Abstract
Members of the lymphocyte antigen-6 (Ly6)/urokinase-type plasminogen activator receptor (uPAR) superfamily of proteins are cysteine-rich proteins characterized by a distinct disulfide bridge pattern that creates the three-finger Ly6/uPAR (LU) domain. Although the Ly6/uPAR family proteins share a common structure, their expression patterns and functions vary. To date, 35 human and 61 mouse Ly6/uPAR family members have been identified. Based on their subcellular localization, these proteins are further classified as GPI-anchored on the cell membrane, or secreted. The genes encoding Ly6/uPAR family proteins are conserved across different species and are clustered in syntenic regions on human chromosomes 8, 19, 6 and 11, and mouse Chromosomes 15, 7, 17, and 9, respectively. Here, we review the human and mouse Ly6/uPAR family gene and protein structure and genomic organization, expression, functions, and evolution, and introduce new names for novel family members.
Similar content being viewed by others
Introduction
The lymphocyte antigen-6 (Ly6)/urokinase-type plasminogen activator receptor (uPAR) superfamily of structurally related proteins is characterized by the LU domain, an 80 amino acid domain containing ten cysteines arranged in a specific spacing pattern that allows distinct disulfide bridges which create the three-fingered (3F) structural motif [1, 2]. Ly6/uPAR proteins were first identified in the mouse over 35 years ago using antisera against lymphocytes [3]. Human homologs were subsequently isolated, leading to the recognition that they represent a well-conserved family with wide-ranging expression patterns and important functions. The fully annotated human and mouse genomes contain 35 and 61 Ly6/uPAR family members, respectively. Research over the last decade has begun to unravel the important functions of the encoded proteins. In this review, we provide an overview of the Ly6/uPAR gene family and their genomic organization, evolution, as well as functions, and provide a nomenclature system for the newly identified members of this family.
Inclusion and approved nomenclature for novel Ly6/uPAR family members
Although Ly6/uPAR family members are related by their structure, absence of a uniform naming convention resulted in arbitrary nomenclature for these genes as they were discovered. As many of the currently approved gene symbols for Ly6/uPAR family members (e.g., CD59 and PLAUR) have been widely used in the scientific literature for many years, we have refrained from a family-wide attempt to standardize their well-established names, avoiding the potential for additional confusion. In compiling this update, we came across many novel members of the Ly6/uPAR gene family, especially in the mouse genome, that did not yet have a systematic name. We named these novel family members in line with the Ly6/uPAR genes that they are most related to, based on a phylogenetic analysis (see below) using either the established LY6/Ly6# root for those that fell within the LY6 clades, or the LYPD/Lypd# (LY6/PLAUR domain-containing) root for those outside the LY6 clades. The new symbols for these genes (1 human and 18 mouse), approved by the HGNC (HUGO Gene Nomenclature Committee) [4, 5] and MGNC (Mouse Genomic Nomenclature Committee) [6], are listed in Tables 1 and 2, respectively. We use the newly approved names for these genes in the rest of this update. HGNC have also created a gene family web-page for the human Ly6/uPAR family members (http://www.genenames.org/cgi-bin/genefamilies/set/1226).
Genomic organization of the Ly6/uPAR gene family
The Ly6/uPAR gene family currently includes 35 well-characterized human members, while the mouse gene family is considerably larger with 61 genes. Information including the name, chromosomal location, numbers of exons and LU domains for human and mouse family members is summarized in Tables 1 and 2, respectively. Twelve human Ly6 genes are clustered together within a short span of about 500 kb on chromosome 8 (8q24) (moving outward from the center of the chromosome: PSCA, LY6K, SLURP1, LYPD2, LYNX1/SLURP2, LY6D, GML, LY6E, LY6L, LY6H, and GPIHBP1) (http://genome-euro.ucsc.edu). The syntenic region on mouse Chromosome 15 (15D3-15E1) contains Psca, Slurp1, Lypd2, Slurp2, Lynx1, Ly6d, Ly6g6g, Ly6k, Gml, Gml2, Ly6m, Ly6e, Ly6i, Ly6a, Ly6c1, Ly6c2, Ly6a2, Ly6g, Ly6g2, Ly6f, Ly6l, Ly6h, and Gpihbp1. Other smaller clusters are seen on human chromosome 19 (19q13) (LYPD4, CD177, TEX101, LYPD3, PINLYP, PLAUR, LYPD5, and SPACA4 with syntenic region on mouse Chromosome 7 containing Lypd5, Plaur, Pinlyp, Lypd3, Tex101, Lypd10, Lypd11, Cd177, Lypd4, and Spaca4), human chromosome 11 (11q24.2) (ACRV1, PATE1, PATE2, PATE3, and PATE4 with syntenic region on mouse Chromosome 9 containing Pate4, Pate2, Pate13, Pate3, Pate1, Pate10, Pate7, Pate6, Pate5, Pate12, Pate11, Pate9, Pate8, Pate14, and Acrv1), and human chromosome 6 (6p21) (LY6G6C, LY6G6D, LY6G6F, LY6G5C, and LY6G5B with syntenic region in the MHC class III region of the mouse Chromosome 17 containing Ly6g6c, Ly6g5c, Ly6g5b, Ly6g6d, Ly6g6f, and Ly6g6e), while the remaining family members are found on other chromosomes (Tables 1 and 2).
Typical Ly6/uPAR gene structure
Ly6/uPAR family members typically contain one LU domain, with the exception of LYPD3 [7] and CD177 [8] which contain two, and PLAUR [9], which contains three direct repeats of the LU domain (Tables 1 and 2). The mouse Cd177 differs from its human ortholog in that it contains four direct repeats of the LU domain. A typical Ly6/uPAR family gene consists of three exons and two introns (Fig. 1a), with the signal peptide being encoded in the first exon. The mature polypeptide is encoded by the last two exons, with the GPI-anchor domain encoded by the third exon.
Structure of a typical Ly6/UPAR family gene and alignment of amino acid sequences of selected LU domains. a Structure of a typical LY6/UPAR family gene, showing three exons (E-1, E-2 and E-3), two introns (I-1 and I-2), and the location of signal peptide as well as GPI-anchor domain. b Alignment of LU domain amino acid sequences of selected human LY6/UPAR proteins. GPI-anchored (top) and secreted (bottom) proteins are clustered together, with an empty line in between. Alignments were performed using ProbCons in Bioinformatics toolkit provided by Max-Planck Institute for Developmental Biology (http://toolkit.tuebingen.mpg.de). Conserved cysteines linked by non-variant disulfide bridges are highlighted in similar colors. Isoforms are denoted with a dash and the isoform name (e.g. LY6G6D-A). LYNX1-C and SLURP2 are precursor forms of the final protein. NTS, Non-translated sequence
Based on their subcellular localization, Ly6/uPAR family members are further subdivided into two groups: membrane-tethered (through a GPI-anchor domain) or secreted (lacking the GPI-anchor domain). GPI-anchored Ly6/uPAR family members tend to congregate on lipid rafts on the cell surface, which promotes their interactions with other proteins. A fraction of the GPI-anchored Ly6/uPAR family proteins such as PLAUR are secreted after their GPI-anchor domain is proteolytically cleaved [10–12]. Experimental evidence supports the presence of a GPI-anchoring signal peptide in a majority of Ly6/uPAR family members, while it is absent in a few (Table 3). For those with no experimental evidence, the GPI-anchor signal predictor ‘PredGPI’ program (http://gpcr.biocomp.unibo.it/predgpi/) [13] predicted the presence of a GPI-anchor signal within mouse and human LYPD8 and LY6L, and in mouse LYPD10, LYPD11, LYPD9, LY6F, and LY6M, while predicting its absence in mouse and human LYPD4, LY6G6F, and PINLYP, and in mouse GML2 and LY6G6 (Table 3).
Structure of the LU domain
The Ly6/uPAR family members have a well-conserved LU domain with a characteristic three-finger structure formed by disulfide bridges connecting the conserved cysteine residues in a specific pattern. LU domains are topologically similar to the three-finger structure of snake venom neurotoxins, which have three β-sheet loops fixed in space by virtue of their unique disulfide bridges. The structure of the extracellular region of CD59 was first solved by 2D NMR methods [14, 15] and further refined by crystallography [16] revealing it to be a flat, disk-shaped molecule consisting of a two-stranded beta-sheet finger loosely packed against a protein core formed by a three-stranded beta-sheet and a short helix.
Alignment of LU domain amino acid sequences of selected human LY6/UPAR proteins performed using ProbCons (http://toolkit.tuebingen.mpg.de) revealed the location of conserved cysteines (Fig. 1b). Five well-conserved disulfide bridges between cysteine pairs 3 and 26, 6 and 13, 19 and 39, 45 and 63, and 64 and 69 stabilize the hydrophobic core, from which three β-sheet-based fingers protrude (Fig. 1b). The sequence of the amino acids exposed at the tips of each finger as well as the length of each of the fingers is variable, providing the three-finger motif with the flexibility for a wide range of intermolecular interactions. In addition to the LU domain, Ly6/uPAR family proteins possess a well-conserved LeuXxxCysXxxXxxCys motif at the amino-terminus and CysCysXxxXxxXxxXxxCysAsn motif at the carboxyl-terminus (Fig. 1b). Functional relevance of these motifs is not yet known.
