Abstract
The sorption of uranium and thorium fluorides by activated carbon from the eutectic was studied. The sorption isotherms of both fluorides at a temperature of 650°C have a pronounced convex character and are described by the Langmuir equation. Experiments were carried out on the sorption of protactinium by activated carbon AG-3 at 650°С from a melt of alkali metal fluorides LiF–NaF–KF containing thorium and neodymium fluorides. It was found that with an increase in the concentrations of neodymium or thorium fluorides, the value of protactinium sorption diminishes, and in the case of thorium fluoride, the decrease occurs to a greater extent. When protactinium is sorbed by activated carbon with metallic sodium, the sorption enhances by a factor of 20 at the 30% sodium content in the carbon. The separation factors of protactinium from other actinides rise with increasing sodium content in the carbon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Based on the production and subsequent use of 233U, the thorium fuel cycle has a number of advantages over the conventional uranium fuel cycle. First of all, the fission of 233U practically does not produce long-lived transplutonium actinides, which are one of the main components of high-level waste (HLW) requiring geological disposal. However, during irradiation with 232Th, the formation of 232U occurs in parallel, which, together with its daughter radionuclides, has a high level of radioactivity hindering practical use in the nuclear power industry due to the need to employ protective chambers for handling at all stages of nuclear fuel manufacture.
Recently, interest in the thorium fuel cycle has increased [1]. Approaches have appeared to the possible production of 233U with a minimum 232U content, including the use of blankets in a thermonuclear fusion–fission reactor [2, 3]. This approach lies in the fact that the continuous extraction of 233Pa, which generates 233U upon decay, from the thorium blanket can significantly reduce the accumulation of 231Pa, from which 232U is formed. With an increase in the irradiation time of a thorium blanket to produce a relatively high amount of 233U (for example, upon irradiation up to 200 days), 232U and 234U will simultaneously accumulate up to 1.7 and 0.19% of the total uranium, respectively [4], while the total dose rate will be 2.2 Sv/h for 1.7% 232U [5].
A melt of fluorides of alkaline elements with thorium fluoride in the composition can be employed as a thorium blanket [3]. A feature of the continuous extraction of 233Pa from a fluoride melt is that during a short cycle of 232Th irradiation, a small accumulation of 233Pa will occur. Therefore, the most studied methods such as reductive extraction of 233Pa into liquid Bi with Li, electrorefining on a cathode, deposition by oxides, and other methods [1] will be ineffective. For the continuous extraction of low concentrations of 233Pa, it would be reasonable to use sorption processes capable of extracting microconcentrations of various elements from melts.
Recently, the authors of [6] showed the possibility of activated carbon application for recovering Nd(III) from a melt of alkali metal fluorides (FLiNaK). At the same time, AG-3 activated carbon demonstrated stability in the studied temperature range of 550–700°C.
This work is aimed at studying the recovery of Pa, U, and Th from the FLiNaK eutectic with activated carbon and carbon modified with Na metal for additional fixation of Pa on carbon.
EXPERIMENTAL
We used a eutectic based on alkali metal fluorides with the composition, mol %: LiF (46.5)–NaF (11.5)–KF (42.0) (FLiNaK). Its quality was checked by melting temperature (Tm = 454°C) by a derivatograph. The setup scheme for carrying out sorption with activated carbon AG-3 from FLiNaK was described in detail earlier [6].
Dried activated carbon was placed in a special metallic copper basket with holes and immersed in the molten salts. The experiments were carried out at 650°C under static conditions, the sorption time was 1.5 h. The basket with activated carbons was periodically subjected to vertical translational motion to mix the salt with carbon. After removal of the basket, the content of target elements in carbon and salt eutectic was examined.
The concentration of Th, U and Nd was analyzed by the following procedure. Samples with carbon and eutectics were treated with sulfuric acid to remove HF. Then, after evaporation, they were diluted with water and analyzed using arsenazo III in hydrochloric acid by a PE-5300VI spectrophotometer at a wavelength of 650 nm according to the procedure [7].
Activated carbon AG-3 before enrichment in metallic sodium in order to increase the affinity of its surface toward the metal was subjected to drying in air at 200°C, heat treatment under vacuum and in an inert gas at 600°C. Samples of activated carbon enriched in metallic sodium were prepared by the setup shown in Fig. 1.
