not treated with 1 μM rSLURP-1 for 72 h; (b) content of cells in different phases of the cell cycle. Data are presented as a percentage of cells in each phase of the cell cycle ± standard error of the mean (n = 5). * p < 0.05 and **** p < 0.0001 (significant difference between groups on two-tailed t test); (c) analysis of the morphology of cell nuclei after incubation with rSLURP-1, staining with Hoechst 33342 and propidium iodide (there was no signal in the propidium iodide channel). Scale bar, 10 µm.
rSLURP-1 suppresses the activation of ERK, p38 MAPK, and AKT kinases, but not JNK, in U251 MG cells. We analyzed the effect of rSLURP-1 on the activity of the main intracellular signaling pathways in U251 MG cells mediating glioma progression and associated with α7 nAChR activity [41–43]. The results of analysis using Bio-Plex magnetic particles showed that a 72-h incubation of glioma cells with 1 μM rSLURP-1 led to a significant decrease in the activity of ERK, p38 MAPK, and AKT kinases, but not JNK (Fig. 4).
Effect of the recombinant rSLURP-1 protein on the activity of the main mitogenic signaling pathways in U251 MG glioma cells. Cells were incubated with 1 μM rSLURP-1 for 72 h, and phosphorylation of ERK, p38 MAPK, JNK, and AKT kinases was analyzed using MagPix technology (BioPlex Pro kit; Bio-Rad, United States). Data are presented as phosphorylation level ± standard error of the mean (n = 5). * p < 0.05 and **** p < 0.0001 (significant difference between groups according to two-tailed t test).
We have previously shown that rSLURP-1 in A549 lung adenocarcinoma cells modulates intracellular signaling through inositol-1,4,5-trisphosphate (IP3) and the PI3K/AKT/mTOR signaling pathway. However, rSLURP-1 does not change the activity of the main mitogenic pathway MEK/ERK and the pathways associated with p38 MAPK and AKT [35], whereas Lynx1 modulates the intracellular cascades associated with PKC/IP3, MEK/ERK, p38 MAPK, and JNK signaling pathways in A549 cells [40]. The comparison of the data obtained in this work with the results of previous studies suggests that the cell cycle arrest in the G2/M phase, observed upon the incubation of cancer cells with rSLURP-1 or Lynx1, can be achieved through different molecular interactions. The suppression of the activity of ERK, p38 MAPK, and AKT kinases mediates the cell cycle arrest in glioma cells, whereas in carcinomas the same process is due to inhibition of other kinases.
Previously, on the basis of the SLURP-1 loop I fragment, we obtained a synthetic peptide that inhibits the current through α7 nAChR and inhibits the growth and migration of A549 lung adenocarcinoma cells [35]. The data obtained in this work indicate the need for further studies of the antitumor effect of rSLURP-1 and its peptide mimetic in glioma cells. Since α7 nAChR activation may promote glioma progression [44, 45], the use of inhibitors of this receptor may become a new strategy for the treatment of these malignancies.
EXPERIMENTAL
Cell culturing. U251 MG glioma cells (Institute of Cytology, Russian Academy of Sciences, Russia) were cultured in RPMI-1640 medium (PanEco, Russia) supplemented with 10% fetal bovine serum (Hyclone, United Kingdom).
The animals were kept under standard conditions at the Laboratory Animal Breeding Facility of the Institute of Bioorganic Chemistry of the Russian Academy of Sciences (the unique scientific facility Bio-Model, Institute of Bioorganic Chemistry, Russian Academy of Sciences; Bioresource collection “Collection of Laboratory Rodents of SPF Status for Basic, Biomedical, and Pharmacological Research,” agreement no. 075-15-2021-1067), having the AAALACi international accreditation. Astrocytes of newborn rats were obtained as described previously [46] and cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (Hyclone, United Kingdom). Astrocytes were grown on plastic plates treated with poly-L-lysine (PanEco, Russia). Cells were cultured at 37°C and 5% CO2 and passaged at least twice a week.
Obtaining rSLURP-1. The recombinant SLURP-1 specimen was prepared as described previously [47]. The purity and correct spatial structure of the specimen were confirmed using mass spectrometry, high-performance liquid chromatography, and 1H NMR spectroscopy.