Most Ly6/uPAR family proteins maintain the ten cysteines characteristic of the LU domain, with some notable exceptions. In PLAUR, which consists of three LU domains (designated D1, D2 and D3), only domain D2 is fully intact with ten cysteines, while domains D1 and D3 have seven and eight cysteines, respectively. Isoforms of proteins such as human LY6G5C maintain conservation throughout the LU domain in almost every isoform. In contrast, different isoforms of human LYNX1 maintain the necessary cysteines, but little else is conserved (Fig. 1b).
Expression of Ly6/uPAR family genes
The expression pattern, interacting factors, and cellular functions of the mouse and human Ly6/uPAR family members are summarized in Table 3. Expression of Ly6/uPAR proteins is (i) widespread and variable across diverse cell types and tissues, (ii) tightly regulated in a spatiotemporal manner, and (iii) often correlated with cellular differentiation. Although the Ly6/uPAR family protein structures are well-conserved across species, their expression patterns tend to vary, indicating divergence among their regulatory networks. Many Ly6/uPAR family members are expressed in hematopoietic precursors in a lineage-specific fashion making them useful cell surface markers for leukocytes, facilitating identification of individual leukocyte subgroups [17–19]. For example, mouse myeloid differentiation marker LY6G (also called Gr-1) is expressed by the myeloid lineage cells in a developmentally regulated manner in the bone marrow. Anti-LY6G antibodies are routinely used to identify neutrophils in the mouse but not humans as there is no human ortholog for Ly6g. Ly6/uPAR family members are generally upregulated during inflammatory conditions or infections and in cancerous cells, with a notable exception of SLURP1, which is invariably downregulated in pro-inflammatory conditions [9, 20–24].
Functions of Ly6/uPAR family proteins
Commensurate with their varied expression patterns, Ly6/uPAR proteins have a wide range of functions in cell proliferation, migration, cell-cell interaction, immune cell maturation, macrophage activation, and cytokine production. They typically exert their influence by targeting nicotinic acetylcholine receptors (nAChRs) (reviewed in [1]). GPI-anchored Ly6/uPAR proteins lacking a cytoplasmic tail are unable to directly participate in intracellular signaling but can initiate signaling by interacting with other transmembrane proteins. Such interactions of GPI-anchored proteins are further facilitated by their tendency to congregate in lipid rafts on the cell surface, where other signaling molecules also are enriched. While GPI-anchored Ly6/uPAR proteins control signaling through interaction with their ligand(s), secreted Ly6/uPAR proteins may serve as agonists for other receptors including nAChR and/or competing scavengers of their ligands [1, 20, 21, 25–27]. Many Ly6/uPAR family members have a prominent role in neutrophils (Table 3) [28]. Below, we summarize the functions of a few well-studied members.
Prostate and testis expressed genes
Human chromosome 11 contains 5 prostate and testis expressed (PATE) genes while the syntenic region on murine Chromosome 9 contains 15 genes [29]. Recent evidence demonstrates that PATE proteins are much more predominantly expressed in the epididymis with a significantly lower expression in the prostate and testis, suggesting that their names are misnomers [30]. PATE proteins secreted by epithelial cells to the epididymal lumen facilitate spermatozoan maturation as they leave the testis and travel through the epididymis. PATE proteins localized in the sperm head assist in sperm-oolemma fusion and penetration [31]. Defects in PATE1 result in decreased sperm motility in aged men and young asthenozoospermia patients, revealing the molecular basis for the decline in sperm quality with age [32]. PATE4 is abundantly expressed in the mouse prostate, spermatozoa, and seminal vesicles. Pate4−/− mice remain fertile and do not display any histological abnormalities [33]. PATE proteins are also expressed in neuron-rich tissues, where they function by modulating nAChR activities [29].
Plasminogen activator, urokinase receptor
Also known as the urokinase-type plasminogen activator receptor (uPAR), plasminogen activator, urokinase receptor (PLAUR) is the most well-studied family member [9]. It is widely expressed in different cell types and plays a key regulatory role in cell surface plasminogen activation, influencing many normal and pathologic processes [9, 23]. PLAUR consists of three direct repeats of the LU domain, which together bind urokinase-type plasminogen activator (PLAU/uPA) in both the pro-protein and mature forms. PLAUR (i) expression is regulated by KLF4 [34] and is upregulated in cancer cells [35, 36] and in response to pro-inflammatory conditions [37], (ii) facilitates neutrophil recruitment in response to bacterial infection [38], (iii) facilitates clearance of Borrelia infection [39], and (iv) interacts with multiple partners including vitronectin and different integrins. Although the bulk of PLAUR exists as GPI-anchored, some of it is known to be secreted as “soluble uPAR” (suPAR), the expression level of which is correlated with disease conditions [10–12, 40].
PLAUR is a multi-functional protein with important roles in regulating cell-matrix interaction, motility, and immune response. PLAUR expression levels directly correlate with the invasive potential of endometrial carcinomas, suggesting that it is a valuable prognostic marker for aggressive endometrial tumors [35]. PLAUR expression is normally low in healthy glomeruli and is elevated in glomeruli from individuals with focal segmental glomerulosclerosis, consistent with its role in regulating renal permeability [41]. PLAUR is required for neutrophil recruitment into alveoli and lungs in response to S. pneumoniae infection [42]. Plaur−/− macrophages display an enhanced ability to engulf wild-type neutrophils, but Plaur−/− neutrophils do not, suggesting that PLAUR plays an essential role in recognition and clearance of neutrophils [43]. Plaur−/− mice exhibit abnormal interneuron migration from the ganglionic eminence, and reduced interneurons in the frontal and parietal cortex [44, 45].
CD177
Expressed by neutrophils, neutrophilic metamyelocytes, and myelocytes, CD177 mediates neutrophil migration across the endothelium by binding PECAM1 (CD31). Anti-CD177 antibodies inhibit neutrophil transmigration across the endothelial monolayer, potentially by interfering with an interaction between CD177 and PECAM1 [46]. Mutations in CD177 or its dysregulated expression are associated with myeloproliferative diseases, secondary to a gain-of-function mutation in JAK2 [8]. Exposure of human neutrophils to pulmonary endotoxin results in strong upregulation of CD177 [47]. Expression of CD177 mRNA is highly upregulated following endotoxin exposure. Overexpression of CD177 is a biomarker for thrombocythemia patients with elevated risk of thromboembolic complications [8]. While human CD177 contains nine exons that encode a protein with two LU domain repeats, mouse Cd177 is substantially larger with 17 exons that encode a larger protein with four LU domain repeats. Surprisingly, Cd177−/− mice displayed no discernible phenotype or any change in immune cells, other than decreased neutrophil counts in peripheral blood [47]. Absence of CD177 had no significant impact on CXCL1/KC- or fMLP-induced mouse neutrophil migration, but led to significant cell death [47].
Complement regulatory protein CD59
CD59 is an essential regulatory protein that protects hematopoietic and neuronal cells against complement-mediated osmolytic pore formation by binding C8 and/or C9 and inhibiting the incorporation of C9 into the membrane attack complex [17, 48–51]. CD2-mediated CD59 stimulation results in secretion of IL1A (IL-1α), IL6, and CSF2 (GM-CSF) in keratinocytes [52]. Inadequate complement regulation is associated with age-related macular degeneration [53]. Mutations in CD59 cause uncontrolled complement activation in hemolytic anemia, thrombosis, and cerebral infarction in paroxysmal nocturnal hemoglobinuria [54]. The mouse genome contains two homologs of CD59, termed Cd59a, and Cd59b. Mouse CD59B has approximately a sixfold higher specific activity than CD59A and is considered a true ortholog of human CD59. Cd59a deficiency exacerbated the skin disease and lymphoproliferative characteristic of the MRL/lpr murine lupus model suggesting that CD59A inhibits systemic autoimmunity in the MRL/lpr lupus model through a complement-independent mechanism [55]. Consistent with its higher specific activity, Cd59b−/− mice display a stronger phenotype including hemolytic anemia, anisopoikilocytosis, echinocytosis, schistocytosis, hemoglobinuria with hemosiderinuria, and platelet activation [56]. Cd59b−/− males suffer from progressive loss of fertility after 5 months of age [56].
Prostate stem cell antigen
Prostate stem cell antigen (PSCA) is a 123 amino acid protein with an N-terminal signal sequence, and a C-terminal GPI-anchoring sequence [57]. It was initially identified as a prostate-specific cell surface antigen in normal male tissues and found to be highly expressed in human prostate cancer [58, 59]. Later studies have revealed it to be more widely expressed. A genome-wide association study of Japanese patients with gastric cancer revealed that genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer [60]. Psca−/− mice are viable, and fertile, with similar rates of spontaneous or radiation-induced primary epithelial tumor formation as the wild-type mice. However, Psca−/− mice display an increased frequency of metastasis suggesting that PSCA may play a role in limiting tumor progression, and deletion of Psca promotes tumor migration and metastasis [61].
GPI-anchored high density lipoprotein-binding protein 1
GPI-anchored high density lipoprotein-binding protein 1 (GPIHBP1) is an endothelial cell protein expressed on the luminal face of capillaries in brown adipose tissue, heart, lung, and liver. GPIHBP1 binds high density lipoprotein and provides a platform for lipoprotein lipase (LPL)-mediated processing of chylomicron lipoprotein particles which transport dietary lipids from the intestines to other locations in the body. GPIHBP1 mutations that affect its ability to bind LPL or chylomicrons are associated with chylomicronemia [62–64]. Gpihbp1−/− mice cannot transport lipoprotein lipase to the capillary lumen, resulting in mislocalization of lipoprotein lipase within tissues, defective lipolysis of triglyceride-rich lipoproteins, and chylomicronemia [62–64]. Defective lipolysis causes reciprocal metabolic perturbations in Gpihbp1−/− mouse adipose tissue and liver. The essential fatty acid content of triglycerides is decreased and lipid biosynthetic gene expression is increased in adipose tissue, while the opposite changes occur in the liver [65].