Scheme of the setup used for the enrichment of activated carbon in metallic sodium. (1) body; (2) removable threaded cover; (3) collet seal of the mesh rod; (4) ceramic insert of the reactor; (5) stainless steel mesh for coal with a rod; (6, 7) temperature sensors; (8) temperature meter—potentiometer; (9) oven; (10) autotransformer; (11) ball valve; (12) rotameter with a needle throttle; (13) membrane pressure regulator with a pressure gauge; (14) balloon; (15) vacuum pump.
The preliminarily prepared carbon was placed in grid 5, the grid rod was fixed with seal 3. Metallic sodium was loaded in the reactor in an amount not exceeding the volume of ceramic insert 4, heating was carried out in argon.
The temperature in the reactor and the time of the enrichment process were chosen based on the required amount of metal in the resulting carbon. It was experimentally established that to obtain samples with a metal content in the range from 1 to 10 wt %, it is necessary to contact the activated carbon with the melt at a temperature of 200–300°C, and heating to 400–600°С for 2 h is necessary to reach higher (up to 30 wt %) metal contents.
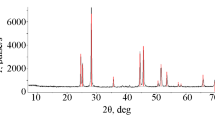
Fluorides of thorium, 231Pa and 241Am, and also Nd were yielded jointly from the corresponding nitrates according to the procedure in [6]. The content of 231Pa, 241Am was analyzed on a γ-spectrometer with a resolution of 1.8 keV equipped with a GTV45P4-83 detection unit and on an ORTEC DSPec jr 2.0 spectrometer. UF4 was obtained by electrochemical reduction of uranyl nitrate, precipitation with ammonium fluoride, and subsequent calcination of the precipitate at 600°С in a mixture with ammonium bifluoride for 4 h. X-ray phase analysis (XRD) was performed on a D2 Phaser diffractometer (Bruker, Germany) using CuKα radiation, X-ray tube voltage 30 kV, current 10 mA. The survey was carried out in the angle range 2θ 7°–70° in the scanning mode with a step of 0.02° at a speed of 0.5 deg/min. The results were processed by the DIFFRAC.EVA.V5.0 package. The quality of the resulting product was controlled by the diffraction pattern (Fig. 2).
RESULTS AND DISCUSSION
To implement the thorium fuel cycle, it is necessary to extract 233Pa directly from the eutectic containing fluoride of irradiated natural 232Th. Previously it was shown that, at 650°С thorium fluoride is sorbed by AG-3 carbon from the FLiNaK eutectic by an order of magnitude less than neodymium fluoride under similar conditions [6]. It is important to clarify the mechanism of sorption under similar conditions of tetravalent uranium fluoride, which is formed during the decay of 233Pa. Data on the sorption of U(IV) and Th(IV) fluorides (Figs. 3) evidence that the recovery of U(IV) is slightly higher than that of Th(IV).
The data of the sorption isotherm of uranium and thorium fluorides (Fig. 3) are satisfactorily approximated by the Langmuir equation:
where C is the current concentration of the element in the eutectic, A0 is the limiting content in the sorbent, and K is a constant.
Figure 3 shows the calculation (line) according to Eq. (1) for U(IV) at A0 = 0.68 mg/g and K = 0.30, for Th(IV) at A0 = 0.30 mg/g and K = 0.15.
As can be seen from Fig. 3 the selective extraction of uranium isotopes formed from protactinium isotopes in the framework of the thorium cycle with activated carbon over the U(IV)–Th(IV) pair is not feasible due to the lack of a significant difference in sorption isotherms.
In the case of continuous extraction of the micro amount of 233Pa formed during irradiation of thorium dissolved in the eutectic, it is necessary to evaluate the role of thorium fluoride, whose solubility in the eutectic is very high and reaches 32.8 mol % [8]. Figure 4 shows the effect of thorium and neodymium fluorides on the sorption of protactinium by AG-3 activated carbon. Figure 4 evidences that with an increase in the concentration of both fluorides, the sorption capacity for protactinium diminishes. Probably, the sorption of Pa from FLiNaK by activated carbon is significantly affected by the processes occurring in the melt associated with the complex formation.
It is known that Nd(III) in LiF–NaF–KF melts generates anionic complexes NdF63– [9], while Th(IV) forms more complicated fluoride complexes (for example, almost 18% [ThF9]5–) with a predominance of [ThF8]4– [10, 11]. It can be assumed that protactinium generates stronger complexes with thorium than with neodymium, for example [Pa]4+[ThF8]4–, which prevent the recovery of protactinium with activated carbon. The valence state of protactinium in FLiNAK and some other melts is 4 [12, 13]. The formation of protactinium compounds with anionic complexes of thorium and neodymium explains the decrease in the sorption extraction of protactinium with an increase in the concentrations of elements in the melt.