Effect of rSLURP-1 on the viability of glioma cells and astrocytes. Cells were seeded in 96-well culture plates (5 × 103 cells per well in 100 μL of medium); 1 μM rSLURP-1 (from 250 μM stock solution in 100% DMSO (Applichem, Germany)) was dissolved in the culture medium and added to the cells. Then, the cells were incubated for 72 h; the medium was replaced with a fresh one every 24 h. The maximum concentration of DMSO did not exceed 0.5%; the added DMSO did not affect cell growth, which was confirmed in a separate experiment. In each experiment, the control wells were supplemented with DMSO at the same concentration as that in the rSLURP-1 samples. Cell viability was assessed using the MTT assay. MTT (PanEco, Russia) at a final concentration of 0.1 mg/mL was added to the cells, the mixture was incubated for 4 h, and the formed formazan crystals were dissolved in isopropanol with 75 mM HCl. The absorbance was determined in the wells of the plate at 540 nm with equalization to the background at 655 nm using a Bio-Rad 680 microplate reader (Bio-Rad, United States). The absorbance of the plate wells was normalized to the absorbance of the wells with the untreated cells, and the results were analyzed using the Graphpad Prism 8.0 software (GraphPad Software, United States).
Effect of rSLURP-1 on U251 MG cell migration. The effect of rSLURP-1 on the migration of U251 MG cells in the in vitro wound healing model (scratch assay) was analyzed as described by Bychkov et al. [35]. U251 MG cells were seeded in 96-well plates (5 × 104 cells/well) and cultured for 24 h. Then, to minimize possible cell proliferation, the medium from the wells was replaced with a serum-free medium. After 8 h of incubation, a vertical scratch was made with a sterile 10-μL pipette tip at the bottom of the well (GenFollower nozzle, E-FTB10S, China). Then, the cells were washed with PBS and treated with rSLURP-1 diluted in the medium from 250 μM stock in 100% DMSO. DMSO without rSLURP-1 was added to the control wells. The images of the wells with scratches were analyzed at 0 and 24 h at 20× magnification using a CloneSelect Imager cell analysis system (Molecular Devices, United States). The center of the plate well was marked as the central reference point to ensure that the photograph of the well was not shifted. After taking digital images, the scratch area was estimated using ImageJ (NIH, United States) and MS Excel software by calculating the percentage of scratch surface occupied by migrating cells. In each experiment, repeated measurements were averaged.
Cell cycle analysis. For cell cycle analysis, U251 MG cells were seeded in 6-well culture plates (25 × 104 cells/well) and incubated with 1 μM rSLURP-1 for 72 h, replacing the medium with a fresh one every 24 h. Then, the cells were fixed in 70% ethanol for 12 h (–20°C), washed twice with Earl’s solution, and incubated in a buffer to facilitate the penetration of propidium iodide into the cells. Cell membranes were permeabilized with a permeabilization buffer (200 mM Na2HPO4 with 0.004% Triton X-100, pH 7.8) for 5 min. Thereafter, the cells were washed, resuspended in Earl’s solution supplemented with 50 mg/mL of propidium iodide and 0.2 mg/mL of RNase A, and analyzed with an Attune NxT cytofluorimeter (Life Technologies, United States). The percentage of cells in different cell cycle phases was determined using the Attune NxT Software (Life Technologies, United States).
Analysis of intracellular kinase phosphorylation. Phosphorylation of cell signaling proteins was analyzed using Bio-Plex magnetic particles with the Bio-Plex Pro cell signaling reagent kit (Bio-Rad, United States). Cells were incubated for 48 h with 1 μM rSLURP-1 from 250 μM stock in 100% stock DMSO and then lysed using the buffer from the kit. The analysis was performed with a Bio-Plex 200 instrument (Bio-Rad, United States) in accordance with the manufacturer’s instructions. The following sets of particles and detection antibodies were used to determine the levels of phosphorylation: ERK (171V50006M), p38 MAPK (171V50014M), JNK (171V50011M), and AKT (171V50001M).
CONCLUSIONS
This is the first study to show that the recombinant rSLURP-1 protein inhibits the growth of glioma cells, suppresses their migration, and induces cell cycle arrest in U251 MG glioma cells in the G2/M phase. The effects caused by rSLURP-1 in glioma cells are due to the suppression of the activity of ERK, p38 MAPK, and AKT kinases. Thus, the recombinant rSLURP-1 protein can be a prototype of new antiglioma drugs, modulators of nicotinic acetylcholine receptors.