Ly6/neurotoxin-1
As an allosteric modulator of nAChR function, Ly6/neurotoxin-1 (LYNX1) serves as a cholinergic brake that limits neuronal plasticity, balancing neuronal activity, and survival in the adult visual cortex [25, 66–68]. LYNX1 also inhibits SRC activation, suppressing mucin expression in the airway epithelium [69]. The LYNX1 gene is positioned in close proximity to SLURP2, leading to the mistaken idea that they are alternatively spliced isoforms of the same gene, a theory which was disproved recently [70]. LYNX1 is one of the genes that has shown accelerated evolution in humans relative to other primates, correlating with the increased brain size and complexity [71]. The juvenile brain exhibits high plasticity which is severely restricted in adulthood. Adult Lynx1−/− mice exhibited visual cortex plasticity similar to that of juveniles, suggesting that LYNX1 serves as a break for cortical plasticity [68]. Using the mouse model, it was demonstrated that LYNX1 plays a modulatory role in the aging brain, and that soluble LYNX1 may be useful for adjusting cholinergic-dependent plasticity and learning mechanisms [72–74].
Secreted Ly6/urokinase-type plasminogen activator receptor-related protein 1
Secreted Ly6/urokinase-type plasminogen activator receptor-related protein 1 (SLURP1) is expressed in a variety of cells including immune cells [75], sensory neurons [76], and epithelial cells [77–80], and secreted into plasma, saliva, sweat, urine, and tears [22, 81]. SLURP1 is downregulated in corneal neovascularization [82], asthmatic lungs [83], Barrett’s esophagus [84, 85], malignant melanomas [86], and squamous cell carcinomas [87, 88]. Mutations or deletions in SLURP1 cause autosomal recessive palmoplantar hyperkeratotic disorder ‘mal de Meleda’ [78, 81, 89–93]. SLURP1 is structurally similar to the snake and frog cytotoxin α-bungarotoxin, and acts as a CHRNA7 (α7nAChR)-ligand, regulating keratinocytes through cholinergic pathways [78, 94]. It modulates signal transduction, activation of the immune response, and cell adhesion, and blocks malignant transformation [75, 79, 95–97]. SLURP1 is proposed to modulate acetylcholine signaling through CHRNA7 [98]. We have documented that SLURP1 serves as an important immunomodulatory molecule at the ocular surface by acting as a soluble scavenger of urokinase (PLAU) [20–22]. Slurp1−/− mice develop signs of palmoplantar keratoderma including elevated keratinocyte proliferation, accumulation of lipid droplets in the stratum corneum, and defective epidermal barrier function reminiscent of mal de Meleda. Slurp1−/− mice also display decreased adiposity, low plasma lipid levels, and a neuromuscular abnormality (hind-limb clasping), suggesting additional functions for SLURP1 [99].
Secreted Ly6/urokinase-type plasminogen activator receptor-related protein 2 (SLURP2)
SLURP2 is expressed by human epidermal and oral keratinocytes, from where it is secreted into sweat and saliva, respectively [100]. SLURP2 expression is strongly induced in psoriatic skin lesions possibly by IL22, and is blocked by IFNG [70, 101]. SLURP2 blocks the effect of acetylcholine by binding CHRNA3 (α3nAChR), and delays keratinocyte differentiation and prevents apoptosis [100]. Although the SLURP2 and LYNX1 genes are closely linked leading to a mistaken idea that they are isoforms, it is now clear that they constitute separate transcription units that are differently regulated [70]. Slurp2−/− mice also develop signs of palmoplantar keratoderma and neuromuscular abnormality (hind-limb clasping) reminiscent of those seen in Slurp1−/− mice [99, 102].
Ly6/Plaur domain containing 1
Ly6/Plaur domain containing 1 (LYPD1), also known as LYNX2, is a prototoxin gene that is expressed in postmitotic central and peripheral neurons including subpopulations of motor neurons, sensory neurons, interneurons, and neurons of the autonomous nervous system [103]. LYPD1 is expressed at high levels in anxiety associated brain areas and plays an important role in regulating anxiety by binding and modulating neuronal nicotinic receptors [104, 105]. Ablation of Lypd1 alters the actions of nicotine on glutamatergic signaling in the prefrontal cortex, resulting in elevated anxiety-like behaviors [104].
Evolution of Ly6/uPAR family proteins
Ly6/uPAR family genes are conserved across species suggesting that they are evolutionarily ancient. Organization of the genes in this family in clusters on multiple chromosomes suggests that both gene duplications and translocations have played a role in their evolution. Comparison of the mouse and human Ly6/uPAR family genes reveals that while there are many orthologs, some Ly6 genes are only present in the mouse. The Ly6 gene complexes on human chromosomes 8, 19, 11, and 6 are syntenic with their counterparts on mouse Chromosomes 15, 7, 9, and 17, respectively, suggesting that these gene complexes were already present in their common ancestor. There are no human orthologs for the subcluster of murine Ly6 genes Ly6i, Ly6a, Ly6c1, Ly6c2, Ly6a2, Ly6g, Ly6g2, and Ly6f on Chromosome 15, and Pate10, Pate7, Pate6, Pate5, Pate12, Pate11, Pate9, Pate8, and Pate14 on Chromosome 9, suggesting that these regions may have arisen in the mouse through gene duplication after evolutionary divergence of these two species. What their functions are in the murine neutrophils and epididymis, respectively, where they are abundantly expressed, and how they are compensated in the corresponding human tissues, remains to be determined.
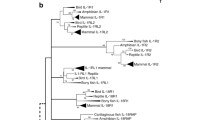
In order to evaluate the evolutionary relatedness of LU family proteins, we generated a phylogram by multiple sequence alignment of their amino acid sequences using web-based Clustal-Omega software, and visualized it with web-based software from Interactive Tree of Life (Fig. 2) [106–108]. Where multiple isoforms exist, we only used the sequence of the longest isoform. Analysis of the phylogenetic relationship among human and mouse Ly6/uPAR family proteins revealed that (i) human LY6K and mouse GML2 are the most ancestral Ly6 proteins with the longest unbranched streak in these two species, (ii) human and mouse LYPD6 are the most recent addition to the family closely followed by mouse LY6C1 and LY6C2, (iii) most of the secreted family proteins (with the notable exception of SLURP1 and SLURP2) form a separate cluster distinct from the GPI-anchored proteins, and (iv) several mouse PATE proteins (PATE4, 5, 6, 7, 8, 9, 10, 13, and 14) have long unbranched streaks suggesting that they have ancient origin and that the important function(s) that they serve have not changed much (Fig. 2).
Phylogram revealing the evolutionary relationship among mouse (ms-) and human (hu-) Ly6/uPAR family proteins. The phylogram was generated using the amino acid sequences in Clustal-Omega web-based program [106, 107] (http://www.ebi.ac.uk/Tools/msa/clustalo/). The display was generated using the methods described [108]. The length of each branch from the most recent branch point indicates the evolutionary distance, or the relative period of time the protein has been in its current state. Known GPI-anchored Ly6/uPAR family proteins are shown in red, and those secreted (without GPI-anchor) are shown in green. Those predicted to contain a GPI-anchor (but not yet experimentally proven) are in purple, and those predicted to not contain a GPI-anchor sequence (but not yet experimentally proven) are in black. Novel genes named in this study are indicated with an asterisk (*)
Concluding remarks
In this gene family update, we have summarized the current literature on the organization, expression patterns, functions, and evolution of human and mouse Ly6/uPLAR family genes. In addition, we identified and named many novel Ly6/uPAR family members. Considering that Ly6/uPLAR family members play critical roles in regulating immunological and physiological responses to infections and varying environmental conditions, it is imperative that we understand them in greater detail. Their involvement in regulating a wide range of functions such as progression of inflammation, complement activity, neuronal activity, angiogenesis, wound healing, and cancer growth indicates that Ly6/uPAR family members will be useful therapeutic targets. Additional insight into (i) the biological functions of individual family proteins, (ii) signaling cascades that regulate their expression and functions, and (iii) the identity of their interacting partners is expected to herald new modalities for diagnosis and treatment of diverse diseases.
References
Tsetlin VI. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: pharmacological tools and endogenous modulators. Trends in Pharmacological Sciences. 2015;36(2):109–23.
Gumley TP, McKenzie IF, Sandrin MS. Tissue expression, structure and function of the murine Ly-6 family of molecules. Immunology and cell biology. 1995;73(4):277–96.
McKenzie IF, Gardiner J, Cherry M, Snell GD. Lymphocyte antigens: Ly-4, Ly-6, and Ly-7. Transplantation proceedings. 1977;9(1):667–9.
Wain HM, Bruford EA, Lovering RC, Lush MJ, Wright MW, Povey S. Guidelines for human gene nomenclature. Genomics. 2002;79(4):464–70.
Gray KA, Seal RL, Tweedie S, Wright MW, Bruford EA. A review of the new HGNC gene family resource. Human genomics. 2016;10(1):6.
Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE, Mouse Genome Database G. The Mouse Genome Database (MGD): facilitating mouse as a model for human biology and disease. Nucleic Acids Res. 2015;43(Database issue):D726–736.
Wurfel J, Seiter S, Stassar M, Claas A, Klas R, Rosel M, Marhaba R, Savelyeva L, Schwab M, Matzku S, et al. Cloning of the human homologue of the metastasis-associated rat C4.4A. Gene. 2001;262(1–2):35–41.
Stroncek DF. Neutrophil-specific antigen HNA-2a, NB1 glycoprotein, and CD177. Current opinion in hematology. 2007;14(6):688–93.
Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11(1):23–36.
Sloand EM. Soluble urokinase activator receptor (suPAR) in stem cell mobilization. Blood. 2005;105(5):1847–8.
Eugen-Olsen J, Giamarellos-Bourboulis EJ. suPAR: the unspecific marker for disease presence, severity and prognosis. International journal of antimicrobial agents. 2015;46 Suppl 1:S33–34.
Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ, Schultz MJ. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive care medicine. 2012;38(9):1418–28.
Pierleoni A, Martelli PL, Casadio R. PredGPI: a GPI-anchor predictor. BMC bioinformatics. 2008;9:392.
Fletcher CM, Harrison RA, Lachmann PJ, Neuhaus D. Sequence-specific 1H-NMR assignments and folding topology of human CD59. Protein science : a publication of the Protein Society. 1993;2(12):2015–27.
Kieffer B, Driscoll PC, Campbell ID, Willis AC, van der Merwe PA, Davis SJ. Three-dimensional solution structure of the extracellular region of the complement regulatory protein CD59, a new cell-surface protein domain related to snake venom neurotoxins. Biochemistry. 1994;33(15):4471–82.
Huang Y, Fedarovich A, Tomlinson S, Davies C. Crystal structure of CD59: implications for molecular recognition of the complement proteins C8 and C9 in the membrane-attack complex. Acta crystallographica Section D, Biological crystallography. 2007;63(Pt 6):714–21.
Davies A, Simmons DL, Hale G, Harrison RA, Tighe H, Lachmann PJ, Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989;170(3):637–54.
Simmons PJ, Zannettino AC, Harrison-Findik D, Swart B, Tomlinson S, Hill B, Javni JA. A novel epitope of CD59 expressed by primitive human hematopoietic progenitors. Experimental hematology. 2001;29(12):1474–83.
Hill B, Rozler E, Travis M, Chen S, Zannetino A, Simmons P, Galy A, Chen B, Hoffman R. High-level expression of a novel epitope of CD59 identifies a subset of CD34+ bone marrow cells highly enriched for pluripotent stem cells. Experimental hematology. 1996;24(8):936–43.
Swamynathan S, Buela KA, Kinchington P, Lathrop KL, Misawa H, Hendricks RL, Swamynathan SK. Klf4 regulates the expression of Slurp1, which functions as an immunomodulatory peptide in the mouse cornea. Invest Ophthalmol Vis Sci. 2012;53(13):8433–46.
Swamynathan S, Swamynathan SK. SLURP-1 modulates corneal homeostasis by serving as a soluble scavenger of urokinase-type plasminogen activator. Invest Ophthalmol Vis Sci. 2014;55(10):6251–61.
Swamynathan S, Delp EE, Harvey SA, Loughner CL, Raju L, Swamynathan SK. Corneal expression of SLURP-1 by age, sex, genetic strain, and ocular surface health. Invest Ophthalmol Vis Sci. 2015;56(13):7888–96.
Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3(12):932–43.
Flanagan K, Modrusan Z, Cornelius J, Chavali A, Kasman I, Komuves L, Mo L, Diehl L. Intestinal epithelial cell up-regulation of LY6 molecules during colitis results in enhanced chemokine secretion. J Immunol. 2008;180(6):3874–81.
Ibanez-Tallon I, Miwa JM, Wang HL, Adams NC, Crabtree GW, Sine SM, Heintz N. Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron. 2002;33(6):893–903.
Jensen MM, Arvaniti M, Mikkelsen JD, Michalski D, Pinborg LH, Hartig W, Thomsen MS. Prostate stem cell antigen interacts with nicotinic acetylcholine receptors and is affected in Alzheimer’s disease. Neurobiology of aging. 2015;36(4):1629–38.
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8.
Lee PY, Wang JX, Parisini E, Dascher CC, Nigrovic PA. Ly6 family proteins in neutrophil biology. J Leukoc Biol. 2013;94(4):585–94.
Levitin F, Weiss M, Hahn Y, Stern O, Papke RL, Matusik R, Nandana SR, Ziv R, Pichinuk E, Salame S, et al. PATE gene clusters code for multiple, secreted TFP/Ly-6/uPAR proteins that are expressed in reproductive and neuron-rich tissues and possess neuromodulatory activity. J Biol Chem. 2008;283(24):16928–39.
Turunen HT, Sipila P, Pujianto DA, Damdimopoulos AE, Bjorkgren I, Huhtaniemi I, Poutanen M. Members of the murine Pate family are predominantly expressed in the epididymis in a segment-specific fashion and regulated by androgens and other testicular factors. Reproductive Biology and Endocrinology. 2011;9(1):128.
Margalit M, Yogev L, Yavetz H, Lehavi O, Hauser R, Botchan A, Barda S, Levitin F, Weiss M, Pastan I, et al. Involvement of the prostate and testis expression (PATE)-like proteins in sperm-oocyte interaction. Human reproduction. 2012;27(5):1238–48.
Liu FJ, Liu X, Han JL, Wang YW, Jin SH, Liu XX, Liu J, Wang WT, Wang WJ. Aged men share the sperm protein PATE1 defect with young asthenozoospermia patients. Human reproduction. 2015;30(4):861–9.
Heckt T, Keller J, Reusch R, Hartmann K, Krasemann S, Hermans-Borgmeyer I, Amling M, Schinke T. No obvious phenotypic abnormalities in mice lacking the Pate4 gene. Biochem Biophys Res Commun. 2016;469(4):1069–74.
Wang H, Yang L, Jamaluddin MS, Boyd DD. The Kruppel-like KLF4 transcription factor, a novel regulator of urokinase receptor expression, drives synthesis of this binding site in colonic crypt luminal surface epithelial cells. J Biol Chem. 2004;279(21):22674–83.
Foca C, Moses EK, Quinn MA, Rice GE. Differential mRNA expression of urokinase-type plasminogen activator, plasminogen activator receptor and plasminogen activator inhibitor type-2 in normal human endometria and endometrial carcinomas. Gynecologic oncology. 2000;79(2):244–50.
Memarzadeh S, Kozak KR, Chang L, Natarajan S, Shintaku P, Reddy ST, Farias-Eisner R. Urokinase plasminogen activator receptor: prognostic biomarker for endometrial cancer. Proc Natl Acad Sci U S A. 2002;99(16):10647–52.
Coleman JL, Gebbia JA, Benach JL. Borrelia burgdorferi and other bacterial products induce expression and release of the urokinase receptor (CD87). J Immunol. 2001;166(1):473–80.
Gyetko MR, Sud S, Kendall T, Fuller JA, Newstead MW, Standiford TJ. Urokinase receptor-deficient mice have impaired neutrophil recruitment in response to pulmonary Pseudomonas aeruginosa infection. J Immunol. 2000;165(3):1513–9.
Hovius JW, Bijlsma MF, van der Windt GJ, Wiersinga WJ, Boukens BJ, Coumou J, Oei A, de Beer R, de Vos AF, van 't Veer C, et al. The urokinase receptor (uPAR) facilitates clearance of Borrelia burgdorferi. PLoS Pathog. 2009;5(5), e1000447.
Hodges GW, Bang CN, Wachtell K, Eugen-Olsen J, Jeppesen JL. suPAR: a New biomarker for cardiovascular disease? The Canadian journal of cardiology. 2015;31(10):1293–302.
Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63.
Rijneveld AW, Levi M, Florquin S, Speelman P, Carmeliet P, van Der Poll T. Urokinase receptor is necessary for adequate host defense against pneumococcal pneumonia. J Immunol. 2002;168(7):3507–11.
Park YJ, Liu G, Tsuruta Y, Lorne E, Abraham E. Participation of the urokinase receptor in neutrophil efferocytosis. Blood. 2009;114(4):860–70.
Dewerchin M, Nuffelen AV, Wallays G, Bouche A, Moons L, Carmeliet P, Mulligan RC, Collen D. Generation and characterization of urokinase receptor-deficient mice. J Clin Invest. 1996;97(3):870–8.
Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30(1):79–89.
Sachs UJ, Andrei-Selmer CL, Maniar A, Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT, Chavakis T, et al. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31). J Biol Chem. 2007;282(32):23603–12.
Xie Q, Klesney-Tait J, Keck K, Parlet C, Borcherding N, Kolb R, Li W, Tygrett L, Waldschmidt T, Olivier A, et al. Characterization of a novel mouse model with genetic deletion of CD177. Protein & cell. 2015;6(2):117–26.
Meri S, Morgan BP, Davies A, Daniels RH, Olavesen MG, Waldmann H, Lachmann PJ. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71(1):1–9.
Meri S, Morgan BP, Wing M, Jones J, Davies A, Podack E, Lachmann PJ. Human protectin (CD59), an 18-20-kD homologous complement restriction factor, does not restrict perforin-mediated lysis. J Exp Med. 1990;172(1):367–70.