Figure 4 demonstrates that the extrapolation of the sorption capacity towards protactinium to zero values of macroelement concentrations leads to approximately the same value of protactinium sorption, equal to 1.4. As can be seen from the comparison of Figs. 3 and 4 it is practically impossible to selectively extract protactinium by sorption at a thorium fluoride content in the eutectic of more than 30 mmol/g, i.e., under conditions of the thorium cycle.
Meanwhile, it is known that protactinium is relatively easily reduced in fluoride melts to the metallic state either by direct introduction of metallic lithium [10] or by extraction into a bismuth melt with a metallic reducing agent (Li, Th, etc.) [13, 14].
The redox potentials of thorium and lanthanide fluorides in the FLiNaK melt are close [15], therewith metallic lithium can reduce lanthanides in this eutectic to a metal at 650°С in contrast to metallic sodium [16, 17]. For this reason, the effect of metallic sodium implemented to activated carbon on the selectivity of protactinium sorption in the presence of thorium and other actinides was studied.
Table 1 shows the values of the distribution coefficients of protactinium and other actinides during sorption by activated carbon AG-3 at a temperature of 650°C and different content of metallic sodium with a fixed content of thorium fluoride in the melt. It follows from Table 1 that in the case of no metallic sodium in AG-3, the distribution coefficients for the considered elements are approximately equal, and the separation factors of protactinium and other actinides are close to 1. With an increase in the content of metallic sodium, the distribution coefficients toward activated carbon increase, but for protactinium, more significant increase compared to other elements is observed. At 30% sodium content in carbon the distribution coefficient of protactinium reaches 12.3, which is much higher than for other elements, and the separation factor of protactinium and thorium reaches 3.85 at comparable values for other actinides.
It can be assumed that the reduction of protactinium with metallic sodium in activated carbon proceeds by analogy with the reduction of lithium dissolved in metallic bismuth during the extraction of protactinium from a fluoride melt [12]:
Alkali metals, unlike metallic bismuth, slightly dissolve actinides (U, Th, Pa, etc.) in the form of a metal [18]. Therefore, the dissolution of protactinium, which is present in the eutectic in an insignificant amount in the form of a metal, in sodium located in the AG-3 carbon, is quite probable.
Another option is also possible, related to the fact that the reduced protactinium will be located in the pore volume of activated carbon. Similarly, nanoparticles of Ag [19], Pd, Cu, and other elements [20] are fixed in the activated carbon pores.
To confirm this assumption, it is necessary to evaluate the nature of the placement of metallic sodium in activated carbon. Graphite and activated carbon have the ability to intercalate Li, Na, and other alkali metals between carbon layers to form C6Na and C8Na compounds [21, 22], and at a high content of alkali metals, disodium and dilithium acetylenides, Na2C2 and Li2C2 are formed [23, 24]. In all these compounds, alkali metals have a positive charge due to the redistribution of electrons with carbon atoms. The reducing properties of metallic sodium may depend on whether sodium is located in the pores or is located in the structure of the graphite layers.
Table 2 presents experimental data on determining the limiting volume of the sorption space (WS) by the absorption of benzene, as well as other parameters calculated based on the filling of pores. It can be seen from Fig. 2 that with an increase in the amount of sodium in AG-3, the value of WS decreases, which indicates that the micro- and mesopores are filled with sodium. If we assume that the decrease in the volume of sorbing pores is associated with the filling of their volume, then, taking into account the density of metallic sodium (0.96842 g/cm3) we can calculate the weight of sodium in the pores of carbon. The remaining amount of sodium can be located between the graphite layers of activated carbon, especially since the temperature of 600°C, at which carbon with the maximum content of metallic sodium was obtained, corresponds to the conditions for its intercalation into graphite [22]. Table 2 shows that the molar ratio carbon to sodium minus it in pores is practically 8.0, i.e., it corresponds to the C8Na compound [21, 22]. For carbon with 5% sodium content, the decrease in the volume WS roughly corresponds to the volume occupied by metallic sodium, i.e., there is no metallic sodium between the layers. This is probably due to the relatively low inclusion temperature of sodium in AG-3.
AG-3 contains 0.9 and 0.35 mequiv./g of acid and basic oxides, respectively [25]. The carbon pores are estimated to contain 8.2 mg-equiv./g of sodium (Table 2), i.e., only a small part of sodium (about 6%) can be in the form of an oxide, which can explain the increase in the distribution coefficient of other elements listed in Table 1 due to the formation of poorly soluble actinide oxyfluorides [26].