REFERENCES
Cahill, D. and Turcan, S., Semin. Neurol., 2018, vol. 38, pp. 5–10. https://doi.org/10.1055/s-0037-1620238
Hu, Y., Jiang, Y., Behnan, J., Ribeiro, M.M., Kalantzi, C., Zhang, M.D., Lou, D., Haring, M., Sharma, N., Okawa, S., Del Sol., A., Adameyko, I., Svensson, M., Persson, O., and Ernfors, P., Sci. Adv., 2022, vol. 8, p. eabm6340. https://doi.org/10.1126/sciadv.abm6340
Ostrom, Q.T., Gittleman, H., Truitt, G., Boscia, A., Kruchko, C., and Barnholtz-Sloan, J.S., Neuro Oncol., 2018, vol. 20, pp. iv1–iv86. https://doi.org/10.1093/neuonc/noy131
Tan, A.C., Ashley, D.M., Lopez, G.Y., Malinzak, M., Friedman, H.S., and Khasraw, M., CA—Cancer J. Clin., 2020, vol. 70, pp. 299–312. https://doi.org/10.3322/caac.21613
Schuller, H.M., Nat. Rev. Cancer, 2009, vol. 9, pp. 195–205. https://doi.org/10.1038/nrc2590
Egleton, R.D., Brown, K.C., and Dasgupta, P., Trends Pharmacol. Sci., 2008, vol. 29, pp. 151–158. https://doi.org/10.1016/j.tips.2007.12.006
Zoli, M., Pucci, S., Vilella, A., and Gotti, C., Curr. Neuropharmacol., 2018, vol. 16, pp. 338–349. https://doi.org/10.2174/1570159X15666170912110450
Wessler, I. and Kirkpatrick, C.J., Br. J. Pharmacol., 2008, vol. 154, pp. 1558–1571. https://doi.org/10.1038/bjp.2008.185
Grando, S.A., Nat. Rev. Cancer, 2014, vol. 14, pp. 419–429. https://doi.org/10.1038/nrc3725
Tsurutani, J., Castillo, S.S., Brognard, J., Granville, C.A., Zhang, C., Gills, J.J., Sayyah, J., and Dennis, P.A., Carcinogenesis, 2005, vol. 26, pp. 1182–1195. https://doi.org/10.1093/carcin/bgi072
Dasgupta, P., J. Clin. Invest., 2006, vol. 116, pp. 2208–2217. https://doi.org/10.1172/JCI28164
Arredondo, J., Chernyavsky, A.I., Jolkovsky, D.L., Pinkerton, K.E., and Grando, S.A., FASEB J., 2007, vol. 22, pp. 1356–1368. https://doi.org/10.1096/fj.07-9965com
Wang, S. and Hu, Y., Oncol. Lett., 2018, vol. 16, pp. 1375–1382. https://doi.org/10.3892/ol.2018.8841
Shulepko, M.A., Bychkov, M.L., Lyukmanova, E.N., and Kirpichnikov, M.P., Dokl. Biochem. Biophys., 2020, vol. 493, pp. 211–214. https://doi.org/10.1134/S1607672920040134
Loughner, C.L., Bruford, E.A., McAndrews, M.S., Delp, E.E., Swamynathan, S., and Swamynathan, S.K., Hum. Genomics, 2016, vol. 10, p. 10. https://doi.org/10.1186/s40246-016-0074-2
Vasilyeva, N.A., Loktyushov, E.V., Bychkov, M.L., Shenkarev, Z.O., and Lyukmanova, E.N., Biochemistry (Moscow), 2017, vol. 82, pp. 1702–1715. https://doi.org/10.1134/S0006297917130090
Shulepko, M.A., Kulbatskii, D.S., Bychkov, M.L., and Lyukmanova, E.N., Russ. J. Bioorg. Chem., 2019, vol. 45, pp. 66–75. https://doi.org/10.1134/S1068162019020122
Liu, J.-F., Mao, L., Bu, L.-L., Ma, S.-R., Huang, C.-F., Zhang, W.-F., and Sun, Z.-J., Am. J. Cancer Res., 2015, vol. 5, pp. 3505–3515.