Chang CP, Husler T, Zhao J, Wiedmer T, Sims PJ. Identity of a peptide domain of human C9 that is bound by the cell-surface complement inhibitor, CD59. J Biol Chem. 1994;269(42):26424–30.
Hochsmann B, Schrezenmeier H. Congenital CD59 deficiency. Hematology/oncology clinics of North America. 2015;29(3):495–507.
Naderi S, Hofmann P, Seiter S, Tilgen W, Abken H, Reinhold U. CD2-mediated CD59 stimulation in keratinocytes results in secretion of IL-1alpha, IL-6, and GM-CSF: implications for the interaction of keratinocytes with intraepidermal T lymphocytes. International Journal of Molecular Medicine. 1999;3(6):609–14.
Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J Pathol. 2013;229(5):729–42.
Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–11.
Miwa T, Zhou L, Maldonado MA, Madaio MP, Eisenberg RA, Song WC. Absence of CD59 exacerbates systemic autoimmunity in MRL/lpr mice. J Immunol. 2012;189(11):5434–41.
Qin X, Krumrei N, Grubissich L, Dobarro M, Aktas H, Perez G, Halperin JA. Deficiency of the mouse complement regulatory protein mCd59b results in spontaneous hemolytic anemia with platelet activation and progressive male infertility. Immunity. 2003;18(2):217–27.
Saeki N, Gu J, Yoshida T, Wu X. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clinical Cancer Research. 2010;16(14):3533–8.
Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, Wahl M, Nisitani S, Yamashiro J, Le Beau MM, et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 1998;95(4):1735–40.
Gu Z, Thomas G, Yamashiro J, Shintaku IP, Dorey F, Raitano A, Witte ON, Said JW, Loda M, Reiter RE. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19(10):1288–96.
Study Group of Millennium Genome Project for C, Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, Matsuno Y, Saito D, Sugimura H, Tanioka F, et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008;40(6):730–40.
Moore ML, Teitell MA, Kim Y, Watabe T, Reiter RE, Witte ON, Dubey P. Deletion of PSCA increases metastasis of TRAMP-induced prostate tumors without altering primary tumor formation. The Prostate. 2008;68(2):139–51.
Beigneux AP, Davies BS, Gin P, Weinstein MM, Farber E, Qiao X, Peale F, Bunting S, Walzem RL, Wong JS, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metabolism. 2007;5(4):279–91.
Beigneux AP, Franssen R, Bensadoun A, Gin P, Melford K, Peter J, Walzem RL, Weinstein MM, Davies BS, Kuivenhoven JA, et al. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(6):956–62.
Franssen R, Young SG, Peelman F, Hertecant J, Sierts JA, Schimmel AW, Bensadoun A, Kastelein JJ, Fong LG, Dallinga-Thie GM, et al. Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circulation Cardiovascular Genetics. 2010;3(2):169–78.
Weinstein MM, Goulbourne CN, Davies BS, Tu Y, Barnes 2nd RH, Watkins SM, Davis R, Reue K, Tontonoz P, Beigneux AP, et al. Reciprocal metabolic perturbations in the adipose tissue and liver of GPIHBP1-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(2):230–5.
Miwa JM, Stevens TR, King SL, Caldarone BJ, Ibanez-Tallon I, Xiao C, Fitzsimonds RM, Pavlides C, Lester HA, Picciotto MR, et al. The prototoxin lynx1 acts on nicotinic acetylcholine receptors to balance neuronal activity and survival in vivo. Neuron. 2006;51(5):587–600.
Miwa T, Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol. 2001;1(3):445–59.
Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330(6008):1238–40.
Fu XW, Rekow SS, Spindel ER. The ly-6 protein, lynx1, is an endogenous inhibitor of nicotinic signaling in airway epithelium. American Journal of Physiology Lung Cellular and Molecular Physiology. 2012;303(8):L661–668.
Moriwaki Y, Takada K, Tsuji S, Kawashima K, Misawa H. Transcriptional regulation of SLURP2, a psoriasis-associated gene, is under control of IL-22 in the skin: a special reference to the nested gene LYNX1. Int Immunopharmacol. 2015;29(1):71–5.
Dorus S, Vallender EJ, Evans PD, Anderson JR, Gilbert SL, Mahowald M, Wyckoff GJ, Malcom CM, Lahn BT. Accelerated evolution of nervous system genes in the origin of Homo sapiens. Cell. 2004;119(7):1027–40.
Miwa JM, Walz A. Enhancement in motor learning through genetic manipulation of the Lynx1 gene. PLoS One. 2012;7(11):e43302.
Lyukmanova EN, Shulepko MA, Buldakova SL, Kasheverov IE, Shenkarev ZO, Reshetnikov RV, Filkin SY, Kudryavtsev DS, Ojomoko LO, Kryukova EV, et al. Water-soluble LYNX1 residues important for interaction with muscle-type and/or neuronal nicotinic receptors. J Biol Chem. 2013;288(22):15888–99.
Bukhari N, Burman PN, Hussein A, Demars MP, Sadahiro M, Brady DM, Tsirka SE, Russo SJ, Morishita H. Unmasking proteolytic activity for adult visual cortex plasticity by the removal of Lynx1. The Journal of Neuroscience. 2015;35(37):12693–702.
Moriwaki Y, Yoshikawa K, Fukuda H, Fujii YX, Misawa H, Kawashima K. Immune system expression of SLURP-1 and SLURP-2, two endogenous nicotinic acetylcholine receptor ligands. Life Sciences. 2007;80(24–25):2365–8.
Moriwaki Y, Watanabe Y, Shinagawa T, Kai M, Miyazawa M, Okuda T, Kawashima K, Yabashi A, Waguri S, Misawa H. Primary sensory neuronal expression of SLURP-1, an endogenous nicotinic acetylcholine receptor ligand. Neuroscience Research. 2009;64(4):403–12.
Horiguchi K, Horiguchi S, Yamashita N, Irie K, Masuda J, Takano-Ohmuro H, Himi T, Miyazawa M, Moriwaki Y, Okuda T, et al. Expression of SLURP-1, an endogenous alpha7 nicotinic acetylcholine receptor allosteric ligand, in murine bronchial epithelial cells. J Neurosci Res. 2009;87(12):2740–7.
Mastrangeli R, Donini S, Kelton CA, He C, Bressan A, Milazzo F, Ciolli V, Borrelli F, Martelli F, Biffoni M, et al. ARS Component B: structural characterization, tissue expression and regulation of the gene and protein (SLURP-1) associated with Mal de Meleda. Eur J Dermatol. 2003;13(6):560–70.
Arredondo J, Chernyavsky AI, Grando SA. SLURP-1 and -2 in normal, immortalized and malignant oral keratinocytes. Life Sciences. 2007;80(24–25):2243–7.
Norman B, Davis J, Piatigorsky J. Postnatal gene expression in the normal mouse cornea by SAGE. Invest Ophthalmol Vis Sci. 2004;45(2):429–40.
Favre B, Plantard L, Aeschbach L, Brakch N, Christen-Zaech S, de Viragh PA, Sergeant A, Huber M, Hohl D. SLURP1 is a late marker of epidermal differentiation and is absent in Mal de Meleda. J Invest Dermatol. 2007;127(2):301–8.
Jia C, Zhu W, Ren S, Xi H, Li S, Wang Y. Comparison of genome-wide gene expression in suture- and alkali burn-induced murine corneal neovascularization. Mol Vis. 2011;17:2386–99.
Narumoto O, Horiguchi K, Horiguchi S, Moriwaki Y, Takano-Ohmuro H, Shoji S, Misawa H, Yamashita N, Nagase T, Kawashima K. Down-regulation of secreted lymphocyte antigen-6/urokinase-type plasminogen activator receptor-related peptide-1 (SLURP-1), an endogenous allosteric alpha7 nicotinic acetylcholine receptor modulator, in murine and human asthmatic conditions. Biochem Biophys Res Commun. 2010;398(4):713–8.
Kimchi ET, Posner MC, Park JO, Darga TE, Kocherginsky M, Karrison T, Hart J, Smith KD, Mezhir JJ, Weichselbaum RR, et al. Progression of Barrett’s metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res. 2005;65(8):3146–54.
Stairs DB, Nakagawa H, Klein-Szanto A, Mitchell SD, Silberg DG, Tobias JW, Lynch JP, Rustgi AK. Cdx1 and c-Myc foster the initiation of transdifferentiation of the normal esophageal squamous epithelium toward Barrett’s esophagus. PLoS One. 2008;3(10):e3534.
Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, Atkins D, Wang Y. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clinical Cancer Research. 2005;11(20):7234–42.
Hu N, Clifford RJ, Yang HH, Wang C, Goldstein AM, Ding T, Taylor PR, Lee MP. Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genomics. 2010;11:576.
Reis PP, Waldron L, Perez-Ordonez B, Pintilie M, Galloni NN, Xuan Y, Cervigne NK, Warner GC, Makitie AA, Simpson C, et al. A gene signature in histologically normal surgical margins is predictive of oral carcinoma recurrence. BMC Cancer. 2011;11:437.
Eckl KM, Stevens HP, Lestringant GG, Westenberger-Treumann M, Traupe H, Hinz B, Frossard PM, Stadler R, Leigh IM, Nurnberg P, et al. Mal de Meleda (MDM) caused by mutations in the gene for SLURP-1 in patients from Germany, Turkey, Palestine, and the United Arab Emirates. Hum Genet. 2003;112(1):50–6.