The study of the effect of metallic sodium introduced into the composition of activated carbon on the sorption of fluorides of various metals, including protactinium and thorium, as the most important elements for the thorium cycle, should be carried out as a separate work, since it will be necessary to developed the technique for producing a given amount of metallic sodium. This study revealed the fact of a significant effect of metallic sodium embedded in activated carbon on its sorption capacity for protactinium and other elements, and considered possible options for such a process that may be caused by the dissolution of reduced protactinium in metallic sodium or its precipitation in the pores of activated carbon .
CONCLUSIONS
Experiments were carried out on the sorption of protactinium, thorium, uranium, and americium from the FLiNaK eutectic by activated carbon AG-3 at 650°C. It is shown that the sorption isotherms of uranium and thorium fluorides have a pronounced convex character and are described by the Langmuir equation, while the sorption of these elements is approximately an order of magnitude less than that for the previously studied neodymium fluoride.
With an increase in the concentration of neodymium and thorium fluorides in the melt, the extraction of protactinium by activated carbon decreases, and in the presence of thorium it is greater than for neodymium under comparable conditions. It has been suggested that the strong Coulomb interaction of the four-charged protactinium cation with the [ThF8]4– anionic complexes prevents the sorption of protactinium. The decrease in the distribution coefficient of protactinium in the presence of neodymium fluoride is less significant, which is probably due to the lower stability of its complex with protactinium.
A rise in the sorption of protactinium by activated carbon saturated with metallic sodium was found. With increasing the sodium content in activated carbon to 30 wt %, the sorption of protactinium enhances by about 20 times compared to the initial carbon without sodium, and at the same time, the separation factor of protactinium from other studied elements increases by more than 3 times.
Change history
24 April 2023
An Erratum to this paper has been published: https://doi.org/10.1134/S1066362223010186
REFERENCES
Molten Salt Reactors and Thorium Energy, Dolan, T.J., Ed., Amsterdam: Elsevier, 2017.
Velikhov, E.P., Koval’chuk, M.V., Il’gisonis, V.I., Ignat’ev, V.V., Tsibul’skii, V.F., and Andrianova, E.A., Energeticheskaya Politika, 2017, no. 3, p. 12.
Velikhov, E.P., Kovalchuk, M.V., Azitov, E.A., Ignatiev, V.V., Subbotin, S.A., and Tsibulskiy, V.F., At. Energy, 2013, vol. 114, no. 3, p. 193. https://doi.org/10.1007/s10512-013-9695-x
Marin, S.V. and Shatalov, G.E., Atom. Energiya, 1984, vol. 56, no. 5, p. 289.
Kang, J. and Hippel, F.N., Sci. Global Secur., 2001, vol. 9, p. 1.
Fedorov, Yu.S., Samonin, V.V., Zotov, A.A., Khrylova, E.D., Spiridonova, E.A., Miroslavov, A.E., and Akatov, A.A., Radiochemistry, 2021, vol. 63, no. 6, p. 754. https://doi.org/10.1134/S1066362221060072
Savin, S.B., Organicheskie reagenty gruppy arsenazo III (Arsenazo III Organic Reagents), Moscow: Atomizdat, 1971.
Lizin, A.A., Tomilin, S.V., Gnevashov, O.E., Gazizov, A.G., Osipenko, A.G., Kormilitsin, M.V., At. Energy, 2013, vol. 115, no. 1, p. 22.
Khokhryakov, A.A., Vershinin, A.O., Paivin, A.S., and Lizin, A.A., Rasplavy, 2015, no. 4, p. 3.
Smith, A.L., Verleg, M.N., Vlieland de Haas, J.D., Ocadiz-Flores, J.A., Martin, P., Rothe, J., Dardenne, K., Salanne, M., Gheribi A., E., Capelli, E., van Eijck, L., and Konings, R.J.M., Synchrotron Radiat., 2019, vol. 26, p. 124. https://doi.org/10.1107/S160057751801648X
Dai, J., Long, D., Huai, P., and Li, Q., J. Mol. Liq., 2015, vol. 211, p. 747. https://doi.org/10.1016/j.molliq.2015.07.076
Zhao, Z., Hu, J., Cheng Z., et al., RSC Adv., 2021, vol. 11, pp. 7436–7441. https://doi.org/10.1039/d0ra09572a
Grimes, W.R., Nucl. Appl. Technol., 1970, vol. 8, pp. 137–155.