Jacobsen, B., Kriegbaum, M.C., Santoni-Rugiu, E., and Ploug, M., World J. Clin. Oncol., 2014, vol. 5, pp. 621–632. https://doi.org/10.5306/wjco.v5.i4.621
Choi, S.H., Kong, H.K., Park, S.Y., and Park, J.H., Int. J. Oncol., 2009, vol. 35, pp. 601–607. https://doi.org/10.3892/ijo_00000371
Su, S.-C., Lin, C.-W., Yang, W.-E., Fan, W.-L., and Yang, S.-F., Expert Opin. Ther. Targets, 2016, vol. 20, pp. 551–566. https://doi.org/10.1517/14728222.2016.1113260
Fu, X.W., Song, P.F., and Spindel, E.R., Int. Immunopharmacol., 2015, vol. 29, pp. 93–98. https://doi.org/10.1016/j.intimp.2015.05.022
Reiter, R.E., Gu, Z., Watabe, T., Thomas, G., Szigeti, K., Davis, E., Wahl, M., Nisitani, S., Yamashiro, J., and Le Beau, M.M., Proc. Natl. Acad. Sci. U. S. A., 1998, vol. 95, pp. 1735–1740. https://doi.org/10.1073/pnas.95.4.1735
Bergqvist, C., Kadara, H., Hamie, L., Nemer, G., Safi, R., Karouni, M., Marrouche, N., Abbas, O., Hasbani, D.J., and Kibbi, A.G., Int. J. Dermatol., 2018, vol. 57, pp. 162–170. https://doi.org/10.1111/ijd.13850
Russo, P., Cardinale, A., Margaritora, S., and Cesario, A., Life Sci., 2012, vol. 91, pp. 1087–1092. https://doi.org/10.1016/j.lfs.2012.05.003
Throm, V.M., Mannle, D., Giese, T., Bauer, A.S., Gaida, M.M., Kopitz, J., Bruckner, T., Plaschke, K., Grekova, S.P., and Felix, K., Oncotarget, 2018, vol. 9, pp. 11734–11751. https://doi.org/10.18632/oncotarget.24312
Luo, L., McGarvey, P., Madhavan, S., Kumar, R., Gusev, Y., and Upadhyay, G., Oncotarget, 2016, vol. 7, pp. 11165–11193. https://doi.org/10.18632/oncotarget.7163
Chernyavsky, A.I., Arredondo, J., Galitovskiy, V., Qian, J., and Grando, S.A., Am. J. Physiol. Cell Physiol., 2010, vol. 299, pp. 903–911. https://doi.org/10.1152/ajpcell.00216.2010
Pettersson, A., Nylund, G., Khorram-Manesh, A., Nordgren, S., and Delbro, D.S., Auton. Neurosci., 2009, vol. 148, pp. 97–100. https://doi.org/10.1016/j.autneu.2009.03.002
Arredondo, J., Chernyavsky, A.I., and Grando, S.A., Life Sci., 2007, vol. 80, pp. 2243–2247. https://doi.org/10.1016/j.lfs.2007.01.003
Lyukmanova, E.N., Shulepko, M.A., Kudryavtsev, D.S., Bychkov, M.L., Kulbatskii, D.S., Kasheverov, I.E., Astapova, M.V., Feofanov, A.V., Thomsen, M.S., Mikkelsen, J.D., Shenkarev, Z.O., Tsetlin, V.I., Dolgikh, D.A., and Kirpichnikov, M.P., PLoS One, 2016, vol. 11, p. e0149733. https://doi.org/10.1371/journal.pone.0149733
Lyukmanova, E.N., Bychkov, M.L., Sharonov, G.V., Efremenko, A.V., Shulepko, M.A., Kulbatskii, D.S., Shenkarev, Z.O., Feofanov, A.V., Dolgikh, D.A., and Kirpichnikov, M.P., Br. J. Pharmacol., 2018, vol. 175, pp. 1973–1986. https://doi.org/10.1111/bph.14194
Lyukmanova, E.N., Shulepko, M.A., Bychkov, M.L., Shenkarev, Z.O., Paramonov, A.S., Chugunov, A.O., Arseniev, A.S., Dolgikh, D.A., and Kirpichnikov, M.P., Acta Nat., 2014, vol. 6, pp. 60–66.