Fischer J, Bouadjar B, Heilig R, Huber M, Lefevre C, Jobard F, Macari F, Bakija-Konsuo A, Ait-Belkacem F, Weissenbach J, et al. Mutations in the gene encoding SLURP-1 in Mal de Meleda. Hum Mol Genet. 2001;10(8):875–80.
Hu G, Yildirim M, Baysal V, Yerebakan O, Yilmaz E, Inaloz HS, Martinez-Mir A, Christiano AM, Celebi JT. A recurrent mutation in the ARS (component B) gene encoding SLURP-1 in Turkish families with mal de Meleda: evidence of a founder effect. J Invest Dermatol. 2003;120(6):967–9.
Ward KM, Yerebakan O, Yilmaz E, Celebi JT. Identification of recurrent mutations in the ARS (component B) gene encoding SLURP-1 in two families with mal de Meleda. J Invest Dermatol. 2003;120(1):96–8.
Marrakchi S, Audebert S, Bouadjar B, Has C, Lefevre C, Munro C, Cure S, Jobard F, Morlot S, Hohl D, et al. Novel mutations in the gene encoding secreted lymphocyte antigen-6/urokinase-type plasminogen activator receptor-related protein-1 (SLURP-1) and description of five ancestral haplotypes in patients with Mal de Meleda. J Invest Dermatol. 2003;120(3):351–5.
Arredondo J, Chernyavsky AI, Webber RJ, Grando SA. Biological effects of SLURP-1 on human keratinocytes. J Invest Dermatol. 2005;125(6):1236–41.
Arredondo J, Chernyavsky AI, Grando SA. Overexpression of SLURP-1 and −2 alleviates the tumorigenic action of tobacco-derived nitrosamine on immortalized oral epithelial cells. Biochem Pharmacol. 2007;74(8):1315–9.
Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J, Huber M, Bertrand D, Hohl D. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet. 2003;12(22):3017–24.
Grando SA. Basic and clinical aspects of non-neuronal acetylcholine: biological and clinical significance of non-canonical ligands of epithelial nicotinic acetylcholine receptors. J Pharmacol Sci. 2008;106(2):174–9.
Adeyo O, Oberer M, Ploug M, Fong LG, Young SG, Beigneux AP. Heterogeneity in the properties of mutant secreted lymphocyte antigen 6/urokinase receptor-related protein 1 (SLURP1) in Mal de Meleda. The British Journal of Dermatology. 2015;173(4):1066–9.
Adeyo O, Allan BB, Barnes 2nd RH, Goulbourne CN, Tatar A, Tu Y, Young LC, Weinstein MM, Tontonoz P, Fong LG, et al. Palmoplantar keratoderma along with neuromuscular and metabolic phenotypes in Slurp1-deficient mice. J Invest Dermatol. 2014;134(6):1589–98.
Arredondo J, Chernyavsky AI, Jolkovsky DL, Webber RJ, Grando SA. SLURP-2: a novel cholinergic signaling peptide in human mucocutaneous epithelium. J Cell Physiol. 2006;208(1):238–45.
Tsuji H, Okamoto K, Matsuzaka Y, Iizuka H, Tamiya G, Inoko H. SLURP-2, a novel member of the human Ly-6 superfamily that is up-regulated in psoriasis vulgaris. Genomics. 2003;81(1):26–33.
Allan CM, Procaccia S, Tran D, Tu Y, Barnes 2nd RH, Larsson M, Allan BB, Young LC, Hong C, Tontonoz P, et al. Palmoplantar keratoderma in Slurp2-deficient mice. J Invest Dermatol. 2016;136(2):436–43.
Dessaud E, Salaun D, Gayet O, Chabbert M, de Lapeyriere O. Identification of lynx2, a novel member of the ly-6/neurotoxin superfamily, expressed in neuronal subpopulations during mouse development. Molecular and Cellular Neurosciences. 2006;31(2):232–42.
Tekinay AB, Nong Y, Miwa JM, Lieberam I, Ibanez-Tallon I, Greengard P, Heintz N. A role for LYNX2 in anxiety-related behavior. Proc Natl Acad Sci U S A. 2009;106(11):4477–82.
Wu M, Puddifoot CA, Taylor P, Joiner WJ. Mechanisms of inhibition and potentiation of alpha4beta2 nicotinic acetylcholine receptors by members of the Ly6 protein family. J Biol Chem. 2015;290(40):24509–18.
McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res. 2013;41(Web Server issue):W597–600.
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology. 2011;7:539.
Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011;39(Web Server issue):W475–478.
Palfree RG. Ly-6-domain proteins—new insights and new members: a C-terminal Ly-6 domain in sperm acrosomal protein SP-10. Tissue antigens. 1996;48(2):71–9.
Hamatani T, Tanabe K, Kamei K, Sakai N, Yamamoto Y, Yoshimura Y. A monoclonal antibody to human SP-10 inhibits in vitro the binding of human sperm to hamster oolemma but not to human Zona pellucida. Biology of Reproduction. 2000;62(5):1201–8.
Foster JA, Klotz KL, Flickinger CJ, Thomas TS, Wright RM, Castillo JR, Herr JC. Human SP-10: acrosomal distribution, processing, and fate after the acrosome reaction. Biology of Reproduction. 1994;51(6):1222–31.
von Vietinghoff S, Tunnemann G, Eulenberg C, Wellner M, Cristina Cardoso M, Luft FC, Kettritz R. NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils. Blood. 2007;109(10):4487–93.
Bauer S, Abdgawad M, Gunnarsson L, Segelmark M, Tapper H, Hellmark T. Proteinase 3 and CD177 are expressed on the plasma membrane of the same subset of neutrophils. J Leukoc Biol. 2007;81(2):458–64.
Stroncek DF, Skubitz KM, McCullough JJ. Biochemical characterization of the neutrophil-specific antigen NB1. Blood. 1990;75(3):744–55.
Mead RJ, Neal JW, Griffiths MR, Linington C, Botto M, Lassmann H, Morgan BP. Deficiency of the complement regulator CD59a enhances disease severity, demyelination and axonal injury in murine acute experimental allergic encephalomyelitis. Laboratory Investigation. 2004;84(1):21–8.
Holt DS, Botto M, Bygrave AE, Hanna SM, Walport MJ, Morgan BP. Targeted deletion of the CD59 gene causes spontaneous intravascular hemolysis and hemoglobinuria. Blood. 2001;98(2):442–9.
Longhi MP, Sivasankar B, Omidvar N, Morgan BP, Gallimore A. Cutting edge: murine CD59a modulates antiviral CD4+ T cell activity in a complement-independent manner. J Immunol. 2005;175(11):7098–102.
Qin X, Miwa T, Aktas H, Gao M, Lee C, Qian YM, Morton CC, Shahsafaei A, Song WC, Halperin JA. Genomic structure, functional comparison, and tissue distribution of mouse Cd59a and Cd59b. Mammalian Genome. 2001;12(8):582–9.
Jiang H, Pillai S. Complement regulatory proteins on the sperm surface: relevance to sperm motility. American Journal of Reproductive Immunology. 1998;39(4):243–8.
Fenichel P, Cervoni F, Hofmann P, Deckert M, Emiliozzi C, Hsi BL, Rossi B. Expression of the complement regulatory protein CD59 on human spermatozoa: characterization and role in gametic interaction. Molecular Reproduction and Development. 1994;38(3):338–46.
Furuhata T, Tokino T, Urano T, Nakamura Y. Isolation of a novel GPI-anchored gene specifically regulated by p53; correlation between its expression and anti-cancer drug sensitivity. Oncogene. 1996;13(9):1965–70.
Ueda K, Miyoshi Y, Tokino T, Watatani M, Nakamura Y. Induction of apoptosis in T98G glioblastoma cells by transfection of GML, a p53 target gene. Oncology Research. 1999;11(3):125–32.
Xue H, O’Neill D, Morrow J, Bank A. A novel mouse gene, HemT, encoding an hematopoietic cell-specific transcript. Gene. 1999;231(1–2):49–58.
Holmes RS, Cox LA. Comparative studies of glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1: evidence for a eutherian mammalian origin for the GPIHBP1 gene from an LY6-like gene. 3 Biotech. 2012;2(1):37–52.
van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A. 1989;86(12):4634–8.
Bailey B, Fransioli J, Gude NA, Alvarez Jr R, Zhang X, Gustafsson AB, Sussman MA. Sca-1 knockout impairs myocardial and cardiac progenitor cell function. Circulation Research. 2012;111(6):750–60.
Hsu YC, Mildenstein K, Hunter K, Tkachenko O, Mullen CA. Acute lymphoid leukemia cells with greater stem cell antigen-1 (Ly6a/Sca-1) expression exhibit higher levels of metalloproteinase activity and are more aggressive in vivo. PLoS One. 2014;9(2):e88966.
Epting CL, King FW, Pedersen A, Zaman J, Ritner C, Bernstein HS. Stem cell antigen-1 localizes to lipid microdomains and associates with insulin degrading enzyme in skeletal myoblasts. J Cell Physiol. 2008;217(1):250–60.
Stanford WL, Haque S, Alexander R, Liu X, Latour AM, Snodgrass HR, Koller BH, Flood PM. Altered proliferative response by T lymphocytes of Ly-6A (Sca-1) null mice. J Exp Med. 1997;186(5):705–17.