Fredrickson, G., Cao, G., Gakhar, R., and Yoo, T., Report INL INL/EXT-18-51033. 2018. https://inldigitallibrary.inl.gov/sites/sti/sti/Sort_7123.pdf.
Bimova, K.C., Tulackova, R., Straka, M., Keppert, M., Lisy, F., and Soucek, P., Abstracts of Papers, Proc. Int. Conf. ATALANTE 2008. Montpellier (France), 2008. Paper P1_22, p. 1. https://inis.iaea.org/collection/NCLCollectionStore/_Public/40/003/40003958.pdf.
Wang, Y., Ge, J., Zhuo, W., Guo, S., and Zhang, J., J. Nucl. Mater., 2019, vol. 518, p. 162. https://doi.org/10.1016/j.jnucmat.2019.03.007
Wang, Y., Species Chemistry and Electrochemical Separation in Molten Fluoride Salt, PhD Dissertation, Blacksburg, VA (USA), 2019. https://vtechworks.lib.vt.edu/bitstream/handle/10919/102614/Wang_Y_D_2019.pdf?sequence=1&isAllowed=y.
Diagrammy sostoyaniya dvoinykh metallicheskikh sistem: Spravochnik (State Diagrams of Binary Metallic Systems: A Handbook), Myakishev, N.P., Ed., Moscow: Mashinostroenie, 2001.
Van, H.T., Nguyen, T.M.P., Thao, V., Vu, T.H., Nguyen, T.V., and Nguyen, L.H., Water Air Soil Pollut, 2018, vol. 229, p. 293. https://doi.org/10.1007/s11270-018-4043-3
Bahri, M.A., Calvo, L., Gilarranz, M.A., Rodriguez, J.J., and Epron, F., Appl. Catal. B: Environmental, 2013, vol. 138–139, p. 141. https://doi.org/10.1016/j.apcatb.2013.02.048
Khomenko, V., Raymundo-Pinero, E., and Beguin, F., J. Power Sources, 2008, vol. 177, pp. 643–651. https://doi.org/10.1016/j.jpowsour.2007.11.101
Wang, G., Yu, M., and Feng, X., Chem. Soc. Rev., 2021, vol. 50, p. 2388. https://doi.org/10.1039/d0cs00187brsc.li/chem-soc-rev
Atoji, M., J. Chem. Phys., 1974, vol. 60, no. 8, p. 3324. https://doi.org/10.1063/1.1681524
Benson, D., Li, Y., Luo, W., Ahuja, R., and Svensson, G., Inorg. Chem., 2013, vol. 52, no. 11, p. 6402. https://doi.org/10.1021/ic4002219
Fedorov, Yu.S., Samonin, V.V., Zotov, A.S., Khrylova, E.D., Spiridonova, E.A., Miroslavov, A.E., and Akatov, A.A., Radiochemistry, 2022, vol. 64, no. 3, p. 1 https://doi.org/10.1016/j.apcatb.2013.02.048
Fergus, J.W., Mater. Res. Bull., 1996, vol. 31, no. 11, p. 1317. https://doi.org/10.1016/0025-5408(96)00138-9
Funding
The study was carried out with the financial support of the Russian Foundation for Basic Research within the framework
of the RFBR scientific project no. 19-29-02010\19.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated from Radiokhimiya, No. 6, pp. 561–567, December, 2022 https://doi.org/10.31857/S0033831122060090
The original online version of this article was revised: The article “Sorption of Protactinium, Thorium, and Other Actinides from the LiF–NaF–KF Melt by Activated Carbon”, written by Yu.S. Fedorov, V.V. Samonin, A.S. Zotov, E.D. Khrylova, E.A. Spiridonova, A.E. Miroslavov, and A.A. Akatov, was originally published electronically in Springer-Link on February 8, 2023 without Open Access. After publication in volume 64, issue 6, pages 728–734, the authors decided to make the article an Open Access publication. Therefore, the copyright of the article has been changed to © The Author(s) 2022 and the article is forthwith distributed under the terms of a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/, CC BY), which permits use, duplication, adaptation, distribution and reproduction of a work in any medium or format, as long as you cite the original author(s) and publication source, provide a link to the Creative Commons license, and indicate if changes were made. The original article can be found online at https://doi.org/10.1134/S1066362222060091
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fedorov, Y.S., Samonin, V.V., Zotov, A.S. et al. Sorption of Protactinium, Thorium, and Other Actinides from the LiF–NaF–KF Melt by Activated Carbon. Radiochemistry 64, 728–734 (2022). https://doi.org/10.1134/S1066362222060091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362222060091