Shulepko, M.A., Bychkov, M.L., Shlepova, O.V., Shenkarev, Z.O., Kirpichnikov, M.P., and Lyukmanova, E.N., Int. Immunopharmacol., 2020, vol. 82, p. 106303. https://doi.org/10.1016/j.intimp.2020.106303
Bychkov, M.L., Shulepko, M.A., Shlepova, O.V., Kulbatskii, D.S., Chulina, I.A., Paramonov, A.S., Baidakova, L.K., Azev, V.N., Koshelev, S.G., Kirpichnikov, M.P., Shenkarev, Z.O., and Lyukmanova, E.N., Front. Cell Dev. Biol., 2021, vol. 9, p. 739391. https://doi.org/10.3389/fcell.2021.739391
Chernyavsky, A.I., Shchepotin, I.B., and Grando, S.A., Int. Immunopharmacol., 2015, vol. 29, pp. 36–44. https://doi.org/10.1016/j.intimp.2015.05.033
Kirichenko, A.V., Shlepova, O.V., Bychkov, M.L., Mikhaylova, I.N., Shulepko, M.A., and Lyukmanova, E.N., J. Invest. Dermatol., 2021, vol. 141, p. 9. https://doi.org/10.1016/j.jid.2021.02.065
Thompson, E.G. and Sontheimer, H., Cells, 2019, vol. 8, p. 1203. https://doi.org/10.3390/cells8101203
Kajstura, M., Halicka, H.D., Pryjma, J., and Darzynkiewicz, Z., Cytom. Part J. Int. Soc. Anal. Cytol., 2007, vol. 71, pp. 125–131. https://doi.org/10.1002/cyto.a.20357
Bychkov, M.L., Shenkarev, Z.O., Shulepko, M.A., Shlepova, O.V., Kirpichnikov, M.P., and Lyukmanova, E.N., PLoS One, 2019, vol. 14, p. e0217339. https://doi.org/10.1371/journal.pone.0217339
Grave, N., Scheffel, T.B., Cruz, F.F., Rockenbach, L., Goettert, M.I., Laufer, S., and Morrone, F.B., Front. Pharmacol., 2022, vol. 13, p. 975197. https://doi.org/10.3389/fphar.2022.975197
Chen, D., Zuo, D., Luan, C., Liu, M., Na, M., Ran, L., Sun, Y., Persson, A., Englund, E., and Salford, L.G., PLoS One, 2014, vol. 9, p. e87281. https://doi.org/10.1371/journal.pone.0087281
Zhang, Z.-Q., Wang, X., Xue, B.-H., Zhao, Y., Xie, F., Wang, S.-D., Xue, C., Wang, Y., Zhang, Y.-S., and Qian, L.-J., Oncol. Rep., vol. 46, p. 202. https://doi.org/10.3892/or.2021.8153
Pucci, S., Fasoli, F., Moretti, M., Benfante, R., Di Lascio, S., Viani, P., Daga, A., Gordon, T.J., McIntosh, M., and Zoli, M., Pharmacol. Res., 2021, vol. 163, p. 105336. https://doi.org/10.1016/j.phrs.2020.105336
McConnell, D.D., Carr, S.B., and Litofsky, N.S., Expert Rev. Neurother., 2019, vol. 19, pp. 545–555. https://doi.org/10.1080/14737175.2019.1617701
Schildge, S., Bohrer, C., Beck, K., and Schachtrup, C., J. Vis. Exp., 2013, p. e50079. https://doi.org/10.3791/50079
Shulepko, M.A., Lyukmanova, E.N., Paramonov, A.S., Lobas, A., Shenkarev, Z.O., Kasheverov, I.E., Dolgikh, D.A., Tsetlin, V.I., Arseniev, A.S., and Kirpichnikov, M.P., Biochemistry (Moscow), 2013, vol. 78, pp. 204–211. https://doi.org/10.1134/S0006297913020090
Funding
This work was supported by the Russian Science Foundation (project no. 17-74-20161).
Author information
Authors and Affiliations
Contributions
M.A. Shulepko and M.L. Bychkov contributed equally to writing this article.
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Additional information
Translated by M. Batrukova
The article is dedicated to the memory of Academician of the Russian Academy of Sciences Vadim T. Ivanov.
Coresponding author: phone: +7 (495) 336-80-11.
Abbreviations: AKT, protein kinase B; ERK, extracellular signal-regulated kinase; JNK, Jun kinase; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; nAChR, nicotinic acetylcholine receptor; NNN, N-nitrosonornicotine; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; PI3K, phosphoinositide-3-kinase; rSLURP-1 is a recombinant analog of the human SLURP-1 protein.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shulepko, M.A., Bychkov, M.L., Kirpichnikov, M.P. et al. Recombinant SLURP-1 Inhibits Growth and Migration of U251 MG Glioma by Cell Cycle Arrest and Modulation of MAPK and AKT Signaling Pathways. Russ J Bioorg Chem 49, 768–774 (2023). https://doi.org/10.1134/S1068162023040180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162023040180