Reese JT, Mehta H, Chappell CH, Bamezai A. Downregulated expression of Ly-6-ThB on developing T cells marks CD4 + CD8+ subset undergoing selection in the thymus. Developmental Immunology. 2001;8(2):107–21.
Brakenhoff RH, Gerretsen M, Knippels EM, van Dijk M, van Essen H, Weghuis DO, Sinke RJ, Snow GB, van Dongen GA. The human E48 antigen, highly homologous to the murine Ly-6 antigen ThB, is a GPI-anchored molecule apparently involved in keratinocyte cell-cell adhesion. J Cell Biol. 1995;129(6):1677–89.
Inlay MA, Bhattacharya D, Sahoo D, Serwold T, Seita J, Karsunky H, Plevritis SK, Dill DL, Weissman IL. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23(20):2376–81.
Kurosawa M, Jeyasekharan AD, Surmann EM, Hashimoto N, Venkatraman V, Kurosawa G, Furukawa K, Venkitaraman AR, Kurosawa Y. Expression of LY6D is induced at the surface of MCF10A cells by X-ray irradiation. The FEBS Journal. 2012;279(24):4479–91.
Dullens HF, Hilgers J, Van Basten CD, De Weger RA, De Heer E, Den Otter W. Cell surface antigen phenotypes and enzyme expression patterns of two murine T-cell lymphomas derived from early and/or mature thymus cells. Leukemia Research. 1982;6(3):425–8.
Mao M, Yu M, Tong JH, Ye J, Zhu J, Huang QH, Fu G, Yu L, Zhao SY, Waxman S, et al. RIG-E, a human homolog of the murine Ly-6 family, is induced by retinoic acid during the differentiation of acute promyelocytic leukemia cell. Proc Natl Acad Sci U S A. 1996;93(12):5910–4.
Xu X, Qiu C, Zhu L, Huang J, Li L, Fu W, Zhang L, Wei J, Wang Y, Geng Y, et al. IFN-stimulated gene LY6E in monocytes regulates the CD14/TLR4 pathway but inadequately restrains the hyperactivation of monocytes during chronic HIV-1 infection. J Immunol. 2014;193(8):4125–36.
MacNeil I, Kennedy J, Godfrey DI, Jenkins NA, Masciantonio M, Mineo C, Gilbert DJ, Copeland NG, Boyd RL, Zlotnik A. Isolation of a cDNA encoding thymic shared antigen-1. A new member of the Ly6 family with a possible role in T cell development. J Immunol. 1993;151(12):6913–23.
Shan X, Bourdeau A, Rhoton A, Wells DE, Cohen EH, Landgraf BE, Palfree RG. Characterization and mapping to human chromosome 8q24.3 of Ly-6-related gene 9804 encoding an apparent homologue of mouse TSA-1. J Immunol. 1998;160(1):197–208.
Capone MC, Gorman DM, Ching EP, Zlotnik A. Identification through bioinformatics of cDNAs encoding human thymic shared Ag-1/stem cell Ag-2. A new member of the human Ly-6 family. J Immunol. 1996;157(3):969–73.
Fleming TJ, O’HUigin C, Malek TR. Characterization of two novel Ly-6 genes. Protein sequence and potential structural similarity to alpha-bungarotoxin and other neurotoxins. J Immunol. 1993;150(12):5379–90.
Wang JX, Bair AM, King SL, Shnayder R, Huang YF, Shieh CC, Soberman RJ, Fuhlbrigge RC, Nigrovic PA. Ly6G ligation blocks recruitment of neutrophils via a beta2-integrin-dependent mechanism. Blood. 2012;120(7):1489–98.
Mallya M, Campbell RD, Aguado B. Characterization of the five novel Ly-6 superfamily members encoded in the MHC, and detection of cells expressing their potential ligands. Protein Science. 2006;15(10):2244–56.
Mallya M, Campbell RD, Aguado B. Transcriptional analysis of a novel cluster of LY-6 family members in the human and mouse major histocompatibility complex: five genes with many splice forms. Genomics. 2002;80(1):113–23.
De Vet EC, Aguado B, Campbell RD. Adaptor signalling proteins Grb2 and Grb7 are recruited by human G6f, a novel member of the immunoglobulin superfamily encoded in the MHC. Biochem J. 2003;375(Pt 1):207–13.
Horie M, Okutomi K, Taniguchi Y, Ohbuchi Y, Suzuki M, Takahashi E. Isolation and characterization of a new member of the human Ly6 gene family (LY6H). Genomics. 1998;53(3):365–8.
Apostolopoulos J, Chisholm LJ, Sandrin MS. Identification of mouse Ly6H and its expression in normal tissue. Immunogenetics. 1999;49(11–12):987–90.
Pflugh DL, Maher SE, Bothwell AL. Ly-6 superfamily members Ly-6A/E, Ly-6C, and Ly-6I recognize two potential ligands expressed by B lymphocytes. J Immunol. 2002;169(9):5130–6.
Kong HK, Yoon S, Park JH. The regulatory mechanism of the LY6K gene expression in human breast cancer cells. J Biol Chem. 2012;287(46):38889–900.
Choi SH, Kong HK, Park SY, Park JH. Metastatic effect of LY-6 K gene in breast cancer cells. International Journal of Oncology. 2009;35(3):601–7.
de Nooij-van Dalen AG, van Dongen GA, Smeets SJ, Nieuwenhuis EJ, Stigter-van Walsum M, Snow GB, Brakenhoff RH. Characterization of the human Ly-6 antigens, the newly annotated member Ly-6 K included, as molecular markers for head-and-neck squamous cell carcinoma. International Journal of Cancer Journal International Du Cancer. 2003;103(6):768–74.
Fujihara Y, Okabe M, Ikawa M. GPI-anchored protein complex, LY6K/TEX101, is required for sperm migration into the oviduct and male fertility in mice. Biology of Reproduction. 2014;90(3):60.
von Allmen CE, Bauer M, Dietmeier K, Buser RB, Gwerder M, Muntwiler S, Utzinger S, Saudan P, Bachmann MF, Beerli RR. Identification of Ly-6K as a novel marker for mouse plasma cells. Molecular Immunology. 2008;45(10):2727–33.
Miwa JM, Ibanez-Tallon I, Crabtree GW, Sanchez R, Sali A, Role LW, Heintz N. lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23(1):105–14.
Fu XW, Song PF, Spindel ER. Role of Lynx1 and related Ly6 proteins as modulators of cholinergic signaling in normal and neoplastic bronchial epithelium. Int Immunopharmacol. 2015;29(1):93–8.
Yu DH, Fan W, Liu G, Nguy V, Chatterton JE, Long S, Ke N, Meyhack B, Bruengger A, Brachat A, et al. PHTS, a novel putative tumor suppressor, is involved in the transformation reversion of HeLaHF cells independently of the p53 pathway. Exp Cell Res. 2006;312(6):865–76.
Kriegbaum MC, Clausen OP, Laerum OD, Ploug M. Expression of the Ly6/uPAR-domain proteins C4.4A and Haldisin in non-invasive and invasive skin lesions. The Journal of Histochemistry and Cytochemistry. 2015;63(2):142–54.
Oshiro R, Yamamoto H, Takahashi H, Ohtsuka M, Wu X, Nishimura J, Takemasa I, Mizushima T, Ikeda M, Sekimoto M, et al. C4.4A is associated with tumor budding and epithelial-mesenchymal transition of colorectal cancer. Cancer Science. 2012;103(6):1155–64.
Smith BA, Kennedy WJ, Harnden P, Selby PJ, Trejdosiewicz LK, Southgate J. Identification of genes involved in human urothelial cell-matrix interactions: implications for the progression pathways of malignant urothelium. Cancer Res. 2001;61(4):1678–85.
Ngora H, Galli UM, Miyazaki K, Zoller M. Membrane-bound and exosomal metastasis-associated C4.4A promotes migration by associating with the alpha(6)beta(4) integrin and MT1-MMP. Neoplasia. 2012;14(2):95–107.
Kriegbaum MC, Jacobsen B, Hald A, Ploug M. Expression of C4.4A, a structural uPAR homolog, reflects squamous epithelial differentiation in the adult mouse and during embryogenesis. The Journal of Histochemistry and Cytochemistry. 2011;59(2):188–201.
Gardsvoll H, Kriegbaum MC, Hertz EP, Alpizar-Alpizar W, Ploug M. The urokinase receptor homolog Haldisin is a novel differentiation marker of stratum granulosum in squamous epithelia. The Journal of Histochemistry and Cytochemistry. 2013;61(11):802–13.
Zhang Y, Lang Q, Li J, Xie F, Wan B, Yu L. Identification and characterization of human LYPD6, a new member of the Ly-6 superfamily. Molecular Biology Reports. 2010;37(4):2055–62.
Ozhan G, Sezgin E, Wehner D, Pfister AS, Kuhl SJ, Kagermeier-Schenk B, Kuhl M, Schwille P, Weidinger G. Lypd6 enhances Wnt/beta-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains. Developmental Cell. 2013;26(4):331–45.
Darvas M, Morsch M, Racz I, Ahmadi S, Swandulla D, Zimmer A. Modulation of the Ca2+ conductance of nicotinic acetylcholine receptors by Lypd6. European Neuropsychopharmacology. 2009;19(9):670–81.
Ni J, Lang Q, Bai M, Zhong C, Chen X, Wan B, Yu L. Cloning and characterization of a human LYPD7, a new member of the Ly-6 superfamily. Molecular Biology Reports. 2009;36(4):697–703.
Bera TK, Maitra R, Iavarone C, Salvatore G, Kumar V, Vincent JJ, Sathyanarayana BK, Duray P, Lee BK, Pastan I. PATE, a gene expressed in prostate cancer, normal prostate, and testis, identified by a functional genomic approach. Proc Natl Acad Sci U S A. 2002;99(5):3058–63.
Soler-Garcia AA, Maitra R, Kumar V, Ise T, Nagata S, Beers R, Bera TK, Pastan I. The PATE gene is expressed in the accessory tissues of the human male genital tract and encodes a secreted sperm-associated protein. Reproduction. 2005;129(4):515–24.
Rolland T, Tasan M, Charloteaux B, Pevzner SJ, Zhong Q, Sahni N, Yi S, Lemmens I, Fontanillo C, Mosca R, et al. A proteome-scale map of the human interactome network. Cell. 2014;159(5):1212–26.
Li SH, Lee RK, Lin MH, Hwu YM, Lu CH, Chen YJ, Chen HC, Chang WH, Chang WC. SSLP-1, a secreted Ly-6 protein purified from mouse seminal vesicle fluid. Reproduction. 2006;132(3):493–500.
Collen D. The plasminogen (fibrinolytic) system. Thrombosis and Haemostasis. 1999;82(2):259–70.
Stewart CE, Sayers I. Urokinase receptor orchestrates the plasminogen system in airway epithelial cell function. Lung. 2013;191(2):215–25.
Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997;17(4):439–44.
Estreicher A, Muhlhauser J, Carpentier JL, Orci L, Vassalli JD. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990;111(2):783–92.
Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990;110(4):1361–7.
Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267(36):26031–7.
Renaud SJ, Macdonald-Goodfellow SK, Graham CH. Coordinated regulation of human trophoblast invasiveness by macrophages and interleukin 10. Biology of Reproduction. 2007;76(3):448–54.
Sidenius N, Blasi F. The urokinase plasminogen activator system in cancer: recent advances and implication for prognosis and therapy. Cancer Metastasis Reviews. 2003;22(2–3):205–22.
Colman RW, Pixley RA, Najamunnisa S, Yan W, Wang J, Mazar A, McCrae KR. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J Clin Invest. 1997;100(6):1481–7.
Higazi AA, Upson RH, Cohen RL, Manuppello J, Bognacki J, Henkin J, McCrae KR, Kounnas MZ, Strickland DK, Preissner KT, et al. Interaction of single-chain urokinase with its receptor induces the appearance and disappearance of binding epitopes within the resultant complex for other cell surface proteins. Blood. 1996;88(2):542–51.
Czekay RP, Kuemmel TA, Orlando RA, Farquhar MG. Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity. Mol Biol Cell. 2001;12(5):1467–79.
Behrendt N, Jensen ON, Engelholm LH, Mortz E, Mann M, Dano K. A urokinase receptor-associated protein with specific collagen binding properties. J Biol Chem. 2000;275(3):1993–2002.
Nykjaer A, Christensen EI, Vorum H, Hager H, Petersen CM, Roigaard H, Min HY, Vilhardt F, Moller LB, Kornfeld S, et al. Mannose 6-phosphate/insulin-like growth factor-II receptor targets the urokinase receptor to lysosomes via a novel binding interaction. J Cell Biol. 1998;141(3):815–28.
Bugge TH, Flick MJ, Danton MJ, Daugherty CC, Romer J, Dano K, Carmeliet P, Collen D, Degen JL. Urokinase-type plasminogen activator is effective in fibrin clearance in the absence of its receptor or tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1996;93(12):5899–904.
Moller LB, Pollanen J, Ronne E, Pedersen N, Blasi F. N-linked glycosylation of the ligand-binding domain of the human urokinase receptor contributes to the affinity for its ligand. J Biol Chem. 1993;268(15):11152–9.
Pass J, Gardsvoll H, Lund LR, Dano K, Hoyer-Hansen G. Generation of antibodies to the urokinase receptor (uPAR) by DNA immunization of uPAR knockout mice: membrane-bound uPAR is not required for an antibody response. Scandinavian Journal of Immunology. 2003;58(3):298–305.
Taeb J, Asgari M, Abolhasani M, Farajollahi MM, Madjd Z. Expression of prostate stem cell antigen (PSCA) in prostate cancer: a tissue microarray study of Iranian patients. Pathology, Research and Practice. 2014;210(1):18–23.
Bahrenberg G, Brauers A, Joost HG, Jakse G. Reduced expression of PSCA, a member of the LY-6 family of cell surface antigens, in bladder, esophagus, and stomach tumors. Biochem Biophys Res Commun. 2000;275(3):783–8.
Wang L, Sang Y, Tang J, Zhang RH, Luo D, Chen M, Deng WG, Kang T. Down-regulation of prostate stem cell antigen (PSCA) by Slug promotes metastasis in nasopharyngeal carcinoma. J Pathol. 2015;237(4):411–22.
Adermann K, Wattler F, Wattler S, Heine G, Meyer M, Forssmann WG, Nehls M. Structural and phylogenetic characterization of human SLURP-1, the first secreted mammalian member of the Ly-6/uPAR protein superfamily. Protein Science. 1999;8(4):810–9.
Chernyavsky AI, Galitovskiy V, Shchepotin IB, Grando SA. Anti-inflammatory effects of the nicotinergic peptides SLURP-1 and SLURP-2 on human intestinal epithelial cells and immunocytes. BioMed Research International. 2014;2014:609086.
Chernyavsky AI, Arredondo J, Galitovskiy V, Qian J, Grando SA. Upregulation of nuclear factor-kappaB expression by SLURP-1 is mediated by alpha7-nicotinic acetylcholine receptor and involves both ionic events and activation of protein kinases. Am J Physiol Cell Physiol. 2010;299(5):C903–911.
Lyukmanova EN, Shulepko MA, Kudryavtsev D, Bychkov ML, Kulbatskii DS, Kasheverov IE, Astapova MV, Feofanov AV, Thomsen MS, Mikkelsen JD, Shenkarev ZO, Tsetlin VI, Dolgikh DA, Kirpichnikov MP. Human Secreted Ly-6/uPAR Related Protein-1 (SLURP-1) Is a Selective Allosteric Antagonist of α7 Nicotinic Acetylcholine Receptor. PLoS One. 2016;11(2):e0149733.
Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W, et al. A mouse knockout library for secreted and transmembrane proteins. Nature Biotechnology. 2010;28(7):749–55.
Shetty J, Wolkowicz MJ, Digilio LC, Klotz KL, Jayes FL, Diekman AB, Westbrook VA, Farris EM, Hao Z, Coonrod SA, et al. SAMP14, a novel, acrosomal membrane-associated, glycosylphosphatidylinositol-anchored member of the Ly-6/urokinase-type plasminogen activator receptor superfamily with a role in sperm-egg interaction. J Biol Chem. 2003;278(33):30506–15.
Teng X, Yang J, Xie Y, Ni Z, Hu R, Shi L, Lin Z, Hu L, Zhao G, Ding X, et al. A novel spermatogenesis-specific uPAR gene expressed in human and mouse testis. Biochem Biophys Res Commun. 2006;342(4):1223–7.
Yin L, Chung CM, Huo R, Liu H, Zhou C, Xu W, Zhu H, Zhang J, Shi Q, Wong HY, et al. A sperm GPI-anchored protein elicits sperm-cumulus cross-talk leading to the acrosome reaction. Cellular and Molecular Life Sciences. 2009;66(5):900–8.
Yin LL, Chung CM, Chen J, Fok KL, Ng CP, Jia RR, Ren X, Zhou J, Zhang T, Zhao XH, et al. A suppressor of multiple extracellular matrix-degrading proteases and cancer metastasis. Journal of Cellular and Molecular Medicine. 2009;13(9B):4034–41.
Kurita A, Takizawa T, Takayama T, Totsukawa K, Matsubara S, Shibahara H, Orgebin-Crist MC, Sendo F, Shinkai Y, Araki Y. Identification, cloning, and initial characterization of a novel mouse testicular germ cell-specific antigen. Biology of Reproduction. 2001;64(3):935–45.
Acknowledgements
Work in the Swamynathan lab was supported by R01EY022898 grant from the National Eye Institute (NEI), National Institutes of Health (NIH) (SKS), NEI Core Grant P30 EY08098, unrestricted grants from Research to Prevent Blindness, the Eye and Ear Foundation of Pittsburgh and the PA Lions Eye Research Sight Conservation Foundation (SKS), and “Fight for Sight/The Eye Bank for Sight” (EED). EAB is supported by NIH Grant U41HG003345 and Wellcome Trust grant 099129/Z/12/Z. MSM is supported by program project grant HG000330 from the National Human Genome Research Institute (NHGRI), NIH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
C.L. Loughner, None; E. A. Bruford, None; M.S. McAndrews, None; E.E. Delp, None; S. Swamynathan (Patent); S.K. Swamynathan, (Patent).
Authors’ contributions
CLL, EAB, MSM, EED, SS, and SKS were involved in drafting the manuscript and revising it critically for important intellectual content. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content. All authors read and approved the final manuscript. Each author has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Loughner, C.L., Bruford, E.A., McAndrews, M.S. et al. Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum Genomics 10, 10 (2016). https://doi.org/10.1186/s40246-016-0074-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40246-016-0074-2